Mycosis fungoides (MF) and Sézary syndrome (SS) are the most common forms of cutaneous T-cell lymphoma (CTCL).1 Despite being malignancies, the clinical picture of CTCL is characterized by erythematous, eczematous, and papulosquamous skin changes reminiscent of inflammatory skin diseases, such as atopic dermatitis and psoriasis.2 Indeed, lesional skin of MF and SS is often strongly pruritic and shares histological, molecular, and microbial features with lesional skin of inflammatory skin disease. How malignant CTCL T cells cause such profound inflammatory changes in the skin has been a long-standing question in the field. Although the T helper type 2 (TH2)–skewed phenotype of clonal T cells has long been suspected to be responsible,3 mechanistic insight was lacking. In this issue of Blood, Gluud et al provide evidence that malignant T cells in CTCL secrete the cytokines interleukin-13 (IL-13), IL-22, and oncostatin M (OSM) to induce JAK-STAT signaling in surrounding keratinocytes, downregulate filaggrin expression, and impair skin barrier function (see figure).4 Blocking these cytokines or inhibiting downstream JAK-STAT signaling restored these epidermal changes in human skin models. These findings provide formal proof that T-cell–derived cytokines are key mediators of the cutaneous manifestations of CTCL and provide a mechanistic rationale for cytokine and/or JAK targeting in MF and SS.
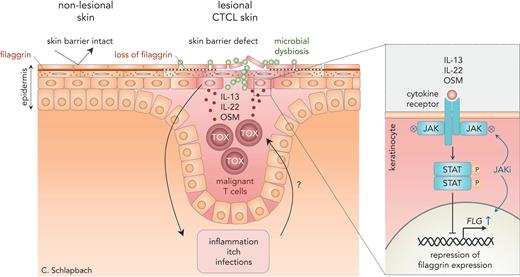
To study skin barrier integrity in CTCL skin lesions, Gluud et al first determined the protein expression of filaggrin, a key structural protein of the epidermis, and measured transepidermal water loss, a proxy for skin barrier function. Strikingly, filaggrin expression was markedly reduced in the immediate vicinity of malignant Thymocyte selection associated high mobility group box (TOX+) T cells infiltrating the epidermis of patients with CTCL, which correlated with increased skin barrier permeability. This suggested that malignant T cells might be secreting factors that impacted barrier function, either directly or via an inflammatory micromilieu. This hypothesis was further supported by the STAT3 activation seen in keratinocytes surrounding the malignant T cells. STAT3 signals downstream of several proinflammatory cytokine receptors, thus implying a mechanism involving T-cell–derived cytokines. By studying the secretome of CTCL cell lines and by analyzing an integrated set of single-cell RNA sequencing data, the authors honed in on the cytokines IL-13, IL-22, and OSM as the prime suspects causing STAT3 activation and downregulation of filaggrin expression. Indeed, interference with signaling of these cytokines, by either blocking antibodies or inhibiting the receptor signaling cascade, restored filaggrin expression in in vitro models of human skin. The results indicated that the combination of IL-13, IL-22, and OSM, rather than one single cytokine, was responsible for the changes seen in CTCL skin. Therefore, the authors studied the effect of the JAK1/3 inhibitor tofacitinib, which blocks signaling of all of these cytokines and is widely used in clinical practice to treat inflammatory diseases, on the barrier defects induced by tumor cell–derived cytokines. As expected, tofacitinib restored filaggrin expression and epidermal hyperproliferation in reconstructed human epidermis and in MF skin ex vivo (see figure). Taken together, Gluud et al describe for the first time a cytokine-mediated cross talk between malignant T cells and surrounding keratinocytes that causes defects in the skin barrier of patients with CTCL that is, in principle, amendable by JAK inhibition.
Cytokines from malignant T cells in CTCL downregulate filaggrin and suppress skin barrier function. In healthy skin, filaggrin promotes integrity of the skin barrier. In cutaneous T-cell lymphoma, malignant T cells produce the cytokines IL-13, IL-22, and OSM, which bind to cytokine receptors on surrounding keratinocytes. This activates the JAK-STAT pathway and downregulates filaggrin expression. Filaggrin deficiency in the epidermis causes defects in the skin barrier, which, in turn, promotes inflammation, microbial dysbiosis, and infections, and may even fuel malignant T-cell growth. JAK inhibitors, such as tofacitinib, reverse these effects and can help restore skin barrier function in CTCL.
Cytokines from malignant T cells in CTCL downregulate filaggrin and suppress skin barrier function. In healthy skin, filaggrin promotes integrity of the skin barrier. In cutaneous T-cell lymphoma, malignant T cells produce the cytokines IL-13, IL-22, and OSM, which bind to cytokine receptors on surrounding keratinocytes. This activates the JAK-STAT pathway and downregulates filaggrin expression. Filaggrin deficiency in the epidermis causes defects in the skin barrier, which, in turn, promotes inflammation, microbial dysbiosis, and infections, and may even fuel malignant T-cell growth. JAK inhibitors, such as tofacitinib, reverse these effects and can help restore skin barrier function in CTCL.
The findings by Gluud et al not only help explain some of the clinical phenomena of CTCL but also raise intriguing questions regarding future therapies. First, they lend further support to the notion that cytokines from malignant T cells are both necessary and sufficient to drive the clinical picture of CTCL.2 This is a direct parallel to inflammatory skin diseases such as atopic dermatitis, where blocking T-cell–derived cytokines with monoclonal antibodies leads to dramatic improvements of disease. Such cytokine-blocking therapies could be repurposed to treat CTCL. Indeed, the anti–IL-4/IL-13 antibody dupilumab has already been used in patients with CTCL, albeit with controversial results. Both progression of CTCL and rapid control of itch and reversal of TH2 bias in the skin have been reported.5,6 Regardless, the article by Gluud et al should spur further research to better understand how cytokine blockade can be leveraged to the benefit of patients with CTCL. Second, the data presented by Gluud et al help explain the abnormal skin microbiome and the high incidence of infections in CTCL. Skin dysbiosis, enabled by skin barrier defects, is a known contributor to skin inflammation, clinical burden, and infectious complications in CTCL.7 Given that infection is arguably the most common cause of death in CTCL, restoration of the skin barrier is of utmost importance in CTCL care. Third, the study by Gluud et al adds to the growing body of evidence that positions JAK-STAT signaling at the center of CTCL pathogenesis.2 On the one hand, hyperactivating mutations in the JAK-STAT pathway are common in clonal T cells, where they promote proliferation and apoptosis resistance.1,2 On the other hand, JAK-STAT signaling is also key in mediating the detrimental effects of the malignant T cells’ cytokines on their environment. This puts the JAK-STAT pathway into the limelight for CTCL therapy. Malignant T cells could, for instance, be targeted by mutation-specific JAK inhibitors or STAT3 degraders.8 More broadly, JAK inhibitors could be used to inhibit the effect of tumor cell–derived cytokines, restore the epithelial barrier, normalize microbial dysbiosis, decrease the risk of infection, and improve the quality of life of patients with CTCL.
Despite these intriguing findings, utmost caution is in order when considering immunosuppressive treatment in CTCL. As long as we lack a detailed understanding of each cytokine’s role and the pharmacological tools to specifically inhibit malignant T cells and their products, there is a high risk of inadvertently impairing anti-tumor immunity and promoting tumor progression. In particular, interfering with JAK-STAT signaling using current JAK inhibitors will also affect signaling of key anti-tumor cytokines, such as type I and type II interferons. Therefore, further study and new therapeutic tools are needed in CTCL before we can expect the therapeutic success we have become accustomed to with immunomodulation in inflammatory skin disease.
Conflict-of-interest disclosure: C.S. has received honoraria as adviser for Abbvie, LEO Pharma, Kyowa Kirin, Lilly, and Novartis and has received research funding from PPM Services.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal