Abstract
Human T-cell leukemia virus type 1 (HTLV-1), also known as human T-lymphotropic virus type 1, causes the aggressive malignancy known as adult T-cell leukemia/lymphoma (ATL) in 5% of infected people and a chronic progressive inflammatory disease of the central nervous system, HTLV-1–associated myelopathy, in ∼0.3% to 4% of them, varying between regions where it is endemic. Reliable treatments are lacking for both conditions, although there have been promising recent advances in the prevention and treatment of ATL. Because ATL typically develops after several decades of infection, it is necessary to understand how the virus persists in the host despite a strong immune response, and how this persistence results in oncogenesis.
HTLV-1
Human T-cell leukemia virus type 1 (HTLV-1) is a retrovirus1,2 distantly related to HIV-1. It has been present in the human population for more than 20 000 years,3,4 and is estimated to infect at least 10 million people globally,5 but the true number is likely to be greater because systematic epidemiology has not been carried out in some large areas of endemic HTLV-1 infection. Although HTLV-1 mainly infects CD4+ (helper) T cells, ∼5% of the proviral load (PVL) is present in CD8+ (cytotoxic) T cells6; the adult T-cell leukemia/lymphoma (ATL) clone is typically a CD4+ cell. The virus induces a T helper cell 1–like phenotype in the infected cell,7 which may contribute to the pathogenesis of the inflammatory disease HTLV-1–associated myelopathy. The virus is almost 100% cell associated, and infection is propagated both within and between individuals through a specialized cell-to-cell contact called the virological synapse.8,9 The infection is, therefore, spread in the population by the transfer of lymphocytes via 2 main routes: breastfeeding and sexual intercourse; transfer of cellular blood products (including occupational exposure) and solid organ transplantation10 account for a small proportion of cases.11 Three closely related retroviruses that infect humans have been discovered: HTLV-2 infection is endemic to certain indigenous populations12 and HTLV-3 and HTLV-4, present in nonhuman primates in sub-Saharan Africa,13 infect humans only sporadically. However, none of these viruses appear to cause malignant disease in humans.
The strongest correlate of the risk of both HTLV-1–associated myelopathy14 and ATL15 is the PVL, that is, the percentage of peripheral blood mononuclear cells (PBMCs) infected with the virus. The PVL is usually higher than that seen in HIV-1 infection, and often exceeds 5% of PBMCs. The PVL is stable in each host within a factor of approximately fivefold but differs between individuals by more than 1000-fold.14 The PVL is proportional to the total number of HTLV-1+ T-cell clones in the individual,16 which typically lies between 103 and 105 but can exceed 106.17 Each clone carries a single copy of the HTLV-1 provirus integrated in a unique position in the host cell genome.18 HTLV-1 replication does not kill the host T cell, and many infected clones can be repeatedly detected for at least 10 years.16,19 It is likely that most of them survive for the remaining lifetime of the host. This clonal longevity is key to understanding the oncogenesis of ATL.
Persistence of HTLV-1 in the host
Like other retroviruses, HTLV-1 can, in principle, remain latently integrated in the host genome, and in this way it can escape recognition and destruction by the immune system. However, a proportion of cells must retain the capacity to re-express the virus, both to maintain the cell’s proliferative advantage over uninfected CD4+ T cells and to enable transmission to another host. It is now known that the replicative genes of the virus, encoded on the plus-strand of the provirus, are expressed in each cell in rare, intense, self-limiting bursts.20,21 By contrast, the single gene, HBZ, encoded on the proviral minus-strand, is expressed by each cell ∼50% of the time.22 At the cell population level, the provirus is persistently expressed, as evidenced by the persistent activation of the HTLV-1–specific immune response.23
The PVL reaches an equilibrium or set point in each host, partly determined by the efficiency of that person’s immune response, which, in turn, depends on the host’s genotype, in particular the HLA class 1 and killer immunoglobulin-like receptor complexes.24,25 The CD8+ cytotoxic T-lymphocyte (CTL) response plays an especially important part in determining the outcome of infection.23 A detectable CTL response to the frequently expressed HBZ protein is associated with a low PVL and a low risk of disease.26-28 By contrast, even a strong CTL response to the immunodominant antigens encoded on the plus-strand, particularly the viral transactivator protein Tax, makes less impact on the PVL27 because the rare transcriptional bursts make only a small proportion of infected cells susceptible to killing by the anti-Tax CTLs at a given instant. However, the critical CTL target antigen HBZ is poorly immunogenic and is expressed at a very low level.20,29 Therefore, the low frequency of the proviral plus-strand bursts and the low-level expression of HBZ contribute to the persistence of the virus in vivo by limiting the effectiveness of immune surveillance. Cell stress can trigger a transcriptional burst of the proviral plus-strand,30 but it is not known whether stress or other factors govern the frequency of bursting in vivo.
Selective survival of HTLV-1+ clones in nonmalignant HTLV-1 infection
Three observations indicate that there is selective clonal survival of certain HTLV-1–infected T cells in the nonmalignant cell population during chronic HTLV-1 infection. Firstly, T cells infected with HTLV-1 in vitro carry the single-copy provirus integrated in any chromosome, with a frequency proportional to chromosome size.31 But in vivo, the clones that survive carry the provirus disproportionately often in 1 of the 5 acrocentric chromosomes,31-33 especially chromosome 13.32 Secondly, the wide variation between individual HTLV-1 clones in the PVL,14 which depends on the host’s immune response,23 indicates that a host who controls the virus efficiently, that is, with a low PVL, may possess 1000-fold fewer HTLV-1+ clones than a host with an inefficient immune response to the virus.17 Thirdly, the PVL rises quickly during primary infection, doubling every ∼1.4 days,34 but many clones are then lost before the PVL settles to the set point.35 We recently reported the unexpected observation that selective clonal survival of HTLV-1+ cells is strongly associated with 2 spatial factors, that is, the radial position of the provirus in the nucleus and the genomic distance of the provirus from the centromere on each chromosome.31 These factors correlate with clonal survival independently of the transcriptional intensity of the host genome flanking the provirus, suggesting that transcriptional latency is not the sole factor that is selected for clonal survival. How the spatial factors confer a survival advantage on the infected cell is not known; the virus appears to exploit topological attributes of the genome that are not yet identified.
Oncogenic mutations in ATL
There is no hotspot of integration of the HTLV-1 provirus in the host genome.32 Integration near a number of potential oncogenes occurs more often than by chance in ATL clones32 and may cause transactivation of these genes,36 but such clones account for only a small fraction of ATL cases.
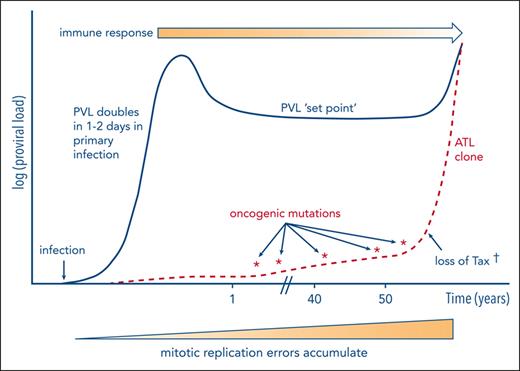
HTLV-1 oncogenesis (schematic). During chronic infection, HTLV-1 replicates persistently via the turnover of existing HTLV-1–infected T-cell clones and a low rate of infection of new clones. Each infected cell expresses HBZ ∼50% of the time and the plus-strand genes (notably tax) in rare intermittent bursts. The host immune response, particularly the CTL response, reaches an equilibrium with the persistent replication of HTLV-1 at the set point of PVL. The efficacy of the immune response is diminished as ATL develops; Tregs induced or maintained by HBZ may contribute to the immune suppression. The main source of oncogenic mutations appears to be a mitotic replication error. In addition, Tax protein may exert direct oncogenic effects (see “Oncogenic actions of HTLV-1 products”); genome-wide deposition of the transcriptionally repressive mark H3K27me3 also often contributes to ATL oncogenesis. † indicates that in ∼40% of cases of ATL, Tax expression is lost, probably as a result of immune-mediated selection.
HTLV-1 oncogenesis (schematic). During chronic infection, HTLV-1 replicates persistently via the turnover of existing HTLV-1–infected T-cell clones and a low rate of infection of new clones. Each infected cell expresses HBZ ∼50% of the time and the plus-strand genes (notably tax) in rare intermittent bursts. The host immune response, particularly the CTL response, reaches an equilibrium with the persistent replication of HTLV-1 at the set point of PVL. The efficacy of the immune response is diminished as ATL develops; Tregs induced or maintained by HBZ may contribute to the immune suppression. The main source of oncogenic mutations appears to be a mitotic replication error. In addition, Tax protein may exert direct oncogenic effects (see “Oncogenic actions of HTLV-1 products”); genome-wide deposition of the transcriptionally repressive mark H3K27me3 also often contributes to ATL oncogenesis. † indicates that in ∼40% of cases of ATL, Tax expression is lost, probably as a result of immune-mediated selection.
The provirus of HTLV-1 in the malignant clone is usually single copy, as in the majority of nonmalignant HTLV-1+ cells, but 2 or more proviral copies are found more often in malignant than in nonmalignant clones.32,37 In cells with >1 provirus, 1 or more copies are often defective but may retain the ability to express HBZ, contributing to clonal dominance (see “Oncogenic actions of HTLV-1 products”).
Like the majority of malignancies of adults, ATL results from a multistage process of oncogenesis; that is, the progressive acquisition of a number of somatic mutations, each conferring on the clone a progressive advantage in survival, proliferation, or both. A landmark study in 2015 reported the identification by whole-exome sequencing of putative oncogenic drivers in 426 cases of ATL in Japan.38 A high proportion of recurrently detected somatic mutations were found in genes in the signaling pathway of the T-cell antigen receptor (TCR), NF-κB, and other genes involved in pathways that are characteristically expressed in T cells. The most frequent changes included activating mutations in PRKCB, PLCG1, VAV, and CARD11. More recently, these authors carried out whole-genome sequencing of 150 cases of ATL.39 The results revealed 10 oncogenic drivers that had been previously unrecognized, which were present in 56 recurrently mutated genes. The median number of oncogenic driver events identified mutated in each case was 9, and 149 of 150 cases had >1 driver alteration. An important outcome of this study39 was to identify the contribution of point mutations in intergenic (noncoding) regions of the genome, which were not identified in the preceding whole-exome study. Other repeatedly observed mutations in pathways that confer an advantage to healthy CD4+ T cells include those in genes that encode certain transcription factors (IRF4, GATA3, and IKZF2) and chemokine receptors (CCR4, CCR7, and GPR183).
The most surprising finding made by Kogure et al39 was a remarkably high frequency of mutations (53% of cases) in a transcriptional repressive complex, CIC-ATXN1. This mutation had gone unrecognized in the earlier study because the genetic structure of the complex was not completely understood then. Experiments in mice showed that such mutations in the CIC-ATXN1 complex can increase the number of cells with a phenotype (CD4+CD25+CD127−FoxP3+) characteristic of the immunosuppressive regulatory T cells (Tregs) in the circulation. This Treg-like phenotype is frequent in both ATL clones and nonmalignant HTLV-1–infected cells,40,41 and secretion of the chemokine CCL22 by HTLV-1–infected T cells maintains a high population of CCR4+ Tregs among both infected and uninfected CD4+ T-cell populations.42 It is likely that the phenotype confers an advantage to the virus and the malignant clone by diminishing the effectiveness of the cellular immune response.41 The products of the HBZ gene of HTLV-1 also confer a Treg-like phenotype on the cell.43 This function of HBZ has presumably conferred an evolutionary advantage to the virus. ATL may arise from a cell that already possesses Treg function, but in most cases it is likely that Treg functions are acquired through the actions of HBZ43 and as a result of successive mutations or epigenetic changes.44
The importance of the immune response in surveillance against ATL is emphasized by 3 further observations. Firstly, driver mutations in ATL are also frequently observed in class 1 HLA genes (HLA-A and -B), which are key genes that determine the specificity and efficiency of the CTL response, and certain other genes involved in immune surveillance, including CD58 and FAS.38,39 Secondly, immune suppression increases the risk of developing ATL.45 Thirdly, structural mutations are frequently found in ATL38,46 in the 3′-untranslated region of the PD-L1 gene, which encodes the immunosuppressive PD-L1 protein familiar as a target of checkpoint-inhibitor therapy. Koya et al,47 in a single-cell analysis of nonmalignant and malignant HTLV-1–infected cells, obtained evidence that the upregulated PD-L1 expression observed in certain cases of ATL can promote the expression of PD-L1 in other cell types, perhaps contributing further to the severe immune suppression observed in ATL. However, it is not yet clear whether treatment with anti–PD-1/PD-L1 antibodies can be of therapeutic benefit in ATL because of the small numbers of patients and the diverse subtypes of disease included in clinical trials to date.48,49 Also, Misawa et al, in 2022,50 reported a case in which treatment of a patient with PD-1/PD-L1 blockade for nonsmall-cell lung cancer was followed by the emergence of ATL.
Kataoka et al51 found that specific driver mutations carry a different prognostic significance in ATL. Mutations in PRKCB, TP53, IRF4, and CDKN2A were more common in the aggressive forms of the disease, whereas STAT3 mutations were more likely to be found in the indolent form. The combination of mutation in PRKCB and amplification of chromosome 9p24, which contains the PD-L1 gene, was associated with a particularly poor prognosis. The authors identified 2 groups of cases based on the mutations present. Patients with chronic ATL were mainly in group 1, characterized by mutations in genes encoding molecules upstream in the TCR/NF-κB signaling pathway, including PLCG1, VAV1, CD28, RHOA, and STAT3. Patients with the lymphomatous form of ATL were found in group 2, with a larger number of mutations, particularly in genes downstream in the TCR/NF-κB signaling pathway, including PRKCB and IRF4, and in HLA class 1, CD58, TET2, and EP300. Patients with acute ATL or smoldering ATL were found in both groups.
Evolution of the ATL clone, abnormal oligoclonality, and early detection of disease
The unique genomic integration site of the HTLV-1 provirus can serve as a clone-specific marker. Consequently, in some cases in which samples of PBMCs from an individual have been stored before the clinical presentation of ATL, the same clone can be identified, and the timing of the successively acquired driver mutations can be documented. Using this approach, Rowan et al19 and Wolf et al52 reported the time course of appearance of driver mutations in the premalignant clone in 6 incident cases of ATL, from each of whom several PBMC samples were stored before the emergence of the disease. The results showed that, although the mutational burden in the premalignant clone typically increased in the 6 months before diagnosis, driver mutations could be detected in the clone up to 10 years earlier.52 Yamagishi et al53 also reported the sequential appearance of mutations during the evolution of the malignant clone, and these authors reviewed54 the contribution of both genetic and epigenetic modifications to this evolution. The evolution of the ATL clone is depicted schematically in Figure 1.
The ability to detect potential driver mutations before the development of ATL raises the question whether it is possible to identify HTLV-1–infected individuals at high risk of progression to malignancy, who might benefit from treatment to halt or slow the progression. The following 4 criteria of high risk can be identified in a patient who is immunocompetent15,19,45,55: age >60 years, a PVL >4% of PBMCs, mutations in known oncogenic drivers, and disproportionate proliferation of 1 clone.
A cardinal feature of HTLV-1 infection is oligoclonal proliferation of the infected cells, that is, the growth to high abundance of a small number of infected T-cell clones in the circulation.56,57 This oligoclonal proliferation, which is present in some degree in all HTLV-1–infected persons, does not necessarily presage the onset of ATL.32 However, early detection of exceptional clonal proliferation might allow for early intervention in the emergence of a premalignant clone to prevent or slow down the progression to frank malignancy. Early identification of people who are infected and at risk of progression requires objective quantification of the degree of oligoclonality. Rowan et al19,52 have developed a practical assay to quantify oligoclonality in the laboratory: the oligoclonality index16 measured via flow cytometry, based on the Gini index, quantifies the degree to which 1 or a small number of clones dominates in the population with PBMC. These authors showed that an oligoclonality index measured via flow cytometry >0.77 was associated with a high risk of subsequent development of ATL.
Molecular mechanisms in oncogenesis of ATL: mitotic replicative error
It was previously believed that the HTLV-1 tax gene was the primary cause of ATL. Tax transactivates many host genes as well as the proviral plus-strand; persistent Tax expression can immortalize cells in vitro and induce tumors of certain cell types in transgenic mice. Persistent expression of tax, under the control of the lck promoter, resulted in T-cell tumors in transgenic mice.58 However, the relevance of these observations to the oncogenesis of ATL in humans is not clear. A dominant role of tax in ATL oncogenesis now appears unlikely, for the following reasons: firstly, tax is expressed in rare, intermittent bursts in each nonmalignant cell; secondly, tax expression is lost in ∼50% of the ATL cases, by either mutation, deletion, or DNA methylation59; and thirdly, ATL can develop in a clone with provirus that was defective in tax expression at the time of integration60; such type 2 defective proviruses, which are found in both asymptomatic carriers of HTLV-161 and in some ATL clones, lack the 5′ long terminal repeat and part of the adjacent region of the provirus. However, direct effects of Tax may contribute to oncogenesis in some cases of ATL (see “Oncogenic actions of HTLV-1 products”).
Two chief factors are associated with the acquisition of driver mutations and the disproportionate expansion of a clone that culminates in ATL: the duration of infection and a high PVL.15 As outlined earlier, a high PVL is made up of a large number of HTLV-1+ clones: the clones appear to survive indefinitely and turn over more frequently than uninfected CD4+ T cells62; that is, they are effectively immortalized in vivo. Furthermore, most cases of ATL in immunocompetent individuals arise in those infected during infancy, usually via breastfeeding. Each clone has therefore passed through a large number of mitotic events in a person aged >60 years who is infected with HTLV-1. Each mitotic event is accompanied by a low frequency of mutations, most commonly single-nucleotide misincorporations, known as replicative errors. Mutations therefore commonly arise, probably randomly, in HTLV-1–infected T-cell clones19 and do not necessarily presage ATL; only certain mutations contribute to the oncogenesis. Tomasetti and Vogelstein63,64 showed that the risk of malignant transformation in a wide range of cell types is strongly correlated with the total number of mitoses that the cell lineage has undergone. Therefore, a simple explanation for the progressive generation of the oncogenic driver mutations that culminate in ATL is mitotic replicative error. The probability that 1 or more infected clones undergoes malignant transformation will depend on the number of clones present in that person and on the time since infection, explaining the observed correlations with age and with the PVL. The trajectory of the PVL and the emergence of an ATL clone are shown schematically in Figure 1.
Each HTLV-1–infected T-cell clone has a proliferative rate and, therefore, a malignant potential that is partly determined by the proviral integration site, the antigen specificity of the clone, and the epigenetic modifications and mitotic errors that it acquires. There is, therefore, a continuum of potential for malignant transformation across the whole population of infected cells in each individual. Consequently, there are well described cases in which >1 clone has transformed65-68: this is most clearly observed among patients in whom the dominant clone has been eliminated, either by drug treatment or, in rare cases, by spontaneous regression,67 and in whom a second clone becomes fully transformed. This phenomenon of clonal succession, in which 1 clone is replaced by a second, unrelated clone, differs fundamentally from the progressive subclonal diversification of 1 clone that commonly occurs in solid tumors.
Oncogenic actions of HTLV-1 products
For the aforementioned reasons, it is likely that the dominant mechanism of oncogenic mutations in ATL is mitotic replicative error. However, although the 2 key regulatory genes of HTLV-1, tax and HBZ, are not rapidly transforming oncogenes, such as myc or src, their products may contribute to oncogenesis. The accessory regulatory proteins1 of HTLV-1 (p12, p13, and p30) have not been shown to play a direct part in ATL oncogenesis. The impact of HTLV-1 Tax protein has been extensively studied.69,70 Tax is well-documented to cause double-strand DNA breaks, micronuclei,71,72 and clastogenesis.73 Giam and Pasupala74 recently reported that NF-κB hyperactivation by Tax led to the formation of R-loops, which, when removed by transcription-coupled nucleotide excision repair, resulted in double-strand DNA breaks and cellular senescence. However, HBZ prevents senescence of the infected cell,75 perhaps by its known action in inhibiting canonical NF-κB signaling.76 HBZ also promotes clonal longevity by inducing telomerase (hTERT) expression.77 Tax also suppresses expression of DNA polymerase β78 and inhibits both certain cell-cycle checkpoints79,80 and the DNA damage responses.81 Finally, Tax functionally inactivates p53,82,83 and TP53 is mutated in a proportion of cases of ATL, especially in the rapidly progressive form.51 These effects cause genetic instability, which, in turn, may confer a survival or proliferative advantage to the clone. Furthermore, 2 endonucleases involved in this repair mechanism, xeroderma pigmentosum F and G, are often deficient in ATL.84
Tax expression is lost via either deletion, mutation, or DNA methylation in ∼50% of ATL clones,59 presumably as a result of immune-mediated selection. However, Tax may contribute in the early stages of oncogenesis and, possibly, to the maintenance21 of the malignant clone in cases in which it is retained.
In contrast, HBZ appears to be retained in all ATL clones, and although each nonmalignant infected cell expresses HBZ ∼50% of the time,22 its expression in ATL cells is more sustained and may be constant. These observations suggest that HBZ plays a necessary part in ATL oncogenesis85 and imply that HBZ is a critical component of a possible future vaccine.26-28 Paradoxically, HBZ opposes many of the actions of Tax.1 For example, HBZ inhibits Tax-mediated transactivation of the proviral plus-strand by blocking the binding of the transcription factors CREB and CBP/p300.86 This modulation of Tax-mediated effects by HBZ is thought to be essential in regulating the proviral expression and in promoting the longevity of the infected clone.
HBZ may contribute to ATL oncogenesis by 2 chief mechanisms. Firstly, both HBZ protein and HBZ messenger RNA promote proliferation of the cell.87 This effect may be critical in maintaining the proliferative advantage of both nonmalignant HTLV-1–infected cells and ATL cells over uninfected T cells. The molecular mechanisms of these effects are not fully understood, but there is recent evidence that HBZ binds to a superenhancer of BATF3, a master regulator of ATL cell survival.88 Secondly, HBZ increases the expression of several molecules characteristic of Tregs, particularly FoxP3, TIGIT, and CCR4.89 The resulting Treg-like phenotype may contribute to the severe immune suppression observed in ATL; moreover, impairment of the immune response is likely to confer a further survival advantage to the ATL clone. Thus, the chief role of HBZ in both nonmalignant cells and ATL oncogenesis is to promote the survival and proliferation of the clone. Finally, epigenetic modification of the host genome contributes to ATL oncogenesis. There is frequent widespread deposition of the repressive histone modification H3K27me3,90,91 as observed in many solid tumors, and genome-wide hypermethylation of DNA may be a target for therapeutic intervention.92
The HTLV-1 provirus binds the chromatin architectural protein CTCF,93 resulting in the formation of unusual chromatin loops and clone-specific deregulation of host genes flanking the provirus94; this deregulation may contribute to oncogenesis in certain cases. It is likely that CTCF confers an evolutionary advantage to the virus by regulating either proviral expression95 or the spatial position of the provirus in the nucleus31 or both; however, further work is needed to elucidate the mechanisms of this putative advantage.96,97
Coinfection with the nematode Strongyloides stercoralis may increase the risk of ATL, but it is difficult to distinguish the cause and effect in this complex coinfection.98
Implications for clinical management of ATL
If the main mechanism of oncogenesis in ATL is persistent mitotic replicative error in the long-lived HTLV-1–infected T-cell clones, what are the implications for clinical management? Current guidance on therapeutic options for the different forms of ATL has been recently reviewed99; here, I consider the underlying principles. These principles are not fundamentally different from those in other malignancies; what is unusual in ATL is that the premalignant clone can, in some cases, be identified at an early stage in the oncogenic process,52 making possible an understanding of the order and the trajectory of the successive steps in oncogenesis19,53,54 and raising the possibility of early detection and intervention in individuals at high risk.
Perhaps the chief reason for the refractoriness to treatment of ATL is the huge diversity of HTLV-1–infected clones in each person.16 If 1 clone has undergone transformation to ATL, it is likely that other clones in that individual have acquired driver mutations. There is evidence for this in the well-documented phenomenon of clonal succession.68
As in other malignancies, an important therapeutic principle is to target more than 1 pathway that confers a proliferative or survival advantage to the clone, in order to minimize the rate of emergence of resistance mutations. The AKT/mammalian target of rapamycin pathway plays an important part in ATL cell survival and proliferation.100 Daenthanasanmak et al101 showed that simultaneous treatment with 3 agents, which respectively inhibit BET (bromodomain and extraterminal motif protein), phosphatidylinositol 3-kinase, and NF-κB, strongly inhibited the growth of ATL cells both in vitro and in xenografts of ATL cells in mice, reducing c-myc expression and increasing apoptosis. Similarly, inhibition by valemetostat of both enhancers of zeste homolog 1 and 2, central enzymes in the polycomb transcriptional complexes, has theoretical potential as a new therapeutic agent in ATL.102
Prevention of ATL is not only preferable in principle but is also more likely to be successful in practice. The rationale of prevention is to minimize the rate of acquisition of potential driver mutations by reducing the total number of mitotic events in the infected cell population. This is effected by reducing both the PVL and the rate of proliferation of the infected cells. The discovery that combination treatment with type 1 interferon and zidovudine could prevent or slow the progression of ATL, especially in indolent cases of the disease, provided the first sign that this approach can be successful.103-105 More recently, treatment with the anti-CCR4 monoclonal antibody mogamulizumab has led to significant improvement in overall survival rates,106-108 especially if it is included in first-line therapy with a CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone)–like regimen.109,110 Mogamulizumab has the following 2 important therapeutic effects in ATL: in addition to a direct lytic effect on the CCR4-expressing ATL clone, the antibody also reduces the population of the immunosuppressive Treg population, allowing the recovery of a stronger antitumor cell-mediated immune response.111
Cheminant et al112 reported that the inhibitory receptor KIR2DL3 is frequently highly expressed in ATL, especially in the acute type of the condition. Therefore, this molecule may offer a further potential therapeutic target.
Successful intervention to prevent the development of ATL depends on the early identification of individuals who are infected with HTLV-1 and are at high risk of progression to allow for effective intervention while avoiding unnecessary treatment of the majority of hosts. Further work is needed to define the best combination of criteria for early intervention; these criteria may include high PVL (eg, >4% PBMCs),15 disproportionate oligoclonality,52 and presence of specific driver mutations.51
Acknowledgments
The author thanks Masao Matsuoka, Lucy Cook, Aileen Rowan, and Graham Taylor for their helpful comments on the manuscript.
Authorship
Contribution: C.R.M.B. wrote the manuscript and designed the figure.
Conflict-of-interest disclosure: C.R.M.B. declares no competing financial interests.
Correspondence: Charles R. M. Bangham, Institute of Infection, Imperial College London, Exhibition Rd, London SW7 2AZ, United Kingdom; e-mail: c.bangham@imperial.ac.uk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal