In this issue of Blood, Grover et al report that deficiency of the plasma serpin C1 inhibitor (C1-INH) enhances thrombin generation in human plasma and venous thrombus growth in mice.1 Last year this group presented epidemiologic evidence indicating patients with hereditary angioedema (HAE), most of whom have a deficiency of C1-INH, are at moderately increased risk for venous thromboembolism (VTE).2 In the current study, they convincingly demonstrate that C1-INH deficiency is procoagulant in vitro and prothrombotic in a rodent model. However, the proposition that C1-INH deficiency significantly increases VTE risk in humans is likely to be controversial, as it runs counter to the clinical impression that patients with HAE, despite having recurrent episodes of soft-tissue edema due to hyperactivity of the plasma kallikrein-kinin system (KKS), rarely have thrombotic events.3-5
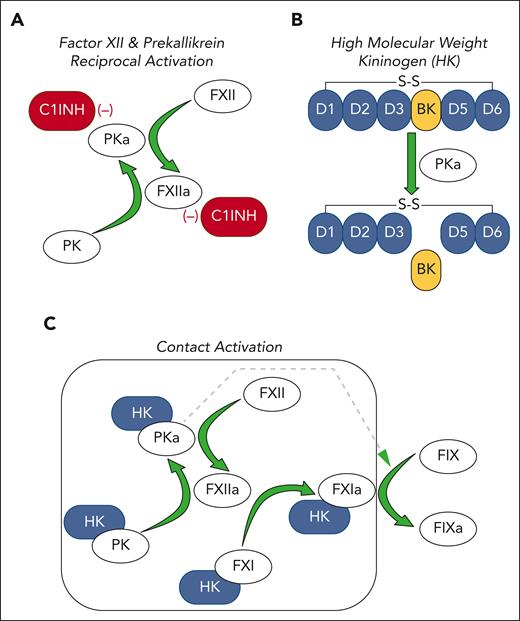
The KKS comprises the protease zymogens factor XII (FXII) and prekallikrein (PK) and the cofactor/substrate high molecular weight kininogen (HK).3-5 In plasma, FXII and PK reciprocally convert each other to the proteases FXIIa and plasma kallikrein (PKa), respectively (see figure panel A).6 PKa cleaves HK, releasing the vasoactive peptide bradykinin (see figure panel B), which binds to the G protein–coupled bradykinin B2 receptor, promoting vasodilatation and vascular permeability, among its several effects.3-5 C1-INH is the main regulator of FXII/PK reciprocal activation. Patients with HAE typically have 5% to 35% of the normal C1-INH concentration in plasma,3,5 rendering them susceptible to processes that accelerate FXII and PK activation. This leads to episodes of bradykinin-induced soft-tissue edema involving the face, oropharynx, hands, genitals, and/or gastrointestinal tract that may be life threatening.
The plasma KKS and contact activation. (A) The plasma zymogens FXII and PK undergo reciprocal activation in solution to the proteases FXIIa and PKa, respectively. This process is restricted by C1-INH, which inhibits both FXIIa and PKa. (B) Most PK in plasma circulates in a complex with HK. HK has 6 domains (D1-D6), the fourth of which contains the 9 amino acid bradykinin (BK) sequence. PKa cleaves HK at 2 locations to release bradykinin. (C) FXII, PK, and HK bind to a variety of surfaces (gray box). This enhances FXIIa and PKa formation and BK generation by a process called contact activation. HK serves as a cofactor for PK binding to the surface, in addition to being a substrate for PKa. During contact activation, FXI also binds to the surface in an HK-dependent manner and is converted to the active protease FXIa. FXIa activates the coagulation protease FIX to FIXa, which drives thrombin generation and plasma clot formation. PKa also activates FIX but much less efficiently than FXI. In all panels, green arrows indicate protease-mediated reactions.
The plasma KKS and contact activation. (A) The plasma zymogens FXII and PK undergo reciprocal activation in solution to the proteases FXIIa and PKa, respectively. This process is restricted by C1-INH, which inhibits both FXIIa and PKa. (B) Most PK in plasma circulates in a complex with HK. HK has 6 domains (D1-D6), the fourth of which contains the 9 amino acid bradykinin (BK) sequence. PKa cleaves HK at 2 locations to release bradykinin. (C) FXII, PK, and HK bind to a variety of surfaces (gray box). This enhances FXIIa and PKa formation and BK generation by a process called contact activation. HK serves as a cofactor for PK binding to the surface, in addition to being a substrate for PKa. During contact activation, FXI also binds to the surface in an HK-dependent manner and is converted to the active protease FXIa. FXIa activates the coagulation protease FIX to FIXa, which drives thrombin generation and plasma clot formation. PKa also activates FIX but much less efficiently than FXI. In all panels, green arrows indicate protease-mediated reactions.
Reciprocal FXII/PK activation is enhanced by a process called contact activation, which occurs when KKS proteins bind to certain macromolecules or “surfaces” (see figure panel C).6 Bradykinin generation increases as a consequence of the increased PKa. A variety of organic (eg, nucleic acids, glycosaminoglycans) and inorganic (eg, polyphosphates, silicates) substances support contact activation in vitro.5-7 High local levels of bradykinin, probably driven by contact activation, contribute to tissue edema at injury sites. It is assumed that a surface-driven process (either local or systemic) is involved in angioedema in HAE patients, although this is not certain in all cases, and the nature of the contact-inducing substances has not been firmly established.
During contact activation, FXI, a homolog of PK, binds to the surface and is converted to the protease FXIa by FXIIa (see figure panel C).5,6 FXIa is a potent activator of the coagulation protease FIX. In the activated partial thromboplastin time assay, clotting is induced by adding a surface such as silica to plasma that drives massive activation of the KKS, leading to FXIa production and clot formation. Contact activation does not appear to be required for hemostasis at a site of injury and may have a limited role in common thrombotic disorders such as myocardial infarction, stroke, and VTE. However, nonbiological surfaces of medical devices, such as those used in cardiopulmonary bypass, extracorporeal membrane oxygenation, and renal dialysis, support the reactions shown in figure panel C.7 The requirement for systemic anticoagulation to prevent thrombus formation during procedures involving these devices reflects the prothrombotic nature of contact activation.
Grover et al show that thrombin generation is significantly increased in C1-INH–deficient plasmas from patients with HAE than in plasmas from healthy controls when silica-induced contact activation is used to initiate clotting through the intrinsic pathway. In contrast, thrombin generation is similar in normal and HAE plasmas supplemented with tissue factor, which promotes clotting through the extrinsic pathway. These findings support the hypothesis that KKS dysregulation contributes to clotting in HAE plasma. Then, using an inferior vena cava stenosis model, they showed that mice lacking C1-INH (C1inh−/−) developed significantly larger thrombi than wild-type mice. This difference disappeared if C1inh−/− mice were first treated with human C1-INH. Interestingly, at baseline C1inh−/− mice have elevated plasma levels of markers of thrombin generation, such as thrombin-antithrombin complex. Similar findings have been reported in plasmas from patients with HAE at baseline, with levels increasing during episodes of angioedema.8,9
In their earlier study of patients with HAE,2 Grover et al suggested C1-INH deficiency confers a modest increase in VTE risk, on the order of that associated with heterozygosity for the factor V-Leiden polymorphism. Considering this, and the rarity of HAE (∼1 in 50 000 individuals), most clinicians taking care of patients with HAE will see few thrombotic events. Perhaps this explains the impression that thrombosis risk is not increased in HAE. Yet, given the marked increase in KKS activity during bouts of angioedema, it is surprising that we do not see more thrombosis in these patients. Unlike reciprocal activation of FXII and PK, which occurs without a surface, FXI activation by FXIIa is surface dependent.6 Perhaps a classic surface-driven process is not responsible for bradykinin production in many patients with HAE. Indeed, a form of HAE associated with a FXII mutation, rather than reduced C1-INH, appears to promote bradykinin generation in a surface-independent manner.10 Alternatively, the types of surfaces involved in triggering HAE may interact poorly with FXI.3,4 Even if some FXIa is formed, it can be inhibited by several plasma serpins other than C1-INH, blunting its thrombotic potential. Yet another possibility is that bursts of intense thrombin generation during most HAE attacks are of insufficient duration to support thrombus growth to the point that it becomes clinically apparent.
The therapeutic implications of the work by Grover et al are not clear at this point, but it seems reasonable to consider prophylactic anticoagulation with illnesses or procedures that may precipitate attacks of angioedema in patients with HAE. Long-term prophylaxis for patients with thrombotic events may be difficult with traditional infusions of C1-INH, but newer agents with long half-lives such as anti-FXII(a) or anti-PK(a) antibodies or chronic therapy with oral PKa inhibitors may be useful for reducing thrombotic risk.
Conflict-of-interest disclosure: D.G. is a consultant for pharmaceutical companies (Anthos Therapeutics; Aronora, Inc; Bayer Pharma; Bristol-Myers Squibb; Ionis Pharmaceuticals; Janssen Pharmaceuticals) with interests in targeting factor XI, factor XII, and prekallikrein for therapeutic purposes.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal