Key Points
Patients with CNS-1 and CNS-2 status have similar outcomes across 2 large studies with divergent therapies, including with and without CRT.
Patients with CNS-3 T-ALL treated with nelarabine had similar OS as CNS-1 and CNS-2, and thus should receive nelarabine as standard of care.
Abstract
To determine the prognostic significance of central nervous system (CNS) leukemic involvement in newly diagnosed T-cell acute lymphoblastic leukemia (T-ALL), outcomes on consecutive, phase 3 Children’s Oncology Group clinical trials were examined. AALL0434 and AALL1231 tested efficacy of novel agents within augmented-Berlin-Frankfurt-Münster (aBFM) therapy. In addition to testing study-specific chemotherapy through randomization, the AALL0434 regimen delivered cranial radiation therapy (CRT) to most participants (90.8%), whereas AALL1231 intensified chemotherapy to eliminate CRT in 88.2% of participants. In an analysis of 2164 patients with T-ALL (AALL0434, 1550; AALL1231, 614), 1564 had CNS-1 (72.3%), 441 CNS-2 (20.4%), and 159 CNS-3 (7.3%). The 4-year event-free-survival (EFS) was similar for CNS-1 (85.1% ± 1.0%) and CNS-2 (83.2% ± 2.0%), but lower for CNS-3 (71.8% ± 4.0%; P = .0004). Patients with CNS-1 and CNS-2 had similar 4-year overall survival (OS) (90.1% ± 0.8% and 90.5% ± 1.5%, respectively), with OS for CNS-3 being 82.7% ± 3.4% (P = .005). Despite therapeutic differences, outcomes for CNS-1 and CNS-2 were similar regardless of CRT, intensified corticosteroids, or novel agents. Except for significantly superior outcomes with nelarabine on AALL0434 (4-year disease-free survival, 93.1% ± 5.2%), EFS/OS was inferior with CNS-3 status, all of whom received CRT. Combined analyses of >2000 patients with T-ALL identified that CNS-1 and CNS-2 status at diagnosis had similar outcomes. Unlike B-ALL, CNS-2 status in T-ALL does not impact outcome with aBFM therapy, without additional intrathecal therapy, with or without CRT. Although nelarabine improved outcomes for those with CNS-3 status, novel approaches are needed. These trials were registered at www.clinicaltrials.gov as #NCT00408005 (AALL0434) and #NCT02112916 (AALL1231).
Introduction
Historically, treatment for B- and T-cell lineage acute lymphoblastic leukemia (B-ALL and T-ALL) has been similar.1 In recent years, knowledge of distinct biology between these subtypes has led to a divergence in therapy among some consortia. With the ongoing arc of therapeutic advancement, many consortia now treat most children and young adults with ALL without prophylactic cranial radiation therapy (CRT).2-6 However, some cooperative groups continue to use CRT for patients with T-ALL, despite well-established risks, including neurocognitive deficits, endocrinopathies, and increased incidence of secondary malignancies.7-12 CRT may result in decrease in executive function in dose-dependent fashion, which is especially evident in young children.9,13 CRT can lead to endocrine complications such as anterior pituitary deficits associated with deficiencies in growth hormone and thyroid hormone.12 In addition, the risk of secondary malignancy is elevated with CRT.14 Recent reports from the Children’s Oncology Group (COG) assessing outcomes based on central nervous system (CNS) status in B-ALL (studies AALL0331 and AALL0232) identified worse survival for CNS-2 and CNS-3 than for CNS-1.15,16 Specifically, CNS-3 status portends a worse outcome than CNS-2, which in turn, is worse than CNS-1.17 Based on these findings, CNS-directed therapy is progressively intensified for CNS-2 and CNS-3 B-ALL. Multiple consortia have shown that patients with CNS-3 T-ALL have inferior outcomes, but the impact of CNS-2 status has not been systematically studied in T-ALL.18,19
We investigated the impact of CNS status in T-ALL on 2 large, sequential, international phase 3 trials, COG AALL0434 and AALL1231 (supplemental Tables 1 and 2, available on the Blood website). These trials included the same basic chemotherapy backbone, the COG augmented Berlin-Frankfurt-Münster (aBFM) regimen, with differences in CNS-directed systemic chemotherapy, use of CRT, and randomized testing of the novel agents nelarabine (AALL0434) and bortezomib (AALL1231). Of note, 90.8% of AALL0434 T-ALL participants received CRT, whereas, AALL1231 was designed to eliminate CRT in the majority of participants by incorporating more CNS-directed systemic therapy. This investigation allowed for the assessment of the impact of CNS status in over 2000 participants across different cytotoxic regimens, including those receiving or not receiving CRT, to definitively identify the prognostic significance of CNS status in patients with T-ALL receiving contemporary therapy.
Patients and methods
AALL0434 and AALL1231 study designs
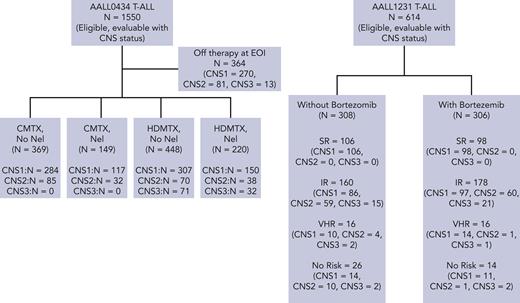
The COG conducted 2 consecutive phase 3 T-ALL trials from 2007 to 2014 (AALL0434) and from 2014 to 2019 (AALL1231), both of which accrued participants aged 1 to 30 years using a COG aBFM treatment regimen, the details of which have been described.20-22 These studies included a 28-day induction period with response to treatment assessed by bone marrow morphology and minimal residual disease that was determined at COG-centralized reference laboratories. These data were used for risk stratification and allocation to postinduction therapy (Table 1 includes risk group definitions on both trials; refer to supplemental Tables 1 and 2 for treatment plans). AALL0434 used a 2 × 2 pseudofactorial randomization for all patients except for induction failures (M3 marrow, >25% blasts at day 29). Patients were randomized to receive either escalating dose methotrexate without leucovorin rescue plus pegaspargase (C-MTX) or high-dose methotrexate with leucovorin rescue (HD-MTX). Capizzi-style methotrexate (C-MTX) is an escalating dose methotrexate regimen without folinic acid rescue that includes pegaspargase. The dosing escalation seeks to achieve impactful doses of methotrexate while also providing a mechanism to limit dose escalation if toxicities arise. The 5 doses of methotrexate are given on days 1, 11, 21, 31, and 41, with starting dose at 100 mg/m2 and a maximum dose of 300 mg/m2, if tolerated. HD-MTX provides uniform doses of higher dose methotrexate regardless of toxicities. Four doses of 5000 mg/m2 with folinic acid rescue are given on days 1, 15, 29, and 43. As recently reported, superior outcomes were seen in those AALL0434 patients randomized to C-MTX.21 These results were unanticipated, but hypothesized to be the result of 2 extra interim maintenance phase doses of pegaspargase and absence of methotrexate rescue from folinic acid. Patients with CNS-3 status were nonrandomly assigned to HD-MTX to potentiate maximal CNS penetration. Intermediate-risk (IR) and high-risk (HR) patients were randomly assigned to six 5-day courses of nelarabine after induction. Patients experiencing induction failure were nonrandomly assigned to HD-MTX and nelarabine. AALL1231 randomized all participants to 4 doses of bortezomib during induction and delayed intensification. Although participants with T-lymphoblastic lymphoma were enrolled on both AALL0434 and AALL1231, the current analyses include only those with T-ALL.
Treatment and risk stratification differences on AALL1231 vs AALL0434
| . | AALL1231 . | AALL0434 . |
|---|---|---|
| Induction corticosteroid | Dexamethasone | Prednisone |
| 6 mg/m2 per d × 28 d | 60 mg/m2 per d × 28 d | |
| Maintenance corticosteroid | Dexamethasone | Prednisone |
| 6 mg/m2 per d × 5 d each 28-d cycle | 40 mg/m2 per d × 5 d each 28-d cycle | |
| Asparginase | 2 doses of pegaspargase during induction | 1 dose of pegaspargase during induction |
| 3 doses of pegaspargase during DI | 2 doses of pegaspargase during DI | |
| Interim maintenance | C-MTX if SR | Randomized to C-MTX or HD-MTX except |
| HD-MTX and C-MTX if IR | Induction failure or CNS-3 and then HD-MTX | |
| VHR intensification blocks and C-MTX if VHR | ||
| Cranial Radiation T-ALL | CNS-3: 18 Gy during maintenance | CNS-3: 18 Gy during DI |
| VHR: 12 Gy during maintenance | IR/HR C-MTX arm: 12 Gy during consolidation | |
| No radiation for SR/IR CNS-1 or CNS-2 | IR/HR HD-MTX arm: 12 Gy during DI | |
| No radiation for LR | ||
| Cranial Radiation T-LLy | CNS-3: 18 Gy during maintenance | CNS-3 not eligible |
| No radiation for patients with CNS-1 or CNS-2 T-LLy | No radiation for patients with CNS-1 or CNS-2 T-LLy | |
| Randomized questions | ± nelarabine (6, 5-d courses): all patients but LR and induction failure. Induction failure nonrandomly assigned to received nelarabine | |
| ± bortezomib during induction and delayed intensification all patients | C-MTX vs HD-MTX interim maintenance as described above | |
| Risk stratification | ||
| LR | National Cancer Institute SR by age (1.00-9.99 y) and WBC (initial ≤50 000/μL); RER, M1 on d 15 and M1 marrow with MRD <0.1% on d 29; CNS-1 status and no testicular disease at diagnosis | |
| SR | CNS-1, lumbar puncture before steroid therapy (not steroid pretreated), d 29 (end of induction) bone marrow M1, d 29 bone marrow MRD ≤0.01%, no testicular leukemia at diagnosis | |
| CNS-2 and CNS-3 cannot be SR and are assigned to IR or VHR based on marrow response | ||
| IR | Not SR or VHR | RER or SER, M1 marrow with MRD <1% on d 29; any CNS status |
| HR | M2 marrow and/or MRD ≥1% on d 29; any CNS status | |
| VHR | M3 marrow at d 29 and/or end of consolidation MRD ≥0.1% |
| . | AALL1231 . | AALL0434 . |
|---|---|---|
| Induction corticosteroid | Dexamethasone | Prednisone |
| 6 mg/m2 per d × 28 d | 60 mg/m2 per d × 28 d | |
| Maintenance corticosteroid | Dexamethasone | Prednisone |
| 6 mg/m2 per d × 5 d each 28-d cycle | 40 mg/m2 per d × 5 d each 28-d cycle | |
| Asparginase | 2 doses of pegaspargase during induction | 1 dose of pegaspargase during induction |
| 3 doses of pegaspargase during DI | 2 doses of pegaspargase during DI | |
| Interim maintenance | C-MTX if SR | Randomized to C-MTX or HD-MTX except |
| HD-MTX and C-MTX if IR | Induction failure or CNS-3 and then HD-MTX | |
| VHR intensification blocks and C-MTX if VHR | ||
| Cranial Radiation T-ALL | CNS-3: 18 Gy during maintenance | CNS-3: 18 Gy during DI |
| VHR: 12 Gy during maintenance | IR/HR C-MTX arm: 12 Gy during consolidation | |
| No radiation for SR/IR CNS-1 or CNS-2 | IR/HR HD-MTX arm: 12 Gy during DI | |
| No radiation for LR | ||
| Cranial Radiation T-LLy | CNS-3: 18 Gy during maintenance | CNS-3 not eligible |
| No radiation for patients with CNS-1 or CNS-2 T-LLy | No radiation for patients with CNS-1 or CNS-2 T-LLy | |
| Randomized questions | ± nelarabine (6, 5-d courses): all patients but LR and induction failure. Induction failure nonrandomly assigned to received nelarabine | |
| ± bortezomib during induction and delayed intensification all patients | C-MTX vs HD-MTX interim maintenance as described above | |
| Risk stratification | ||
| LR | National Cancer Institute SR by age (1.00-9.99 y) and WBC (initial ≤50 000/μL); RER, M1 on d 15 and M1 marrow with MRD <0.1% on d 29; CNS-1 status and no testicular disease at diagnosis | |
| SR | CNS-1, lumbar puncture before steroid therapy (not steroid pretreated), d 29 (end of induction) bone marrow M1, d 29 bone marrow MRD ≤0.01%, no testicular leukemia at diagnosis | |
| CNS-2 and CNS-3 cannot be SR and are assigned to IR or VHR based on marrow response | ||
| IR | Not SR or VHR | RER or SER, M1 marrow with MRD <1% on d 29; any CNS status |
| HR | M2 marrow and/or MRD ≥1% on d 29; any CNS status | |
| VHR | M3 marrow at d 29 and/or end of consolidation MRD ≥0.1% |
Other components of therapy, including type and doses of anthracyclines and alkylating agents were the same in both trials. Supplemental Table 2 provides a detailed description of AALL1231 therapy; the same doses and schedules were used in AALL0434 except those noted in Table 1.
RER: M1 marrow on either day 8 or 15, and M1 marrow with MRD <0.1% on day 29.
SER: M2 or M3 marrow on day 15 or positive MRD ≥0.1% on day 29. M1/M2 marrow on day 29.
LR, low risk; RER, rapid early responder; SER, slow early responder; SR, standard risk; T-LLy, T-cell lymphoblastic lymphoma.
Definitions of CNS status
The definitions of CNS status were uniformly specified for both studies. CNS-1 was defined as the absence of blasts on cytospin preparation, regardless of the number of white blood cells (WBCs). For CNS-2 it was the presence of <5/μL WBCs and cytospin positive for blasts or ≥5/μL WBCs with negative Steinherz-Bleyer algorithm. For CNS-3, the presence of ≥5/μL WBCs and cytospin positive for blasts and/or clinical signs of CNS leukemia (such as facial nerve palsy, brain/eye involvement, or hypothalamic syndrome) were considered.
Therapies directed against CNS relapse
On AALL1231, CNS-directed therapy changes augmented the AALL0434 backbone to eliminate CRT in the majority of patients, including using dexamethasone rather than prednisone as the corticosteroid during induction and maintenance, and inclusion of 2 additional pegaspargase doses, 1 during induction and 1 during delayed intensification. Although both trials included the use of C-MTX, the AALL1231 trial included HD-MTX during the first interim maintenance phase for IR patients and 3 intensification blocks for very HR (VHR) patients (Table 1).
Both studies used 18 Gy CRT for patients with CNS-3. Prophylactic CRT (pCRT, 12 Gy) was given to all patients without CNS-3 on AALL0434, except for those with low risk, who received no CRT (Table 1). In contrast, only VHR patients without CNS-3 status received pCRT on AALL1231. In total, 90.5% of AALL0434 participants received CRT (8.7%, 18 Gy; and 81.8%, 12 Gy) compared with 11.8% of AALL1231 participants (6.8%, 18 Gy; and 5.0%, 12 Gy). Patients who received CRT on either study did so during either the consolidation or delayed intensification phases of AALL0434, and during the first month of maintenance therapy on AALL1231. Intrathecal (IT) chemotherapy varied among risk groups and the total number of prescribed IT doses depended on the risk group and sex of the patient. Participants received IT cytarabine with the diagnostic lumbar puncture whereas subsequent IT therapy used methotrexate. VHR patients on AALL1231 received triple IT therapy with methotrexate, hydrocortisone, and cytarabine during the intensification blocks. The total number of IT doses by study, risk group, sex, and treatment arm are included in supplemental Figure 3.
Study-related approaches to the treatment of CNS-2 status
On AALL0434 and AALL1231, patients with CNS-2 were not stratified into the lowest risk groups (low risk on AALL0434 and standard risk on AALL1231) (Table 1). AALL0434 risk-stratified patients with CNS-2 to either IR or HR arms, and thus they received induction therapy, consolidation therapy ± nelarabine, interim maintenance with C-MTX or HD-MTX, delayed intensification ± nelarabine, and maintenance ± nelarabine, with CRT administered to all. AALL1231 stratified patients with CNS-2 to IR or VHR arms. IR patients received induction ± bortezomib, consolidation, interim maintenance 1 with HD-MTX, delayed intensification ± bortezomib, interim maintenance 2 with C-MTX and maintenance, and no CRT. VHR patients received induction ± bortezomib, consolidation, 3 intensification blocks, delayed intensification ± bortezomib, interim maintenance with C-MTX and maintenance, and CRT.
Statistical analyses
Event-free survival (EFS) was defined as the time from study enrollment to first event (induction death, relapse or second malignant neoplasm, refractory disease defined as persistent disease after intensification blocks, or remission death) or last contact date for those who were event-free. Overall survival (OS) was defined as the time from study enrollment to death or last contact date for those who were alive. Disease-free survival (DFS) was defined as the time from start of consolidation to first event (relapse or second malignant neoplasm or remission death) or last contact date for those who were event-free. Survival rates were estimated using the Kaplan-Meier method and standard errors of Peto et al.23,24 Survival rates are presented as rates ± standard errors. Power calculation for the randomized bortezomib comparison was based on a 1-sided log-rank test (a = 0.05) because the primary objective was to determine whether the addition of bortezomib improved outcome. Unless otherwise specified, 1-sided log-rank tests were used for survival curve comparisons. Multivariable analyses used Cox proportional hazards model. Proportions were compared between groups using a χ2 test or Fisher exact test. Cumulative incidence rates (CIRs) were computed using the cumulative incidence function for competing risks, with comparisons between groups using the K-sample test. A P < .05 was considered significant for all comparisons. All analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC). Graphics were generated using R version 2.13.1 (http://www.r-project.org). AALL1231 completed accrual more recently than AALL0434, and 4-year survival rates are reported for both trials. This report includes data as recent as of 30 June 2021.
Results
Over 2 consecutive phase 3 randomized COG trials, 2164 T-ALL eligible, evaluable patients were enrolled and included in this report. (CONSORT diagram, Figure 1). There were 1564 patients with CNS-1 (72.3%), 441 with CNS-2 (20.4%), and 159 with CNS-3 (7.3%). From early 2007 to mid-2014, 1550 patients with T-ALL were enrolled and were evaluable for analyses on AALL0434, including 1128 patients with CNS-1 (72.8%), 306 with CNS-2 (19.7%), and 116 with CNS-3 (7.5%). From 2014 to early closure in 2017, 614 participants with T-ALL were enrolled on AALL1231 and were evaluable for analyses, including 436 with CNS-1 (71.0%), 135 with CNS-2 (22.0%), and 43 with CNS-3 (7.0%).
AALL0434 outcomes by CNS status
Four-year EFS rates for patients with CNS-1, 2, and 3 treated on AALL0434 were 86.0% ± 1.1%, 83.1% ± 2.3%, and 71.4% ± 4.6%, respectively (P = .0007) (Figure 2A). Four-year OS rates were 91.0% ± 0.9% (CNS-1), 89.7% ± 1.9% (CNS-2), and 84.3% ± 3.7% (CNS-3) (P = .0438) (Figure 2B). Four-year DFS from start of consolidation therapy was 89.4% ± 1.2% (CNS-1), 86.5% ± 2.5% (CNS-2), and 76.9% ± 4.6% (CNS-3) (P = .0033) (Figure 2C). Four-year DFS for arm A (C-MTX without nelarabine) and for arm B (C-MTX+ nelarabine) were similar (Figure 3A-B). Four-year DFS rates for arm C (HD-MTX without nelarabine) differed by CNS status (CNS-1, 87.6% ± 2.0%; CNS-2, 80.0% ± 5.1%; and CNS-3, 70.2% ± 5.8%), with significantly inferior outcomes for patients with CNS-3 status (P = .0006) (Figure 3C). Four-year DFS for arm D (HD-MTX + nelarabine) showed no statistically significant differences in outcome based on CNS status (Figure 3D). Nelarabine significantly improved DFS of participants with CNS-3 who were all nonrandomly assigned to receive HD-MTX (93.1% ± 5.2% with nelarabine [n = 29] vs 70.2% ± 5.8% without [n = 71, P = .0151]) (Figure 3E). The 4-year CIR of relapse of isolated CNS relapse was significantly associated with CNS status (CNS-1, 1.0% ± 0.4%; CNS-2, 4.2% ± 1.4%; and CNS-3, 11.0% ± 3.2% [P < .0001]) (Figure 4C). CNS status was not associated with CIR of isolated marrow relapse or combined marrow and CNS relapse (Figure 4A-B).
EFS, OS, and DFS by CNS status at diagnosis. (A) AALL0434 4-year EFS: CNS-1, 86.0% ± 1.1%; CNS-2, 83.1% ± 2.3%; and CNS-3, 71.4% ± 4.6% (P = .0007). (B) AALL0434 4-year OS: CNS-1, 91.0% ± 0.9%; CNS-2, 89.7% ± 1.9%; and CNS-3, 84.3% ± 3.7% (P = .0438). (C) AALL0434 4-year DFS: CNS-1, 89.4% ± 1.2%; CNS-2, 86.5% ± 2.5%; and CNS-3, 76.9% ± 4.6% (P = .0033). (D) AALL1231 4-year EFS: CNS-1, 82.7% ± 2.2%; CNS-2, 83.2% ± 3.9%; and CNS-3, 71.9% ± 8.7% (P = .37). (E) AALL1231 4-year OS: CNS-1, 87.7% ± 1.9%; CNS-2, 92.4% ± 2.8%; and CNS-3, 78.5% ± 8.1% (P = .022).
EFS, OS, and DFS by CNS status at diagnosis. (A) AALL0434 4-year EFS: CNS-1, 86.0% ± 1.1%; CNS-2, 83.1% ± 2.3%; and CNS-3, 71.4% ± 4.6% (P = .0007). (B) AALL0434 4-year OS: CNS-1, 91.0% ± 0.9%; CNS-2, 89.7% ± 1.9%; and CNS-3, 84.3% ± 3.7% (P = .0438). (C) AALL0434 4-year DFS: CNS-1, 89.4% ± 1.2%; CNS-2, 86.5% ± 2.5%; and CNS-3, 76.9% ± 4.6% (P = .0033). (D) AALL1231 4-year EFS: CNS-1, 82.7% ± 2.2%; CNS-2, 83.2% ± 3.9%; and CNS-3, 71.9% ± 8.7% (P = .37). (E) AALL1231 4-year OS: CNS-1, 87.7% ± 1.9%; CNS-2, 92.4% ± 2.8%; and CNS-3, 78.5% ± 8.1% (P = .022).
AALL0434 4-year DFS by treatment arm and CNS status. (A) Arm A (C-MTX, no nelarabine): CNS-1, 91.4% ± 1.8% and CNS-2, 92.9% ± 3.0% (P = .1756). (B) Arm B (C-MTX with nelarabine): CNS-1, 91.8% ± 3.0% and CNS-2, 89.8% ± 6.1% (P = .6218). (C) Arm C (HD-MTX, no nelarabine): CNS-1, 87.6% ± 2.0% and CNS-2, 80.0% ± 5.1%; and CNS-3, 70.2% ± 5.8% (P = .0006). (D) Arm D (HD-MTX with nelarabine): CNS-1, 86.6% ± 3.6%; CNS-2, 80.0% ± 8.4%; and CNS-3, 93.1% ± 5.2% (P = .35). (E) Only patients with CNS-3: arm C (HD-MTX, no nelarabine) 70.2% ± 5.8% and arm D (HD-MTX with nelarabine) 93.1% ± 5.2% (P = .0151).
AALL0434 4-year DFS by treatment arm and CNS status. (A) Arm A (C-MTX, no nelarabine): CNS-1, 91.4% ± 1.8% and CNS-2, 92.9% ± 3.0% (P = .1756). (B) Arm B (C-MTX with nelarabine): CNS-1, 91.8% ± 3.0% and CNS-2, 89.8% ± 6.1% (P = .6218). (C) Arm C (HD-MTX, no nelarabine): CNS-1, 87.6% ± 2.0% and CNS-2, 80.0% ± 5.1%; and CNS-3, 70.2% ± 5.8% (P = .0006). (D) Arm D (HD-MTX with nelarabine): CNS-1, 86.6% ± 3.6%; CNS-2, 80.0% ± 8.4%; and CNS-3, 93.1% ± 5.2% (P = .35). (E) Only patients with CNS-3: arm C (HD-MTX, no nelarabine) 70.2% ± 5.8% and arm D (HD-MTX with nelarabine) 93.1% ± 5.2% (P = .0151).
Four-year CIRs of relapse by type of relapse and CNS status. (A) AALL0434 CIR of isolated marrow relapse: CNS-1, 3.4% ± 0.6%; CNS-2, 3.2% ± 1.2%; and CNS-3, 3.1% ± 1.8% (P = .977). (B) AALL0434 CIR of combined marrow and CNS relapse: CNS-1, 1.1% ± 0.4%; CNS-2, 1.4% ± 0.8%; and CNS-3, 1.0% ± 1.0% (P = .963). (C) AALL0434 CIR of isolated CNS relapse: CNS-1, 1.0% ± 0.4%; CNS-2, 4.2% ± 1.4%; and CNS-3, 11.0% ± 3.2% (P < .0001). (D) AALL1231 CIR of isolated marrow relapse: CNS-1, 3.4% ± 0.9%; CNS-2, 1.5% ± 1.1%; and CNS-3, 8.4% ± 4.9% (P = .18). (E) AALL1231 CIR of combined marrow and CNS relapse: CNS-1, 0.7% ± 0.4%; CNS-2, 2.3% ± 1.3%; and CNS-3, 0.0% ± 0.0% (P = .45). (F) AALL1231 CIR of isolated CNS relapse: CNS-1, 2.8% ± 0.8%; CNS-2, 5.3% ± 2.0%; and CNS-3, 4.7% ± 3.3% (P = .34).
Four-year CIRs of relapse by type of relapse and CNS status. (A) AALL0434 CIR of isolated marrow relapse: CNS-1, 3.4% ± 0.6%; CNS-2, 3.2% ± 1.2%; and CNS-3, 3.1% ± 1.8% (P = .977). (B) AALL0434 CIR of combined marrow and CNS relapse: CNS-1, 1.1% ± 0.4%; CNS-2, 1.4% ± 0.8%; and CNS-3, 1.0% ± 1.0% (P = .963). (C) AALL0434 CIR of isolated CNS relapse: CNS-1, 1.0% ± 0.4%; CNS-2, 4.2% ± 1.4%; and CNS-3, 11.0% ± 3.2% (P < .0001). (D) AALL1231 CIR of isolated marrow relapse: CNS-1, 3.4% ± 0.9%; CNS-2, 1.5% ± 1.1%; and CNS-3, 8.4% ± 4.9% (P = .18). (E) AALL1231 CIR of combined marrow and CNS relapse: CNS-1, 0.7% ± 0.4%; CNS-2, 2.3% ± 1.3%; and CNS-3, 0.0% ± 0.0% (P = .45). (F) AALL1231 CIR of isolated CNS relapse: CNS-1, 2.8% ± 0.8%; CNS-2, 5.3% ± 2.0%; and CNS-3, 4.7% ± 3.3% (P = .34).
AALL1231 outcomes by CNS status
Four-year EFS for participants enrolled onto AALL1231 for CNS-1, 2, and 3 were 82.7% ± 2.2%, 83.2% ± 3.9%, and 71.9% ± 8.7%, respectively (P = .37) (Figure 2D). There were no statistically significant differences in EFS by CNS status on each treatment arm; without bortezomib, 83.7% ± 3.0% (CNS-1), 78.6% ± 5.8% (CNS-2), and 57.5% ± 18.7% (CNS-3) (P = .118); with bortezomib, 81.8% ± 3.1% (CNS-1), 88.5% ± 5.0% (CNS-2), and 78.7% ± 9.4% (CNS-3) (P = .34) (supplemental Figure 5). Four-year OS was similar for CNS-1 and CNS-2 but inferior for CNS-3 (CNS-1, 87.7% ± 1.9%; CNS-2, 92.4% ± 2.8%; and CNS-3, 78.5% ± 8.1%; P = .022) (Figure 2E). Statistically inferior 4-year OS for CNS-3 was also demonstrated both without bortezomib (CNS-1, 89.6% ± 2.5%; CNS-2, 88.7% ± 4.5%; and CNS-3, 71.9% ± 17.1%; P = .0158) and with bortezomib (CNS-1, 85.9% ± 2.8%; CNS-2, 96.7% ± 2.8%; CNS-3, 83.3% ± 8.8%; and P = .046) (supplemental Figure 5).
IR patients with CNS-1 and CNS-2 on AALL1231 received identical therapy. There were 183 participants with CNS-1, 119 with CNS-2, and 36 with CNS-3 at IR. There were no differences in EFS and OS for IR participants by CNS status; 4-year EFS 86.5% ± 3.0% (CNS-1), 87.1% ± 3.7% (CNS-2), and 85.2% ± 7.5% (CNS-3) (P = .99) (supplemental Figure 5); and 4-year OS 90.6 ± 2.6 (CNS-1), 95.7% ± 2.2% (CNS-2), and 88.7% ± 6.8% (CNS-3) (P = .218) (supplemental Figure 5). Participants who were IR and with CNS-3 had similar outcomes to IR participants with CNS-1 and CNS-2, albeit those with CNS-3 received 18 Gy CRT. The 4-year CIR was not different based on CNS status regardless of the site of relapse. Isolated marrow CIR was 3.4% ± 0.9% (CNS-1), 1.5% ± 1.1% (CNS-2), and 8.4% ± 4.9% (CNS-3) (P = .18) (Figure 4D). The CIR for combined bone marrow and CNS relapse was 0.7% ± 0.4% (CNS-1), 2.3% ± 1.3% (CNS-2), and 0.0% ± 0.0% (CNS-3) (P = .45) (Figure 4E). CIR for isolated CNS was 2.8% ± 0.8% (CNS-1) 5.3% ± 2.0% (CNS-2), and 4.7% ± 3.3% (CNS-3) (P = .34) (Figure 4F). Comparison of similar participants on AALL0434 who received CRT and did not on AALL1231 identified that CRT did not provide a statistically distinct EFS (P = .395) or OS (P = .525) at 4 years.22
AALL0434 and AALL1231 combined cohort
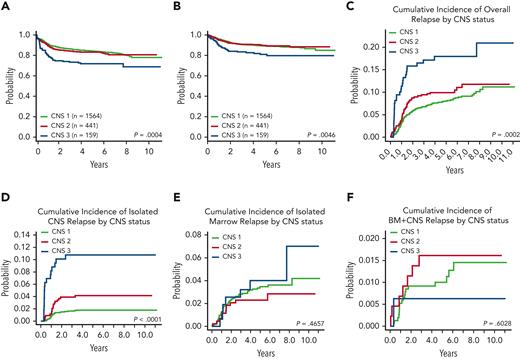
Four-year EFS and OS rates were analyzed combining participants from both studies. Four-year EFS was similar for patients with CNS-1 (85.1% ± 1.0%) and CNS-2 (83.2 ± 2.0), but lower for those with CNS-3 (71.8% ± 4.0%; P = .0004) (Figure 5A). Four-year OS was similar for patients with CNS-1 (90.1% ± 0.8%) and CNS-2 (90.5% ± 1.6%) status, but was significantly lower for those with CNS-3 status (82.7% ± 3.4%; P = .005) (Figure 5B). The 4-year CIR for all types of relapses was similar between patients with CNS-1 (7.6% ± 0.7%) and CNS-2 (9.9% ± 1.4%) status but was significantly worse for those with CNS-3 status (17.9% ± 3.1%; P = .0002) (Figure 5C). The 4-year CIR of isolated CNS relapse was significantly associated with CNS status (CNS-1, 1.8% ± 0.3%; CNS-2, 4.1% ± 1.0%; CNS-3, 10.8% ± 2.5%; and P < .001) (Figure 5D). CIR of isolated marrow relapse and the CIR of combined marrow and CNS relapse were not statistically significantly associated with CNS status (Figures 5E-F).
Pooled AALL0434 plus AALL1231 EFS, OS, and CIR by CNS status. (A) Four-year EFS: CNS-1, 85.1% ± 1.0%; CNS-2, 83.2% ± 2.0%; and CNS-3, 71.8% ± 4.0% (P = .0004). (B) Four-year OS: CNS-1, 90.1% ± 0.8%; CNS-2, 90.5% ± 1.6%; and CNS-3, 82.7% ± 3.4% (P = .005). (C) Four-year CIR: CNS-1, 7.6% ± 0.7%; CNS-2, 9.9% ± 1.4%; and CNS-3, 17.9% ± 3.1% (P = .0002). (D) Four-year CIR of isolated CNS relapse: CNS-1, 1.8% ± 0.3%; CNS-2, 4.1% ± 1.0%; and CNS-3, 10.8% ± 2.5% (P < .001). (E) Four-year CIR of isolated marrow relapse: CNS-1, 3.2% ± 0.5%; CNS-2, 2.3% ± 0.7%; and CNS-3, 4.0% ± 1.6% (P = .466). (F) Four-year CIR of combined CNS and marrow relapse: CNS-1, 0.9% ± 0.2%; CNS-2, 1.6% ± 0.6%; and CNS-3, 0.6% ± 0.6% (P = .603). BM, bone marrow.
Pooled AALL0434 plus AALL1231 EFS, OS, and CIR by CNS status. (A) Four-year EFS: CNS-1, 85.1% ± 1.0%; CNS-2, 83.2% ± 2.0%; and CNS-3, 71.8% ± 4.0% (P = .0004). (B) Four-year OS: CNS-1, 90.1% ± 0.8%; CNS-2, 90.5% ± 1.6%; and CNS-3, 82.7% ± 3.4% (P = .005). (C) Four-year CIR: CNS-1, 7.6% ± 0.7%; CNS-2, 9.9% ± 1.4%; and CNS-3, 17.9% ± 3.1% (P = .0002). (D) Four-year CIR of isolated CNS relapse: CNS-1, 1.8% ± 0.3%; CNS-2, 4.1% ± 1.0%; and CNS-3, 10.8% ± 2.5% (P < .001). (E) Four-year CIR of isolated marrow relapse: CNS-1, 3.2% ± 0.5%; CNS-2, 2.3% ± 0.7%; and CNS-3, 4.0% ± 1.6% (P = .466). (F) Four-year CIR of combined CNS and marrow relapse: CNS-1, 0.9% ± 0.2%; CNS-2, 1.6% ± 0.6%; and CNS-3, 0.6% ± 0.6% (P = .603). BM, bone marrow.
Multivariable analysis for EFS was performed for the combined cohort (supplemental Table 4). Although age, sex, race, and ethnicity had no impact on outcome, CNS-3 status, initial WBC (≥50 000 T-lymphoblasts per μL), and end of induction minimal residual disease (≥0.01%) were associated with worse outcomes. Similarly, CNS-2 status was not an independent risk factor (hazard ratio, 1.04; 95% confidence interval, 0.80-1.37; P = .759).
Discussion
Our combined analyses of CNS status and outcome of 2164 patients with T-ALL treated on 2 sequential COG studies is the largest of its kind. Without adequate treatment, CNS relapse can occur in patients with T-ALL and be very difficult to salvage. Because the CNS can harbor leukemic cells that traffic to other tissue compartments, elimination of residual disease within the CNS compartment remains critical.25,26 COG AALL0434 and AALL1231 showed that outcomes for participants with CNS-1 and CNS-2 status were similar when treated with an aBFM backbone, regardless of the induction steroid, use of pCRT, additional asparaginase, or the addition of nelarabine or bortezomib. Although CNS relapse is more common in T-ALL than B-ALL,17 we found that CIR for all types of relapses was not impacted by CNS-2 status in the combined analysis, or when analyzed independently for either study. Participants with CNS-3 status at diagnosis had worse outcomes than those with CNS-1 or CNS-2, despite the usage of 18 Gy CRT for those with CNS-3 status in both trials. The only exception was in participants who received nelarabine in AALL0434, which had significantly improved outcomes.20
because of the damaging effects of CRT on endocrine and cognitive function, and its greater potential to cause secondary malignant neoplasms, the use of CRT in children and young adults with ALL should be avoided.27,28 Nevertheless, CRT may prevent CNS relapse and based on the difficulty salvaging patients with relapsed T-ALL, it may be warranted in some settings. Because CNS-2 status has been shown to negatively impact outcomes for B-ALL, additional doses of IT chemotherapy during induction therapy, but not adding CRT, have been incorporated into Nordic Society of Pediatric Hematology and Oncology, St. Jude, Dana-Farber Cancer Institute, and COG aBFM–based therapies for patients with CNS-2.3,29-31 Several modifications were made to the AALL1231 backbone to eliminate CRT in most patients and there was no increased incidence of CNS relapse for patients with CNS-2. Despite equally good outcomes on both trials, patients with CNS-1 or CNS-2 disease still suffered CNS relapses, and future trials should continue to investigate novel approaches to prevent relapse. Perhaps more sensitive detection of CNS leukemia using flow cytometry on the cerebrospinal fluid (CSF) may identify those patients who would benefit from more intensive IT therapy or other novel approaches. Recently, the Nordic Society of Pediatric Hematology and Oncology reported that using flow cytometry-based CSF assessments aided in the detection of CNS leukemia, delineated patients at higher risk of relapse, and improved risk stratification to optimize CNS-directed therapy.32 Furthermore, similar results were recently published from a consortium study, ALL-2015, from the Chinese Children’s Cancer Group.33 It is prudent to note that the COG, among others, have learned over sequential treatment protocols that CNS-directed intensification is warranted in T-ALL. It remains possible, therefore, that the intensity of modern therapeutics supersedes biological distinctions between CNS-1 and CNS-2 status in T-ALL.
Except for patients with CNS-3 disease who received nelarabine on the AALL0434 study, EFS/OS for those with CNS-3 was inferior compared with those with CNS-1 and CNS-2. All patients with CNS-3 were nonrandomly assigned to receive HD-MTX on AALL0434, and none received nelarabine on AALL1231. Consequently, nelarabine has not been evaluated for patients with CNS-3 in the context of C-MTX therapy. Nelarabine, C-MTX, and dexamethasone have all been shown to reduce CNS relapse. It is possible that a backbone including all 3 would eliminate the need for CRT for patients with CNS-3 T-ALL.5,20 Importantly, the UK’s Medical Research Council, the Dutch Childhood Oncology Group, and St. Jude Children’s Research Hospital have all eliminated CRT in newly diagnosed patients with ALL, regardless of their CNS status.3,5,6 However, the published results from these groups have limited number of participants with CNS-3 T-ALL. Using a balanced risk/benefit analysis when considering which patients with CNS-3 to treat with CRT will be critical, especially for younger children (aged ≤5 years) based on the increased risk of long-term neurocognitive impairment.8,10,11 In a large analysis of children with ALL across 10 pediatric consortia, Vora et al reported that CRT did not improve outcomes other than for a specific population with CNS-3 disease who had less CNS-only relapse.5 However, those patients with CNS-3 who received CRT did not have improved outcomes when all types of relapses were assessed, and furthermore, patients with T-ALL comprised only a small minority in their review.5 Flow cytometry was not performed on CSF for either study. The COG has not yet developed means to standardize flow cytometry on CSF at the majority of participating institutions in North America.
In conclusion, we found that CNS-2 was not independently prognostic in patients with T-ALL treated on 2 consecutive COG trials using aBFM therapy, and that patients with CNS-2 can be treated similarly to CNS-1 without the use of pCRT. In addition, distinct from B-ALL, patients with T-ALL with CNS-2 status do not require more IT chemotherapy treatments than those with CNS-1 within their risk group cohort. Because relapse involving the CNS remains a common problem for patients with T-ALL, new approaches are needed for those with CNS-3 disease at diagnosis. The use of nelarabine mitigates the poor prognosis of CNS-3 on a backbone that includes CRT, and it is possible that the additional use of C-MTX and induction dexamethasone will eliminate the need for CRT in these patients. The particular benefit of nelarabine for patients with CNS-3 is notable because it provides optimism that novel therapeutic modalities that target the CNS may alleviate the need for CRT and its adverse effects for a difficult-to-treat population. Several novel immunotherapies and targeted therapies have recently translated into the clinic in patients with relapsed and refractory T-ALL. It is anticipated that a portion will progress to frontline therapy, if safe and effective. Some of these, including chimeric antigen receptor T cells targeting antigens including CD5 and CD7 (#NCT05043571 and #NCT04984356) and small molecule inhibitors such as the MEK inhibitor selumetinib (#NCT03705507) penetrate the CNS and if moved to the front line could eliminate the need for CRT in all patients with T-ALL.34 However, other new therapies such as the anti-CD38 monoclonal antibody daratumumab have poor CNS penetration and are unlikely to impact CNS relapse. In our experience, effective therapies will likely be most useful when incorporated early in the treatment course, whereas, it is still possible to consolidate the CNS compartment for patients who are at the highest risk for relapse or disease progression.
Acknowledgments
This work was supported by grants from the National Institutes of Health (grant U10 CA98543) (S.S.W., K.P.D., M.L.L., and S.P.H.), (U10 CA98413) (M.D.), (U10CA180886) (M.L.L. and D.T.T.), (U10CA180899) (M.D. and Z.C.), (U24CA196173) (M.L.L. and D.T.T.), (R01CA193776) (D.T.T. and M.D.), (X01HD100702) (D.T.T., S.P.H., M.D., M.L.L., S.S.W., and K.P.D.); the Leukemia and Lymphoma Society (D.T.T.), (R03CA256550) (D.T.T., M.L.L., S.P.H., M.D., S.S.W., and K.P.D.), (R01CA264837) (D.T.T.); and St Baldrick’s Foundation funding (M.L.L. and S.P.H.). S.P.H. is the Jeffrey E. Perelman Distinguished Chair in Pediatrics at the Children’s Hospital of Philadelphia. E.A.R. is a KiDS of NYU Foundation Professor at NYU Langone Health.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contributions: N.P.G., S.S.W., and D.T.T. contributed to the conception and design of the study; M.D. and Z.C. completed the statistical analyses; N.P.G., S.S.W., and D.T.T. completed data analysis and primary manuscript preparation; and all authors contributed to the data interpretation, drafting, and revision of the manuscript, and provided final approval to submit the manuscript for publication.
Conflict-of-interest disclosure: S.P.H. has received honoraria from Amgen, Jazz Pharmaceuticals, and Servier; consulting fees from Novartis; and owns common stock in Amgen. D.T.T. serves on advisory boards for BEAM, Janssen, and Sobi with honoria from Sobi and has research support from BEAM and NeommuneTech. E.A.R. receives research funding from Pfizer and serves on a data safety monitoring board for Bristol Myers Squibb.
Correspondence: Nathan P. Gossai, Center for Cancer and Blood Disorders, Children’s Minnesota, 2530 Chicago Ave, Minneapolis, MN 55404; e-mail: nathan.gossai@childrensmn.org.
References
Author notes
∗S.S.W. and D.T.T. contributed equally to this study.
The Children’s Oncology Group (COG) data sharing policy describes the release and use of COG’s individual participant data for use in research projects in accordance with National Clinical Trials Network Program and National Cancer Institute Community Oncology Research Program Guidelines. Only data expressly released from the oversight of the relevant COG Data and Safety Monitoring Committee are available to be shared. Data from this study are available following the primary publication. An individual-level deidentified data set containing the variables analyses in the primary results paper can be expected to be available upon request. Requests for access to COG protocol research data should be sent to datarequest@childrensoncologygroup.org. Data are available to researchers whose proposed analysis is found by COG to be feasible and of scientific merit and who agree to the terms and conditions of use.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal