In this issue of Blood,Schnegg-Kaufmann et al1 demonstrate that highly mutated hematopoietic stem and progenitor cells contribute to normal hematopoiesis in myelodysplastic neoplasms (MDS) and chronic myelomonocytic leukemia (CMML) with or without azacytidine (AZA) treatment.
Myeloid malignancies represent a highly diverse set of diseases, including myeloproliferative neoplasms, MDS, and acute myeloid leukemia (AML). Notably, many genetic mutations are shared across different myeloid malignancies (TET2, TP53), whereas others are unique to a specific type (NPM1c). These different mutations ultimately promote abnormal self-renewal of hematopoietic stem and progenitor cells (HSPCs) and/or block their maturation into normal myeloid cells such as granulocytes or monocytes. This block in maturation ultimately results in a major morbidity in MDS/CMML cytopenias, which make patients transfusion dependent.
Importantly, concepts from 1 disease are frequently applied to other myeloid malignancies. For example, minimal residual disease (MRD) measurement in chronic myeloid leukemia is standard of care,2 and a similar concept for risk stratification is emerging in AML.3 The fundamental paradigm to emerge from these 2 diseases is that reduction in disease burden permits unmutated HSPCs to mature, thereby normalizing blood counts. This implies that the mutated HSPCs cannot differentiate with therapy and, therefore, their elimination is vital to disease control. This concept has been applied to other myeloid malignancies such as MDS and CMML. The contradiction is that treatment with DNA hypomethylating agents (HMAs) such as decitabine or AZA can normalize blood counts without a commensurate reduction in disease burden.4,5 Whether this is because highly mutated HSPCs can mature normally in response to AZA or there is increased output from the remaining normal cells or HSPCs with fewer mutations is an important, unresolved question within the literature.5
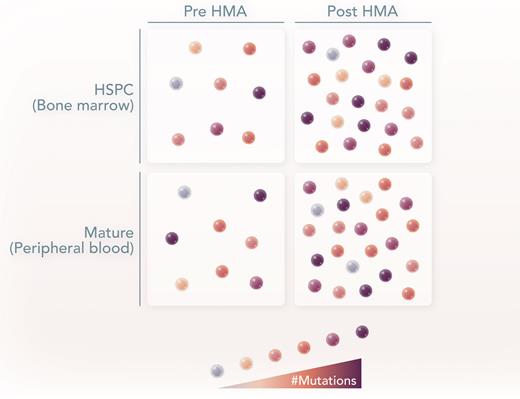
To address this question, Schnegg-Kaufmann et al used multiple approaches on samples from patients with MDS or the MDS/myeloproliferative neoplasm overlap disease CMML, before and after AZA treatment. The authors initially hypothesized based on in vitro colony-forming assays5 that highly mutated clones would fail to differentiate into peripheral blood cells. In addition, they hypothesized that normal HSPCs or clones with fewer mutations would mature in response to DNA hypomethylation. As a first step, 2 patients with CMML and 1 patient with MDS, all harboring TET2 mutations, had stem and progenitor cells fractionated, and quantitative polymerase chain reaction was used to measure the variant allele fraction of different mutations to quantify clonal architecture on both bulk and single-cell populations. Counterintuitively, the authors found that the clonal architecture did not vary as hematopoietic cells differentiated from stem cells to myeloid progenitors and eventually into differentiated cells. These data demonstrate that highly mutated HSPCs can differentiate into mature cells in the absence of treatment.
To understand how HMAs may alter the clonal architecture, a second cohort of 9 MDS patients was analyzed similarly, this time pre– or post–AZA treatment. Again, their results at the single-cell level confirm that highly mutated HSPCs could differentiate into normal mature blood cells. Significantly, the ability of highly mutated HSPCs to differentiate was seen in both AZA responders and non-responders. The only mutation that appeared disfavored during the differentiation process was biallelic mutations in TP53; these clones were depleted by AZA. Collectively, this work implies that highly mutated HSPCs can be induced to mature via AZA therapy, and this ability is independent of clinical response. One important caveat is that given the robust, highly detailed molecular studies performed, the number of patients was overall small, and, therefore, more extensive studies are needed to confirm these findings. However, by leveraging single-cell approaches, the authors have addressed a critical question regarding how HMAs can induce clinical responses without altering disease burden in MDS/CMML.
This work has important implications beyond the use of AZA in MDS/CMML. First, it highlights that not all concepts from 1 myeloid malignancy can be applied broadly across the group. Traditionally, MDS and CMML are considered precursor conditions that ultimately transform into AML in a minority of patients. This work would imply that the same paradigms in measuring response to therapy through MRD may not be applicable in MDS, at least in reversing cytopenia(s) and long-term disease control. Given that many MDS patients are older and not eligible for curative therapy with an allogeneic bone marrow transplantation, this work highlights that following MRD may not be a valuable guide for treatment. Second, as people age, clonal hematopoiesis dominates,6,7 and the bulk of hematopoiesis is driven by a small number of HSPCs with a single mutation. In the case of clonal cytopenia of undetermined significance (CCUS), treatment with HMAs has been proposed and studied.8,9 Schnegg-Kaufmann et al’s work suggests that although HMAs may improve cytopenia(s), they may not alter the clinical trajectory of the disease in terms of progression to MDS or AML. Collectively, this research highlights the need for larger prospective studies in patients with CCUS, MDS, and CMML to contrast their clonal dynamics with distinct myeloid malignancies such as AML (see figure).
In MDS and CMML, there is a mixture of normal (white) and abnormal (brown) HSPCs. Brown color gradient represents the mutational burden. In both pre- and post-HMA treatment, the highly mutated HSPCs can differentiate and contribute to the peripheral blood, with slight variance in the clonal architecture. Professional illustration by Somersault18:24.
In MDS and CMML, there is a mixture of normal (white) and abnormal (brown) HSPCs. Brown color gradient represents the mutational burden. In both pre- and post-HMA treatment, the highly mutated HSPCs can differentiate and contribute to the peripheral blood, with slight variance in the clonal architecture. Professional illustration by Somersault18:24.
Conflict-of-interest disclosure: S.R. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal