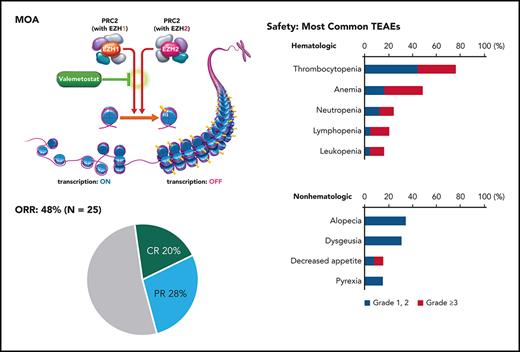
Key Points
This phase 2 study assessed the efficacy and safety of the dual EZH2 and EZH1 inhibitor valemetostat in patients with R/R ATL.
Valemetostat 200 mg orally once daily demonstrated promising efficacy and manageable toxicity in heavily pretreated patients.
Abstract
Adult T-cell leukemia/lymphoma (ATL) is an aggressive non-Hodgkin lymphoma with poor prognosis and few treatment options for patients with relapsed, recurrent, or refractory disease. We evaluated the efficacy and safety of valemetostat, a potent enhancer of zeste homolog 2 (EZH2) and EZH1 inhibitor, in treating relapsed or refractory (R/R) ATL. This multicenter phase 2 trial enrolled patients with R/R aggressive ATL (acute, lymphoma, unfavorable chronic type). Patients received valemetostat 200 mg/day orally until progressive disease or unacceptable toxicity. The primary end point was overall response rate (ORR) centrally assessed by an independent efficacy assessment committee (IEAC). Secondary end points included best response in disease compartments, duration of response (DOR), pharmacokinetics, and safety. Twenty-five patients (median age, 69.0 years) with a median of 3 prior lines of therapy were enrolled; 24 had prior mogamulizumab treatment. The primary end point was met with a centrally reviewed ORR of 48.0% (90% confidence interval [CI], 30.5-65.9), including 5 complete and 7 partial remissions. Patients pretreated with mogamulizumab had an ORR of 45.8% (4 complete and 7 partial remissions). IEAC-assessed median DOR was not reached (NR) (95% CI, 1.87 to NR; months). Treatment-emergent adverse events (TEAEs) were manageable. TEAEs that occurred in ≥20% of patients included thrombocytopenia, anemia, alopecia, dysgeusia, neutropenia, lymphopenia, leukopenia, decreased appetite, and pyrexia. Grade ≥3 TEAEs included thrombocytopenia, anemia, lymphopenia, leukopenia, and neutropenia. Valemetostat demonstrated promising efficacy and tolerability in heavily pretreated patients, warranting further investigation in treating R/R ATL. This trial was registered at www.clinicaltrials.gov as #NCT04102150.
Introduction
Adult T-cell leukemia/lymphoma (ATL) is an aggressive non-Hodgkin lymphoma (NHL) subtype that arises from T cells infected with human T-lymphotropic virus type 1 (HTLV-1).1-3 HTLV-1 is endemic to Japan, the Caribbean, Central and South America, Africa, the Middle East, and Australia. Recent reports indicate that ATL constitutes ≥30% of all T-cell lymphoma cases in Japan.4-6 ATL is classified into 4 clinical subtypes (acute, lymphoma, chronic, and smoldering), with acute, lymphoma, and unfavorable chronic subtypes among the most aggressive.4 Current standard first-line treatment for aggressive ATLs is multiagent chemotherapy, including VCAP-AMP-VECP (vincristine, cyclophosphamide, doxorubicin, and prednisone; doxorubicin, ranimustine, and prednisone; and vindesine, etoposide, carboplatin, and prednisone).1-3,7 Even for those who respond to first-line chemotherapy, the response is usually not durable, and the prognosis of patients with aggressive ATL remains poor (median survival is ≤1 year).8,9 Early upfront allogeneic hematopoietic stem cell transplant (allo-HSCT) is considered for aggressive ATL.10,11 However, the median age at diagnosis (68 years), donor availability, patient comorbidities, and infectious complications during induction treatment severely limit eligibility for allo-HSCT.10,12-14 In addition, more than half of patients who receive allo-HSCT cannot achieve long-term survival owing to relapse and/or treatment-related toxicities, requiring further treatment.9
Recently, new agents have been incorporated into the armamentarium for relapsed or refractory (R/R) ATL.15,16 A phase 2 study of the defucosylated anti-CCR4 antibody, mogamulizumab, resulted in an overall response rate (ORR) of 50%.16 A separate phase 2 study of lenalidomide, an immunomodulator and inhibitor of E3 ubiquitin ligase, yielded an ORR of 42%.15 Another phase 2 study of tucidinostat (HBI-8000; chidamide), a histone deacetylase inhibitor, in R/R ATL with mogamulizumab pretreatment resulted in an ORR of 30%.17 These agents received regulatory approval in Japan. Despite these options, response rates in patients with aggressive ATLs remain low, and patients continue to experience relapse. Development of novel therapies is therefore critical for patients with R/R ATL.
Enhancer of zeste homolog 2 (EZH2) and EZH1 are the principal histone methyltransferases of the polycomb repressive complex 2 and initiate chromatin folding through trimethylation of histone H3 lysine 27, resulting in transcriptional repression.18-22 Although EZH2-selective inhibitors have been developed, EZH1 compensation for EZH2 loss necessitates the implementation of dual inhibitory agents.23,24 Valemetostat tosylate (valemetostat) is a novel, potent, and selective dual inhibitor of EZH2 and EZH1 with strong antitumor properties.24 Interim analyses from a phase 1 study in the United States and Japan of valemetostat monotherapy showed that 200 mg once daily oral valemetostat had an acceptable safety profile with signs of preliminary efficacy in patients with R/R NHLs, including ATL (www.clinicaltrials.gov #NCT02732275; DS3201-A-J101).25 Based on these encouraging results, we conducted a phase 2 trial to assess the efficacy and safety of valemetostat 200 mg once daily in patients with R/R ATL for the purposes of obtaining regulatory approval in Japan.
Methods
Patient eligibility
Patients aged ≥20 years with cytologically or pathologically diagnosed R/R ATL (acute, lymphoma, or unfavorable chronic type as assessed at the time of diagnosis) with antibody-confirmed HTLV-1 infection were eligible. Unfavorable chronic ATL was defined as having ≥1 of the following factors: low serum albumin, high lactate dehydrogenase, or high blood urea nitrogen concentration.7 Patients needed to have relapsed, recurrent, or refractory disease after prior mogamulizumab therapy or, if mogamulizumab was contraindicated or not tolerated, ≥1 systemic therapy with cytotoxic chemotherapy. Patients with an Eastern Cooperative Oncology Group performance status of 0 to 2 and ≥1 measurable lesion were eligible. Eligibility criteria further included a neutrophil count ≥1000/μL, platelet count ≥75 000/μL, hemoglobin ≥8.0 g/dL, serum aspartate aminotransferase and alanine aminotransferase ≤3 × upper limit of normal (ULN), bilirubin ≤1.5 × ULN, and serum creatinine ≤1.5 × ULN or creatinine clearance ≥30 mL/min. Patients with central nervous system involvement of ATL at screening, chemotherapy or molecularly targeted therapy within 21 days, history of allo-HSCT, or recent autologous HSCT within 12 weeks before enrollment were excluded. Corticosteroids over 10 mg/day were not permitted. Patients treated with investigational drugs within 28 days or with a history of EZH inhibitor treatment were excluded.
Study design
This trial (NCT04102150; DS3201-A-J201) was a multicenter, single-arm, open-label phase 2 clinical trial for patients with R/R ATL. The objectives of this study were to evaluate the efficacy and safety of valemetostat monotherapy in patients with R/R ATL. Relapsed disease (RD) was defined as disease progression after achieving complete remission (CR) or unconfirmed CR (CRu) following prior chemotherapy. Recurrent disease was defined as disease progression after achieving partial remission (PR) with prior chemotherapy. Disease was considered refractory if patients required a treatment switch after achieving stable disease (SD) or had experienced disease progression after prior treatment. The primary end point was the centrally reviewed ORR, defined as the proportion of participants whose best response was CR, CRu, or PR as assessed by an independent efficacy assessment committee (IEAC).26 Secondary end points included investigator-assessed ORR, best response in disease compartments, CR rate, tumor control rate (TCR), time to response (TTR), duration of response (DOR), progression-free survival (PFS), overall survival (OS), pharmacokinetics, and safety. Details of pharmacokinetic methodology are described in the supplemental Methods available on the Blood website.
Patients were treated with valemetostat 200 mg once daily orally under fasting conditions (≥2 hours before or ≥1 hour after a meal) on continuous 28-day cycles until progressive disease (PD) or unacceptable toxicity. Patients receiving strong CYP3A inhibitors or P-glycoprotein inhibitors had a valemetostat dose reduction to 100 mg once daily. Those receiving drugs with a strong inhibitory effect on both CYP3A and P-glycoprotein received a reduced dose of 50 mg once daily.
Efficacy and safety assessments
All patients treated with ≥1 dose of valemetostat were included in the efficacy analysis. The initial antitumor response was assessed 4 weeks after the first dose of valemetostat, and the response was subsequently assessed every 8 weeks. After 48 weeks, assessments were conducted every 12 weeks thereafter. Efficacy assessments were conducted by an IEAC. Patient best responses and best change in tumor burden by disease compartment were quantified across treatment. Nodal or measurable extranodal lesions were assessed with computed tomography scans, and sums of the products of the greatest diameters were quantified. Skin lesions were evaluated visually and through calculation using the modified severity-weighted assessment tool.27 Disease in peripheral blood was evaluated based on white blood cell count, lymphocyte count, and abnormal lymphocyte count. The antitumor response was assessed in accordance with the antitumor response assessment criteria, which were slightly modified from the response assessment criteria for ATL.26 The modification was made such that there was no requirement for each criterion to be present for ≥4 weeks.
The safety analysis consisted of patients treated with ≥1 dose of valemetostat. The Medical Dictionary for Regulatory Activities version 23.1 was used to code all adverse events (AEs). Safety was assessed according to the Common Terminology Criteria for Adverse Events version 5.0.
Statistical analyses
All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). The 90% confidence interval (CI) for ORR was calculated using the Clopper-Pearson method. The Kaplan-Meier method was used to estimate the survival distribution function for time-to-event analyses. DOR calculated for responders was defined as the time from first response to RD/PD or death from any cause, whichever occurred first. PFS was measured from the start of treatment until RD/PD based on overall response assessment or death. OS was defined as the time from the start of study treatment to death, regardless of cause. TTR was defined as the time from the start of study treatment to the first assessed response (CR, CRu, or PR). TCR consisted of the proportion of patients whose best response was CR, CRu, PR, or SD.
Sample size
The threshold ORR was set at 5%, primarily because no established treatment exists for target patients. The expected ORR was set at 30% based primarily on a prior study of lenalidomide in R/R ATL.15 A binomial 1-sided exact test was performed to test the null hypothesis at a 5% significance level (H0: ORR <0.05); 21 patients were needed for 90% power.
Study oversight
This study was sponsored by Daiichi Sankyo Co Ltd (Tokyo, Japan) and was conducted in compliance with the Declaration of Helsinki and Good Clinical Practice guidelines as outlined by the International Conference on Harmonisation E6 requirements. All protocols were approved by the institutional review board at each participating center. Academic investigators and sponsors were responsible for the study design. All patients who participated in this trial provided written informed consent before enrollment.
Results
Patients
Twenty-eight patients were screened, and 25 (12 men, 13 women) were enrolled between November 2019 and October 2020 across 12 sites in Japan. Baseline patient and disease characteristics are summarized in Table 1. The median age was 69.0 years (range, 59-84 years). This study enrolled 16, 6, and 3 patients with acute, lymphoma, or unfavorable chronic type R/R ATL, respectively. ATL status included 8 patients (32%) with relapsed, 6 patients (24%) with recurrent, and 11 patients (44%) with refractory disease. The median time since the last ATL treatment was 60 days (range, 23-1400 days). All 25 patients had received treatment for ATL, with a median of 3 prior lines of therapy (range, 1-8). Twenty-four patients had received mogamulizumab treatment; 1 patient with CCR4-negative ATL had no prior mogamulizumab therapy. Six patients (24%) were refractory to mogamulizumab-containing regimens. Eight patients (32%) had received lenalidomide. Seventeen patients (68%) discontinued the study drug, and 14 patients (56%) discontinued because of disease progression. Two patients (8%) discontinued study treatment owing to AEs, and 1 patient (4%) discontinued study treatment per physician decision.
Baseline patient and disease characteristics
| Patient characteristics . | Patients (N = 25) . |
|---|---|
| Age, median (range), y | 69.0 (59-84) |
| Female, n (%) | 13 (52.0) |
| Eastern Cooperative Oncology Group performance status, n (%) | |
| 0 | 13 (52.0) |
| 1 | 10 (40.0) |
| 2∗ | 2 (8.0) |
| Time since last ATL treatment, median (range), d | 60.0 (23-1400) |
| Prior lines of therapy, median (range) | 3 (1-8) |
| Prior mogamulizumab therapy, n (%) | |
| Yes | 24 (96.0) |
| No | 1 (4.0) |
| Refractory to mogamulizumab-containing therapy, n (%) | 6 (24.0) |
| Prior lenalidomide therapy, n (%) | |
| Yes | 8 (32.0) |
| No | 17 (68.0) |
| Prior anthracycline-based therapy, n (%) | |
| Yes | 24 (96.0) |
| No | 1 (4.0) |
| Prior HSCT, n (%) | |
| No | 25 (100.0) |
| ATL subtype, n (%) | |
| Acute | 16 (64.0) |
| Lymphoma | 6 (24.0) |
| Unfavorable chronic | 3 (12.0) |
| Patient characteristics . | Patients (N = 25) . |
|---|---|
| Age, median (range), y | 69.0 (59-84) |
| Female, n (%) | 13 (52.0) |
| Eastern Cooperative Oncology Group performance status, n (%) | |
| 0 | 13 (52.0) |
| 1 | 10 (40.0) |
| 2∗ | 2 (8.0) |
| Time since last ATL treatment, median (range), d | 60.0 (23-1400) |
| Prior lines of therapy, median (range) | 3 (1-8) |
| Prior mogamulizumab therapy, n (%) | |
| Yes | 24 (96.0) |
| No | 1 (4.0) |
| Refractory to mogamulizumab-containing therapy, n (%) | 6 (24.0) |
| Prior lenalidomide therapy, n (%) | |
| Yes | 8 (32.0) |
| No | 17 (68.0) |
| Prior anthracycline-based therapy, n (%) | |
| Yes | 24 (96.0) |
| No | 1 (4.0) |
| Prior HSCT, n (%) | |
| No | 25 (100.0) |
| ATL subtype, n (%) | |
| Acute | 16 (64.0) |
| Lymphoma | 6 (24.0) |
| Unfavorable chronic | 3 (12.0) |
One patient had an Eastern Cooperative Oncology Group performance status of 2 at initial screening but advanced to a status of 3 on day 1 cycle 1.
Efficacy
At data cutoff (24 April 2021), the median follow-up was 6.5 months. The IEAC-assessed/median TTR was 1.4 months (range, 1.0-5.6 months). The study met its primary end point with a centrally reviewed IEAC-assessed ORR of 48.0% (P < .0001; 12/25; 90% CI, 30.5%-65.9%), including a CR rate of 20.0% (5/25) and a PR rate of 28.0% (7/25) (Table 2). The ORR by subtype was 62.5% (10/16) for acute, 16.7% (1/6) for lymphoma, and 33.3% (1/3) for unfavorable chronic. Notably, the ORR of patients pretreated with mogamulizumab was 45.8% (11/24; 90% CI, 28.2%-64.2%). The ORR of patients refractory to mogamulizumab-containing therapies was 50.0% (3/6). An ORR of 50% (90% CI, 15.7%-84.3%) was achieved in patients with prior lenalidomide treatment (4/8). The ORR by disease status was 37.5% (3/8) for relapsed, 66.7% (4/6) for recurrent, and 45.5% (5/11) for refractory disease (Table 2). The TCR was 88.0% (95% CI, 68.8%-97.5%).
Summary of patient best responses as assessed by an IEAC
| Population . | N . | ORR, n (%) . | CR, n (%) . | CRu, n (%) . | PR, n (%) . | SD, n (%) . | PD, n (%) . |
|---|---|---|---|---|---|---|---|
| All patients | 25 | 12 (48.0) | 5 (20.0) | 0 | 7 (28.0) | 10 (40.0) | 3 (12.0) |
| ATL subtype | |||||||
| Acute | 16 | 10 (62.5) | 5 (31.3) | 0 | 5 (31.3) | 4 (25.0) | 2 (12.5) |
| Lymphoma | 6 | 1 (16.7) | 0 | 0 | 1 (16.7) | 5 (83.3) | 0 |
| Unfavorable chronic | 3 | 1 (33.3) | 0 | 0 | 1 (33.3) | 1 (33.3) | 1 (33.3) |
| Disease site | |||||||
| Nodal or extranodal lesions | 20 | 10 (50.0) | 6 (30.0) | 2 (10.0) | 2 (10.0) | 7 (35.0) | 3 (15.0) |
| Skin lesions∗ | 7 | 3 (42.9) | 1 (14.3) | Not evaluable | 2 (28.6) | 3 (42.9) | 0 |
| Peripheral blood | 9 | 8 (88.9) | 2 (22.2) | Not evaluable | 6 (66.7) | 1 (11.1) | 0 |
| Disease status | |||||||
| Relapsed | 8 | 3 (37.5) | 1 (12.5) | 0 | 2 (25.0) | 4 (50.0) | 1 (12.5) |
| Recurrent | 6 | 4 (66.7) | 1 (16.7) | 0 | 3 (50.0) | 2 (33.3) | 0 |
| Refractory | 11 | 5 (45.5) | 3 (27.3) | 0 | 2 (18.2) | 4 (36.4) | 2 (18.2) |
| Prior mogamulizumab treatment | |||||||
| Yes | 24 | 11 (45.8) | 4 (16.7) | 0 | 7 (29.2) | 10 (41.7) | 3 (12.5) |
| No | 1 | 1 (100.0) | 1 (100.0) | 0 | 0 | 0 | 0 |
| Prior lenalidomide treatment | |||||||
| Yes | 8 | 4 (50.0) | 0 | 0 | 4 (50.0) | 3 (37.5) | 1 (12.5) |
| No | 17 | 8 (47.1) | 5 (29.4) | 0 | 3 (17.6) | 7 (41.2) | 2 (11.8) |
| Population . | N . | ORR, n (%) . | CR, n (%) . | CRu, n (%) . | PR, n (%) . | SD, n (%) . | PD, n (%) . |
|---|---|---|---|---|---|---|---|
| All patients | 25 | 12 (48.0) | 5 (20.0) | 0 | 7 (28.0) | 10 (40.0) | 3 (12.0) |
| ATL subtype | |||||||
| Acute | 16 | 10 (62.5) | 5 (31.3) | 0 | 5 (31.3) | 4 (25.0) | 2 (12.5) |
| Lymphoma | 6 | 1 (16.7) | 0 | 0 | 1 (16.7) | 5 (83.3) | 0 |
| Unfavorable chronic | 3 | 1 (33.3) | 0 | 0 | 1 (33.3) | 1 (33.3) | 1 (33.3) |
| Disease site | |||||||
| Nodal or extranodal lesions | 20 | 10 (50.0) | 6 (30.0) | 2 (10.0) | 2 (10.0) | 7 (35.0) | 3 (15.0) |
| Skin lesions∗ | 7 | 3 (42.9) | 1 (14.3) | Not evaluable | 2 (28.6) | 3 (42.9) | 0 |
| Peripheral blood | 9 | 8 (88.9) | 2 (22.2) | Not evaluable | 6 (66.7) | 1 (11.1) | 0 |
| Disease status | |||||||
| Relapsed | 8 | 3 (37.5) | 1 (12.5) | 0 | 2 (25.0) | 4 (50.0) | 1 (12.5) |
| Recurrent | 6 | 4 (66.7) | 1 (16.7) | 0 | 3 (50.0) | 2 (33.3) | 0 |
| Refractory | 11 | 5 (45.5) | 3 (27.3) | 0 | 2 (18.2) | 4 (36.4) | 2 (18.2) |
| Prior mogamulizumab treatment | |||||||
| Yes | 24 | 11 (45.8) | 4 (16.7) | 0 | 7 (29.2) | 10 (41.7) | 3 (12.5) |
| No | 1 | 1 (100.0) | 1 (100.0) | 0 | 0 | 0 | 0 |
| Prior lenalidomide treatment | |||||||
| Yes | 8 | 4 (50.0) | 0 | 0 | 4 (50.0) | 3 (37.5) | 1 (12.5) |
| No | 17 | 8 (47.1) | 5 (29.4) | 0 | 3 (17.6) | 7 (41.2) | 2 (11.8) |
RD: received ≥1 prior chemotherapy, achieved CR or CRu, and subsequently experienced disease progression. Recurrent disease: received ≥1 prior chemotherapy, achieved PR, and subsequently experienced disease progression. Refractory: received ≥1 prior chemotherapy and either achieved SD requiring a treatment switch or subsequently experienced PD.
One patient was not evaluated for skin lesions after baseline assessment.
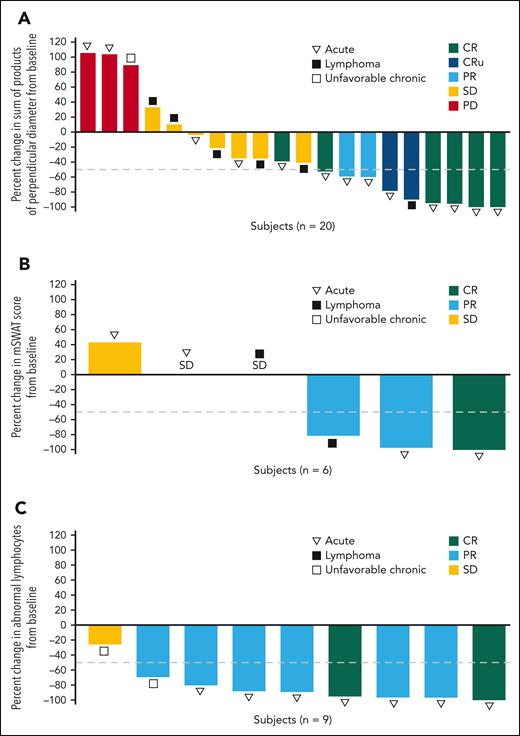
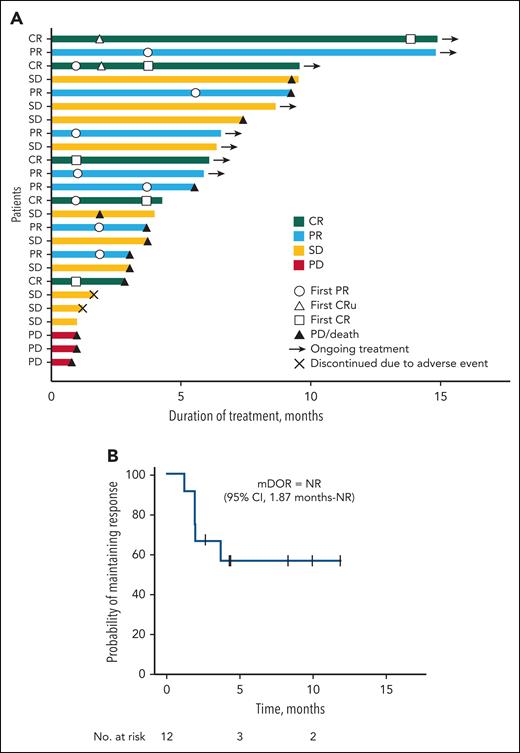
Efficacy was further assessed by disease compartment. The IEAC-assessed best percentage change in tumor burden by disease compartment is depicted in Figure 1. A ≥50% reduction from baseline was observed in the nodal or extranodal lesions in 9 of 20 patients (5 CRs, 2 CRus, and 2 PRs) (Figure 1A), in the skin compartment in 3 of 6 patients (1 CR and 2 PRs) (Figure 1B), and in the peripheral blood in 8 of 9 patients (2 CRs, and 6 PRs) (Figure 1C). One patient with skin lesions did not undergo assessment after the initial baseline measurement and was not included in the analysis. The treatment duration with clinical outcomes of the 25 patients is shown in Figure 2A. At data cutoff, 8 patients were receiving ongoing study treatment. Notably, 4 of 10 patients who achieved SD were able to receive valemetostat for ≥6 months following treatment initiation. Furthermore, responses were converted from PR to CR in 2 patients. The median DOR was NR (95% CI, 1.87 months to NR) (Figure 2B), and 50% (6/12) of responders had an ongoing response. Although immature, the median PFS and OS were 7.4 months (95% CI, 3.0 months to not estimable) and 16.4 months (95% CI, 6.5 months-16.4 months), respectively.
Best percent change in tumor burden by disease compartment as assessed by IEAC. The best percent change in tumor burden is shown for nodal or extranodal lesions (A), skin lesions (B), and peripheral blood (C) in patients treated with valemetostat. The dashed line indicates a 50% reduction in tumor burden from baseline.
Best percent change in tumor burden by disease compartment as assessed by IEAC. The best percent change in tumor burden is shown for nodal or extranodal lesions (A), skin lesions (B), and peripheral blood (C) in patients treated with valemetostat. The dashed line indicates a 50% reduction in tumor burden from baseline.
Treatment duration with clinical outcomes and DOR. (A) The swimmer plot summarizes treatment duration of individual patients and best response. Patients ongoing in the study are denoted by arrows. (B) The Kaplan-Meier plot depicts the DOR. Tick marks denote censored patients. mDOR, median duration of response in months; NR, not reached.
Treatment duration with clinical outcomes and DOR. (A) The swimmer plot summarizes treatment duration of individual patients and best response. Patients ongoing in the study are denoted by arrows. (B) The Kaplan-Meier plot depicts the DOR. Tick marks denote censored patients. mDOR, median duration of response in months; NR, not reached.
Efficacy outcomes per investigator assessment were consistent with IEAC-adjudicated results. Investigator-assessed ORR was 56.0% (90% CI, 37.9%-73.0%), including 24.0% (6/25) with CR, 4.0% (1/25) with CRu, and 28.0% (7/25) with PR. The investigator-assessed CR rate was 28.0% (7/25).
Pharmacokinetics
Serial and trough blood samples were collected for plasma valemetostat concentration measurements (supplemental Figure 1). The mean maximum plasma concentration (Cmax) of total valemetostat (2230 ng/mL on cycle 1 day 1 and 2300 ng/mL on cycle 1 day 15) and unbound valemetostat (81.7 ng/mL on cycle 1 day 1 and 84.9 ng/mL on cycle 1 day 15) was achieved in a median time to Cmax (Tmax) of 2 to 4 hours (supplemental Table 1). At steady state (cycle 1 day 15), the mean area under the plasma concentration time profile during dosing interval (AUCtau) was 20,800 ng·h/mL for total valemetostat and 584 ng·h/mL for unbound valemetostat. The mean accumulation ratios for AUCtau of total and unbound valemetostat were 1.19 and 1.27, respectively, indicating mild accumulation after continuous once-daily dosing at 200 mg.
Safety
Table 3 summarizes TEAEs and the frequency of the most common TEAEs. All patients treated with valemetostat experienced TEAEs. Common hematologic TEAEs were thrombocytopenia (80%), anemia (52%), neutropenia (28%), lymphopenia (24%), and leukopenia (20%). Grade ≥3 hematologic TEAEs reported in ≥10% of patients were thrombocytopenia (32%), anemia (32%), lymphopenia (16%), leukopenia (12%), and neutropenia (12%). Common nonhematologic TEAEs included alopecia (40%), dysgeusia (36%), decreased appetite (20%), and pyrexia (20%). Serious TEAEs occurred in 8 patients (32%). Cardiac failure led to discontinuation in 1 patient; other serious TEAEs resolved without discontinuation of the study drug. This study prespecified 3 AEs of special interest, which included combined elevations of aminotransferases and bilirubin (alanine aminotransferase and/or aspartate aminotransferase ≥3 × ULN and blood bilirubin ≥2 × ULN), secondary malignancy, and thrombocytopenia. No patient met the criteria for AEs of special interest except for thrombocytopenia. Secondary malignancies, including hematologic malignancy or myelodysplastic syndrome, were not observed.
Summary of TEAEs occurring in ≥20% of patients and grade ≥3 events regardless of relation to valemetostat treatment
| All AEs . | ||
|---|---|---|
| AE type . | N = 25, n (%) . | |
| TEAEs | 25 (100.0) | |
| TRAEs | 24 (96.0) | |
| Serious TEAE∗ | 8 (32.0) | |
| Serious TRAEs | 7 (28.0) | |
| Grade ≥3 TEAEs | 15 (60.0) | |
| Grade ≥3 TRAEs | 14 (56.0) | |
| TEAEs leading to reduction | 2 (8.0) | |
| TRAEs leading to reduction | 2 (8.0) | |
| TEAEs leading to interruption | 5 (20.0) | |
| TRAEs leading to interruption | 4 (16.0) | |
| TEAEs leading to discontinuation | 2 (8.0) | |
| TRAEs leading to discontinuation | 2 (8.0) | |
| All AEs . | ||
|---|---|---|
| AE type . | N = 25, n (%) . | |
| TEAEs | 25 (100.0) | |
| TRAEs | 24 (96.0) | |
| Serious TEAE∗ | 8 (32.0) | |
| Serious TRAEs | 7 (28.0) | |
| Grade ≥3 TEAEs | 15 (60.0) | |
| Grade ≥3 TRAEs | 14 (56.0) | |
| TEAEs leading to reduction | 2 (8.0) | |
| TRAEs leading to reduction | 2 (8.0) | |
| TEAEs leading to interruption | 5 (20.0) | |
| TRAEs leading to interruption | 4 (16.0) | |
| TEAEs leading to discontinuation | 2 (8.0) | |
| TRAEs leading to discontinuation | 2 (8.0) | |
| Most common TEAEs . | ||
|---|---|---|
| Hematologic | All grades (≥20%), n (%) | Grade ≥3, n (%) |
| Thrombocytopenia† | 20 (80.0) | 8 (32.0) |
| Anemia‡ | 13 (52.0) | 8 (32.0) |
| Neutropenia§ | 7 (28.0) | 3 (12.0) |
| Lymphopenia|| | 6 (24.0) | 4 (16.0) |
| Leukopenia¶ | 5 (20.0) | 3 (12.0) |
| Nonhematologic | All grades (≥20%), n (%) | Grade ≥3, n (%) |
| Alopecia | 10 (40.0) | 0 |
| Dysgeusia | 9 (36.0) | 0 |
| Decreased appetite | 5 (20.0) | 2 (8.0) |
| Pyrexia | 5 (20.0) | 0 |
| Most common TEAEs . | ||
|---|---|---|
| Hematologic | All grades (≥20%), n (%) | Grade ≥3, n (%) |
| Thrombocytopenia† | 20 (80.0) | 8 (32.0) |
| Anemia‡ | 13 (52.0) | 8 (32.0) |
| Neutropenia§ | 7 (28.0) | 3 (12.0) |
| Lymphopenia|| | 6 (24.0) | 4 (16.0) |
| Leukopenia¶ | 5 (20.0) | 3 (12.0) |
| Nonhematologic | All grades (≥20%), n (%) | Grade ≥3, n (%) |
| Alopecia | 10 (40.0) | 0 |
| Dysgeusia | 9 (36.0) | 0 |
| Decreased appetite | 5 (20.0) | 2 (8.0) |
| Pyrexia | 5 (20.0) | 0 |
TEAE, treatment-emergent adverse event; TRAE, treatment-related adverse event.
Acute kidney injury, cardiac failure, cytomegalovirus chorioretinitis, cytomegalovirus infection reactivation, febrile neutropenia, hepatic function abnormal, hypercalcemia, lower gastrointestinal hemorrhage, overdose, thrombocytopenia, pneumonia, and venous thrombosis limb occurred in 1 patient each.
Encompasses the preferred terms: thrombocytopenia and platelet count decreased.
Encompasses the preferred terms: anemia, hemoglobin decreased, hematocrit decreased, and red blood cell count decreased.
Encompasses the preferred terms: neutropenia and neutrophil count decreased.
Encompasses the preferred terms: lymphopenia and lymphocyte count decreased.
Encompasses the preferred terms: leukopenia and white blood cell count decreased.
Of 3 patients who experienced grade 4 thrombocytopenia (platelet count, <25 × 109/L), the median time to onset of postbaseline platelet reduction occurred early during treatment, at 21 days from the first dose, with a median time to recovery (platelet count, ≥25 × 109/L) of 3 days. Three of 20 patients who experienced thrombocytopenia required dose modification (discontinuation, 1 patient; dose interruption, 2 patients). Thrombocytopenia in most of the remaining 17 patients was transient and resolved without dose modification. Five patients required platelet transfusions for thrombocytopenia, and 3 patients required a red blood cell transfusion for anemia. TEAEs led to a dose reduction in 2 patients (8%). Five patients (20%) experienced TEAEs that required dose interruption. Two patients (8%) who had achieved SD discontinued study treatment owing to AEs such as cardiac failure and thrombocytopenia. No treatment-related deaths occurred.
The median dose intensity of valemetostat was 199.33 mg/day. The median duration of treatment was 4.3 months (range, 0.8-14.9 months).
Discussion
Here, we observed clinically relevant efficacy and tolerable safety of valemetostat in patients with R/R ATL after prior systemic therapy, including mogamulizumab or ≥1 prior systemic therapy with cytotoxic chemotherapy in patients intolerant of, or ineligible for, mogamulizumab. The primary end point was met, with an IEAC-assessed ORR of 48.0%, including a CR rate of 20.0% and a PR rate of 28.0%. Importantly, valemetostat was effective in patients pretreated with mogamulizumab and those with disease refractory to mogamulizumab, ORRs of 45.8% and 50.0%, respectively. We noted an ORR of 50.0% in patients previously treated with lenalidomide.
ATL carries a very poor prognosis among various histologic subtypes of T-cell lymphomas.3,4,8,9,28,29 Few treatment options are available for patients with R/R ATL, highlighting the considerable need for novel therapies. In separate studies that included subsets of patients pretreated with mogamulizumab, the ORRs with lenalidomide and tucidinostat monotherapy were 18% (2/11) and 30% (7/23), respectively.15,17 Our findings demonstrated clinically meaningful efficacy of valemetostat in heavily pretreated patients with R/R ATL and support valemetostat as a treatment option for patients experiencing PD after prior therapy, including mogamulizumab.
In contrast to follicular lymphoma or diffuse large B-cell lymphoma, EZH2 gain-of-function mutations are not observed in ATL; however, overexpression of EZH2 and polycomb repressive complex 2 dysfunction are common in ATL.30-32 Molecular therapeutics targeting EZH2 have been explored in the treatment of NHLs except for ATL.33,34 Compared with the EZH2-selective inhibitor tazemetostat, valemetostat strongly reduced H3K27 trimethylation through inhibition of EZH2 and EZH1, driving re-expression of repressed genes.24 Valemetostat demonstrated greater attenuation of ATL cell growth in vitro at lower concentrations than tazemetostat and GSK126, primarily because of dual inhibition of EZH2 and EZH1.24 We continue to evaluate the biological underpinnings of valemetostat’s mechanism of action through assessments of key biomarkers and comprehensive gene mutation and expression analyses of ATL cells.
Responses to valemetostat were observed across disease compartments, subtypes (acute, lymphoma, or chronic), and statuses (relapsed, recurrent, or refractory). Notably, valemetostat yielded an ORR of 50% in nodal or extranodal lesions, which is higher than that seen in phase 2 studies of mogamulizumab (25%, 3/12) and lenalidomide (31%, 5/16).15,16 In patients with aggressive acute type ATL, the ORR was 62.5% (10/16) in response to valemetostat compared with 43% (6/14) and 33% (5/15) with mogamulizumab and lenalidomide, respectively.15,16 This study included 11 patients with refractory disease (SD or PD) to last prior therapy; the ORR in this population was 45.5%. However, in the phase 2 study of mogamulizumab, only patients who had achieved response to the last previous therapy were included.15 In the phase 2 study of lenalidomide, only 2 patients with SD to prior therapy were included.14 The shorter time since last ATL treatment (median, 60 days) in the current study compared with those in the phase 2 studies for other agents (234.5 days for lenalidomide and 89 days for tucidinostat) reflects the inclusion of patients with refractory disease and more aggressive features. Furthermore, the higher median of 3 prior lines of therapy (range, 1-8) in this study compared with 2 for lenalidomide (range, 1-4) and 2 for tucidinostat is similarly reflective of aggressive ATL.
The safety profile was consistent with the phase 1 study of valemetostat.25 Among the most common TEAEs were cytopenias, which included thrombocytopenia, the most common grade ≥3 severe TEAE. Many thrombocytopenia events resolved without dose reduction or interruption. All other TEAEs were manageable with supportive care and/or dose modification, indicating a manageable and acceptable safety profile for valemetostat in patients with R/R ATL.
This study had several limitations. Patients who had received prior allo-HSCT were excluded as part of the study design because of potential worsening of graft-versus-host disease. Future studies could assess the impact of valemetostat in patients with prior allo-HSCT. In addition, it is unknown how valemetostat therapy would affect outcomes of subsequent allo-HSCT treatment because no patient underwent allo-HSCT after study drug discontinuation. Prior treatment with mogamulizumab in patients undergoing allo-HSCT is associated with high rates of severe graft-versus-host disease and mortality, mainly because of the long-term depletion of CCR4-expressing regulatory T cells.35 The effects of valemetostat on immune function in patients remain to be elucidated. Furthermore, the follow-up period of this study is limited. Long-term follow-up is warranted to fully understand the efficacy and safety of valemetostat in patients with R/R ATL. Finally, the number of patients included in this study was limited, although sample size was sufficient to evaluate our hypothesis, and the number of patients enrolled in this study was comparable with that in the phase 2 studies of other agents for ATL, a relatively rare disease, even in Japan where HTLV-1 is endemic.15-17 Because of this small number of patients, there are limitations to examining the effect of this agent in each subgroup. Future follow-up studies will be necessary to fully understand the efficacy of these agents in the treatment of ATL.
Collectively, valemetostat shows promise in treating relapsed, recurrent, or refractory ATL in patients with an extensive treatment history, including mogamulizumab. The combination of clinical efficacy, antitumor properties, and an acceptable safety profile of valemetostat in our phase 2 study provides rationale for further investigation of this agent in ATL.
Acknowledgments
The authors thank the patients, their families, and their caregivers for their participation, and the study staff and the independent efficacy assessment committee for their contributions.
Medical writing support was provided by Michael C. Holter of SciMentum Inc, a Nucleus Holdings Group Inc company, and was funded by Daiichi Sankyo Inc. Editorial support was provided in accordance with Good Publication Practice guidelines. This study is sponsored by Daiichi Sankyo Co Ltd.
Authorship
Contribution: K. Izutsu, A.U., K. Tsukasaki, H.Y., K. Tobinai, K.Y., and K. Ishitsuka conceived and designed this study; K. Tobinai and K. Ishitsuka supervised the study; K. Izutsu, H.Y., and N.A. wrote the manuscript; K. Izutsu, S. Makita, K.N., M.Y., A.U., S.K., S. Morishima, K. Tsukasaki, T.K., T.O., S.R., H.K., J.I., K.Y., and K. Ishitsuka contributed to patient accrual; K. Izutsu, H.Y., K.K., M.T., and K. Ishitsuka contributed to data analysis and interpretation; Y.K. provided statistical support; and all authors reviewed, edited, and approved the final version of the manuscript.
Conflict-of-interest disclosure: K. Izutsu has received honoraria from Eisai, Chugai, Janssen, AstraZeneca, Novartis, Bristol Myers Squibb, Kyowa Kirin, AbbVie, Ono Pharmaceutical, Eli Lilly, MSD, Daiichi Sankyo, Symbio, and Takeda; research funding from Eisai, Chugai, Janssen, AstraZeneca, Novartis, AbbVie, Daiichi Sankyo, Pfizer, Yakult, Genmab, Beigene, and Incyte; and has had an advisory or consulting role with Eisai, AbbVie, and Genmab. S. Makita has received honoraria from Celgene/Bristol Myers Squibb, Chugai Pharma, Daiichi Sankyo/UCB Japan, Eisai, Novartis, Takeda, CSL Behring, and Meiji Seika Pharma and has had an advisory or consulting role at Celgene/Bristol Myers Squibb and Takeda. M.Y. has received honoraria from Takeda, Bristol Myers Squibb/Medarex, Novartis, CSL Behring, Chugai Pharma, Otsuka, and Daiichi Sankyo and has had an advisory or consulting role at Takeda. A.U. has received honoraria from Bristol Myers Squibb and Meiji Seika Pharma and has had an advisory or consulting role with JIMRO and Otsuka Medical Devices. S.K. has received honoraria and research funding from Chugai Pharmaceutical Co Ltd, Kyowa Kirin Co Ltd, and Daiichi Sankyo/UCB Japan. S. Morishima has received honoraria from Pfizer, Janssen, AbbVie, Nippon Shinyaku, Bayer, Chugai Pharma, Sanofi, Kyowa Kirin, Takeda, and Daiichi Sankyo. K. Tsukasaki has received honoraria from Chugai Pharmaceutical Co Ltd/Roche, Eisai, Kyowa Hakko Kirin, Takeda, HUYA Bioscience International, Meiji Seika Kaisha, and Bristol Myers Squibb Japan; research funding from Eisai, HUYA Bioscience International, Daiichi Sankyo/UCB Japan, Bristol Myers Squibb Japan, Chugai Pharma, Byer, Kyowa Kirin International, and Regeneron Pharmaceutical; and has had an advisory or consulting role at HUYA Bioscience International, Yakult Pharmaceutical, Ono Pharmaceutical, Meiji Seika Kaisha, Solasia Pharma, and Daiichi Sankyo/UCB Japan. T.O. has received honoraria from Kyowa Hakko Kirin, Chugai Pharmaceutical Co Ltd, Novartis, Bristol Myers Squibb, Pfizer, Otsuka Pharmaceutical Co Ltd, Ono Pharmaceutical Co Ltd, Takeda Pharmaceutical Co Ltd, Astellas Pharma, Eisai Pharmaceuticals, Janssen Pharm, Daiichi Sankyo, Mundipharma, and Asahi Kasei Pharma; research funding from Celgene, Kyowa Hakko Kirin, Chugai Pharmaceutical Co, Ltd, TAIHO Pharmaceutical Co Ltd, and Asahi Kasei Pharma; and has participated in speaker’s bureaus at Novartis, Bristol Myers Squibb, Pfizer, and Otsuka Pharmaceutical Co Ltd. S.R. has participated in speaker’s bureaus at Chugai Pharmaceutical, Eisai Pharmaceutical, Ono Pharmaceuticals, and Janssen Pharmaceutical. K.K. has been employed by Bristol Myers Squibb, Daiichi Sankyo, and Shattuck Labs and owns stock in Bristol Myers Squibb and Celgene. K. Tobinai has received honoraria from Zenyaku Kogyo, Mundipharma, HUYA Bioscience International, Celgene, Daiichi Sankyo, and Solasia Pharma and has had an advisory or consulting role at Zenyaku Kogyo, HUYA Bioscience International, Daiichi Sankyo, and Mundipharma. K.Y. has received honoraria from AbbVie, Amgen, Celgene, Daiichi Sankyo, Eisai, Eli Lilly Japan, Janssen, Kaken, Kyowa Kirin, Maruho, Meiji Seika Pharma, Minophagen, Novartis, Sanofi, Sun Pharma, Taiho, Torii, and UCB. K. Ishitsuka has received honoraria from Celgene, Kyowa Hakko Kirin, Bristol Myers Squibb Japan, Eisai, Pfizer, Daiichi Sankyo, Takeda, Chugai Pharma, CSL Behring, Nippon Shinyaku, Janssen, Sanofi, Ono Pharmaceutical, Astellas Pharma, Otsuka, and Meiji Seika Kaisha and research funding from Ono Pharmaceutical, Japan Blood Products Organization, Eisai, Taiho Pharmaceutical, MSD, Chugai Pharma, Dainippon Sumitomo Pharma, Asahi Kasei, Mochida Pharmaceutical Co Ltd, and Takeda. H.Y., M.T., Y.K., and N.A. are current employees of Daiichi Sankyo Co Ltd. The remaining authors declare no competing financial interests.
The current affiliation for K.K. is Shattuck Labs, Inc, Austin, TX.
Correspondence: Koji Izutsu, Department of Hematology, National Cancer Center Hospital, 5-1-1 Tsukiji, Chuo-ku, Tokyo 104-0045, Japan; e-mail: kizutsu@ncc.go.jp.
References
Author notes
Qualified researchers may request access to deidentified individual participant data and applicable supporting clinical trial documents through the clinical study data request platform at https://vivli.org/. In cases where clinical trial data and supporting documents are provided pursuant to Daiichi Sankyo’s company policies and procedures, Daiichi Sankyo will continue to protect the privacy of our clinical trial participants. Details on data sharing criteria and the procedure for requesting access can be found at https://vivli.org/ourmember/daiichi-sankyo/.
Data are available on request from the corresponding author, Koji Izutsu (kizutsu@ncc.go.jp).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal