TO THE EDITOR:
Patients with multiple myeloma (MM) are at increased risk of infection and severe outcomes from COVID-19 infection,1,2 partly owing to inadequate protection by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination.2-7 Their susceptibility stems from both immune-suppressive treatments used for MM and underlying immune-deficiency from the disease itself.4,6,8 However, it is unknown how much risk is shared by patients with monoclonal gammopathy of undetermined significance (MGUS),9,10 a precursor state to MM that does not require immune-suppressive treatment but may be associated with immune-deficiency. Here, we measured the risk of breakthrough infections among vaccinated patients with MM or MGUS relative to the patients without MM or MGUS in the national Veterans Affairs (VA) health care system and described the association of MM-specific treatments with breakthrough risk.
We used a matched cohort design to measure the risk of breakthrough infection among patients with MM or MGUS relative to those without, using a similar matching procedure as recently used in the VA cancer patient population.7 Veterans fully vaccinated with messenger RNA (mRNA)-based vaccine were identified using the VA Corporate Data Warehouse (CDW), which collates electronic health record information from VA facilities nationwide. Full vaccination was defined to start 14 days after receipt of the second dose of BNT162b2 or mRNA-1273 vaccine. Patients vaccinated with Janssen/Johnson & Johnson Ad26.COV2.S or prior SARS-CoV-2 infection were excluded. Patients with MM were identified using the VA CDW using a previously published algorithm.11 They were required to have at least 3 MM International Classification of Disease–coded visits and have received at least 1 MM-specific treatment prior to December 15, 2020. Patients with MGUS were required to have at least 3 MGUS International Classification of Disease–coded visits and to never have had any MM-specific treatment prior to December 15, 2020. We filtered for regular users of the VA system, which is defined as those who had at least 1 outpatient or inpatient visit every year 3 years prior to study start date, to exclude patients whose data may be incomplete. Data were primarily obtained from the VA CDW and SARS-CoV-2 vaccination, and infection status was obtained from the VA COVID-19 Shared Data Resource.
Fully vaccinated patients with MM and MGUS were matched 1:1 with vaccinated controls on factors potentially associated with SARS-CoV-2 exposure: specifically, age, race, VA facility, and rurality of home address. VA facility was defined as the location where the last COVID-19 vaccine dose was administered. Age, race, and rurality of home address are available as structured data in the CDW. We used minimum distance matching for age and exact matching for other variables. For each day of the study period, each new fully vaccinated patient with MM or MGUS was matched with a control patient without MM or MGUS. The primary outcome was reverse transcriptase–polymerase chain reaction or antigen-confirmed SARS-CoV-2 infection. The secondary outcome of severe COVID-19 was defined as SARS-CoV-2 infection within −1 to 14 days of a documented oxygen saturation <94% or supplemental oxygen use, as defined in our previous work.12 Each pair was followed until SARS-CoV-2 infection, death, or end of study. The Fine-Gray subdistribution hazard was used to measure risk of infection with the competing risk of death.
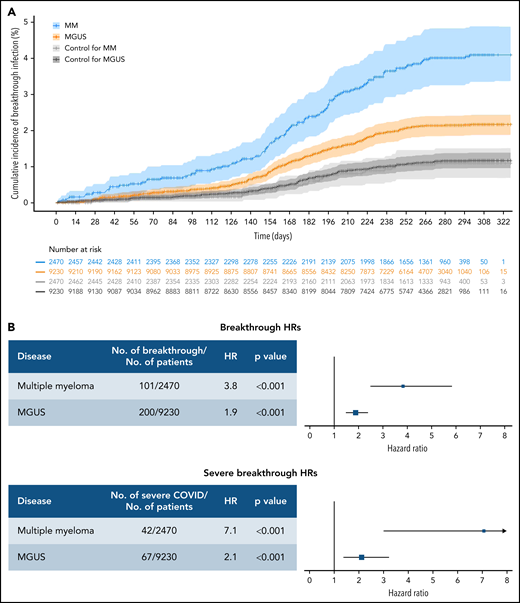
Between December 15, 2020 and December 13, 2021, we identified 2677 patients with MM (median age, 73; non-Hispanic white, 58%) and 9804 patients with MGUS (median age, 74; non-Hispanic white, 56%) who were fully vaccinated from an mRNA-based vaccine prior to the end of study. Matched to non-MM/MGUS controls were 2470 patients with MM and 9230 patients with MGUS. The median follow-up time for the matched vaccinated cohort was 285 days for MM and 280 days among MGUS. Compared with the controls, patients with MM had an increased risk of breakthrough infection (hazard ratio [HR], 3.81; 95% confidence interval [CI], 2.49-5.82; P < .001), and MGUS (HR, 1.88; 95% CI, 1.49-2.37; P < .001) (Figure 1). Similar increased risk was observed for severe COVID-19 (HR, 7.07; 95% CI, 3.01-16.64; P < .001 for MM; and HR, 2.11; 95% CI, 1.39-3.22; P < .001 for MGUS). Of the matched cohort, 1482 patients with MM and 4523 patients with MGUS received booster vaccinations, compared with 691 and 2626 boosted patients in their respective matched controls.
Cumulative incidence and HRs for breakthrough infection in patients with MM or MGUS. (A) Cumulative incidence curves of any breakthrough SARS-COV-2 infection by disease state shown using time zero as 14 days after the day of second dose of vaccination. CIs were calculated by bootstrapping. The number at risk are also shown. (B) HRs for infection separated by disease state and COVID severity. Hazards are calculated separately for risk of any breakthrough infection and risk of severe breakthrough infection relative to matched controls. All hazards were calculated using Fine-Gray competing risk regression with the competing risk of death.
Cumulative incidence and HRs for breakthrough infection in patients with MM or MGUS. (A) Cumulative incidence curves of any breakthrough SARS-COV-2 infection by disease state shown using time zero as 14 days after the day of second dose of vaccination. CIs were calculated by bootstrapping. The number at risk are also shown. (B) HRs for infection separated by disease state and COVID severity. Hazards are calculated separately for risk of any breakthrough infection and risk of severe breakthrough infection relative to matched controls. All hazards were calculated using Fine-Gray competing risk regression with the competing risk of death.
Next, we wanted to determine how cancer treatment affects breakthrough infection risk among fully vaccinated patients with MM. Prior studies have shown decreased vaccine response in patients on anti-CD38–directed therapies and chemotherapy.4,7 However, the effect of less immunosuppressive therapies, such as proteasome inhibitors, on vaccine response is unknown.6 To evaluate impact on breakthrough infection risk, we categorized type of systemic anti-MM treatments received and timing of last therapy before vaccination. We defined anti-MM treatments as systemic chemotherapies and MM-specific therapies using a previously validated algorithm.11 Treatment types were determined by treatments received within 90 days of the initial vaccine dose; patients could have multiple treatment types. All treatment types were combined for subgroup analyses by timing of last dose of systemic therapy prior to vaccination. Χ2 tests were used to determine significance.
A total of 103 patients with MM (3.8%) experienced breakthrough infection. Patients with last therapy within 90 days of initial vaccination tended to experience more breakthrough infections. Among patients with last therapy within 90 days, those receiving anti-CD38 therapy, chemotherapy, or proteasome inhibitor had significantly higher frequency of breakthrough infection (Table 1). These data confirm the prior serological data showing decreased antibody response to vaccination in patients on anti-CD38–directed therapies6 and chemotherapy.7
Treatment characteristics of fully vaccinated patients with MM by breakthrough infection status
| . | Overall . | Breakthrough . | No breakthrough . | P . |
|---|---|---|---|---|
| N | 2677 | 103 (3.8) | 2574 (96.2) | |
| Treatment timing* (%) | ||||
| Within 90 d | 2084 | 88 (4.2) | 1996 (95.8) | .077 |
| 90 to 180 d | 220 | 5 (2.3) | 215 (97.7) | .278 |
| Longer than 180 d | 373 | 10 (2.7) | 363 (97.3) | .264 |
| Treatment type† (%) | ||||
| IMiD | 1976 | 85 (4.3) | 1891 (95.7) | .053 |
| Proteasome inhibitor | 1484 | 71 (4.8) | 1413 (95.2) | .007 |
| Chemotherapy | 839 | 47 (5.6) | 792 (94.4) | .002 |
| Anti-CD38 | 470 | 31 (6.6) | 439 (93.4) | .001 |
| Other | 66 | 1 (1.5) | 65 (98.5) | .501 |
| . | Overall . | Breakthrough . | No breakthrough . | P . |
|---|---|---|---|---|
| N | 2677 | 103 (3.8) | 2574 (96.2) | |
| Treatment timing* (%) | ||||
| Within 90 d | 2084 | 88 (4.2) | 1996 (95.8) | .077 |
| 90 to 180 d | 220 | 5 (2.3) | 215 (97.7) | .278 |
| Longer than 180 d | 373 | 10 (2.7) | 363 (97.3) | .264 |
| Treatment type† (%) | ||||
| IMiD | 1976 | 85 (4.3) | 1891 (95.7) | .053 |
| Proteasome inhibitor | 1484 | 71 (4.8) | 1413 (95.2) | .007 |
| Chemotherapy | 839 | 47 (5.6) | 792 (94.4) | .002 |
| Anti-CD38 | 470 | 31 (6.6) | 439 (93.4) | .001 |
| Other | 66 | 1 (1.5) | 65 (98.5) | .501 |
Defined based on date of the last dose of systemic antimyeloma therapy received prior to the first vaccination dose.
Defined based on all systemic antimyeloma therapies received within 90 d prior to the first vaccination dose. Patients who received multiple treatment types in this time period are included in multiple treatment type categories. IMiD, immunomodulatory drugs.
These results suggest that vaccinated patients with MM and MGUS are at increased risk of breakthrough infection relative to the general population. To our knowledge, this study is the first to demonstrate a significant breakthrough risk for patients with MGUS. Patients with MGUS outnumber patients with MM. However, MGUS, unlike MM, is not listed among the Centers for Disease Control and Prevention COVID-19 high-risk criteria.13 Indeed, a previous report has suggested that risk of SARS-CoV-2 infection is similar in unvaccinated patients with MGUS and the general population.9 Our results suggest a limited vaccine response in patients with MGUS compared with the general population. Future work is needed to determine how risk of COVID-19 in patients with precancerous states, like MGUS, compares with established Centers for Disease Control and Prevention risk criteria.13 Breakthrough incidence increased further in all groups ∼5 months after vaccination, possibly owing to waning immunity, increasing prevalence of vaccine-resistant SARS-CoV-2 variants, or a combination thereof. Future work is needed to determine effective mitigation strategies against waning immunity, such as booster vaccination.
Patients with MM are at higher risk than patients with MGUS, especially for severe COVID-19. This may be partly due to the use of immunosuppressive therapy for MM.8 Consistent with prior serologic vaccination studies,4,8 we find the greatest breakthrough infection risk with recent use of immunosuppressive therapy, such as anti-CD38 therapy. However, we find increased risk with proteasome inhibitors, where existing evidence is less clear.8 Proteasome inhibitors have a complex interaction with the immune system and may impair the innate antiviral immune response.14 Our study shows that the type and timing of antimyeloma therapy affect predisposition to breakthrough infection.
Our observations are limited by the retrospective nature of the study, restriction to the VA population, and limitations inherent in electronic health record data. Specifically, certain predictors, such as antibody titers and COVID variant type, are incompletely captured and have not been included. Also, as many antimyeloma agents are used in combination, the risk from individual agents cannot be fully ascertained. Despite these limitations, our study represents the largest cohort of vaccinated patients with MM and MGUS to date and, as performed on real-world patients, would be expected to have high external validity in clinical practice.
In conclusion, patients with MM, especially those on anti-CD38 therapy, proteasome inhibitors, or chemotherapy, are at high risk of breakthrough COVID-19 infection after vaccination. Patients with MGUS are also at increased risk. Our findings suggest that these vulnerable populations should be considered for additional mitigation strategies, such as preexposure prophylaxis.
Acknowledgments
Funding support for this article was provided by the VA Cooperative Studies Program; VA Merit Review Award (1I01BX001584); National Cancer Institute (P01-155258-07, P50-100707); American Heart Association (870726); Stanford Cancer Institute (SPO #216151).
Authorship
Contribution: J.T.-Y.W., J.L., N.R.F., and N.C.M. designed the research; J.L. and N.R.F. collected and analyzed the data; J.T.-Y.W., N.R.F., and N.C.M. wrote the manuscript; J.L., J.T-Y.W., W. B.-E., L.H., S.S.H., M.B., N.V.D., A.Y.L., N.R.F., and N.C.M. interpreted data and critically read the manuscript.
Conflict-of-interest disclosure: W.B.-E. reported receiving grants (site PI for multicenter study) from Gilead Sciences and funds to institution and grants from VA Health Services Research and Development Service outside of the submitted work. M.B. reported receiving nonfinancial support from the VA Cooperative Studies Program during the conduct of the study. A.Y.L. reported receiving grants from the Department of Defense outside of the submitted work. N.C.M. reported receiving personal fees from Bristol Myers Squibb, Janssen, Amgen, Takeda, OncoPep, AbbVie, Karyopharm, Novartis, Legend, Raqia, Adaptive Biotechnology, and Pfizer outside the submitted work; in addition, N.C.M. had a patent for OncoPep licensed and held stocks in C4 Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Nikhil C. Munshi, VA Boston Healthcare System, Dana-Farber Cancer Institute, Harvard Medical School, Jerome Lipper Multiple Myeloma Center, 450 Brookline Ave, Boston, MA 02215; e-mail: nikhil_munshi@dfci.harvard.edu.
REFERENCES
Author notes
J.L., J.T.-Y.W., N.R.F., and N.C.M. contributed equally to this study.
J.L. and J.T.-Y.W are joint first authors.
N.R.F., and N.C.M. are joint senior authors.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal