TO THE EDITOR:
Hereditary thrombotic thrombocytopenic purpura (hTTP) is a rare (0.5-2.0 patients per 106 population) autosomal recessive disorder caused by a severe deficiency of ADAMTS13, a plasma metalloprotease that cleaves ultralarge von Willebrand factor (ULVWF).1 The persisting ULVWF multimers can create von Willebrand factor–platelet microthrombi causing microvascular thrombosis and organ ischemia.1 The clinical features of hTTP are extremely diverse. Some patients appear to have excellent health, whereas others may die soon after birth or have major morbidities.2,3 Patients with immune-mediated TTP (iTTP) often have disorders of cognitive function, depression,4-10 and silent cerebral infarctions during clinical remission.11-14 Stroke is also frequent in patients who have reduced ADAMTS13 activity (median, 43%) during remission following iTTP.15 Aims of the present study were to determine the neurological long-term effects in patients with hTTP by documenting their rates of stroke, stroke-related disability, and presence of neuropsychiatric symptoms.
Patients living in the United States who had enrolled and previously consented to participate in the International hTTP Registry3 were contacted to schedule an interview. Those interested signed a new consent to participate in this study. Hereditary TTP was confirmed in all patients by identification of biallelic pathogenic ADAMTS13 mutations and ADAMTS13 activity <10%. Author A.B. interviewed the patients and/or parents of minors by telephone for 30 to 60 minutes using discussion guides specific for young children (≤6 years old), older children (7-17 years), or adults (≥18 years old). Participants were asked about neuropsychiatric symptoms (Table 1) and stroke, the age they occurred, and residual symptoms or disability. The occurrence of stroke was accepted from the patients’ descriptions of symptoms, diagnostic evaluations, diagnosis conveyed to them bytreating physicians, and clinical course. Participants were also asked about prophylaxis, when it began, and any adverse reactions. The interview questions regarding hospitalizations,prophylaxis, and stroke are detailed in supplemental Table 1, available on the Blood Web site. Neuropsychiatric symptoms were compared between patients with or without history of stroke.
Recurrent neuropsychiatric symptoms stratified by history of stroke
| Neuropsychiatric symptoms* with associated questions . | All 26 patients† (%) . | 10 patients No stroke (%) . | 16 patients Stroke (%) . | P‡ . |
|---|---|---|---|---|
| Headaches Do you have regular headaches? | 23 (88) | 10 (100) | 13 (81) | .26 |
| Poor concentration Do you feel that you have poor concentration? For example, do you have difficulty focusing on a task or at work/school? | 17 (65) | 6 (60) | 11 (69) | .70 |
| Depression Have you ever had significant depression, now or in the past? | 16 (62) | 6 (60) | 10 (63) | 1.0 |
| Headache with aura (presumed migraine)§ Do you have any vision changes prior to your headaches? | 15 (58) | 7 (70) | 8 (50) | .43 |
| Vision changes Do you ever get blurry vision or changes in your vision? | 12 (46) | 4 (40) | 8 (50) | .70 |
| Forgetfulness Do you feel that you often forget things, or your memory is not as good as others around you? | 12 (46) | 3 (30) | 9 (56) | .25 |
| Fatigue Do you often feel very tired or like your whole body is weak? | 12 (46) | 4 (40) | 8 (50) | 1.0 |
| Neuropathy Do you ever feel numbness or tingling in your hands or feet? | 11 (42) | 2 (20) | 9 (56) | .11 |
| Dysarthria Do you ever have difficulty with speaking, such as slurring of your speech? | 10 (38) | 1 (10) | 9 (56) | .04 |
| Loss of vision Do you ever have loss of vision? | 7 (27) | 3 (30) | 4 (25) | 1.0 |
| Seizure Have you ever had a seizure? | 6 (23) | 2 (20) | 4 (40) | 1.0 |
| Transient weakness Do you ever have sudden weakness in 1 part of the body, such as an arm or leg? | 5 (19) | 1 (10) | 4 (25) | .39 |
| Falls Have you fallen frequently? | 5 (19) | 1 (10) | 4 (25) | .62 |
| Dysphagia Do you ever have difficulty swallowing? | 3 (12) | 0 | 3 (19) | .26 |
| No symptoms‖ | 1 (4) | 0 | 1 (6) | NA |
| Neuropsychiatric symptoms* with associated questions . | All 26 patients† (%) . | 10 patients No stroke (%) . | 16 patients Stroke (%) . | P‡ . |
|---|---|---|---|---|
| Headaches Do you have regular headaches? | 23 (88) | 10 (100) | 13 (81) | .26 |
| Poor concentration Do you feel that you have poor concentration? For example, do you have difficulty focusing on a task or at work/school? | 17 (65) | 6 (60) | 11 (69) | .70 |
| Depression Have you ever had significant depression, now or in the past? | 16 (62) | 6 (60) | 10 (63) | 1.0 |
| Headache with aura (presumed migraine)§ Do you have any vision changes prior to your headaches? | 15 (58) | 7 (70) | 8 (50) | .43 |
| Vision changes Do you ever get blurry vision or changes in your vision? | 12 (46) | 4 (40) | 8 (50) | .70 |
| Forgetfulness Do you feel that you often forget things, or your memory is not as good as others around you? | 12 (46) | 3 (30) | 9 (56) | .25 |
| Fatigue Do you often feel very tired or like your whole body is weak? | 12 (46) | 4 (40) | 8 (50) | 1.0 |
| Neuropathy Do you ever feel numbness or tingling in your hands or feet? | 11 (42) | 2 (20) | 9 (56) | .11 |
| Dysarthria Do you ever have difficulty with speaking, such as slurring of your speech? | 10 (38) | 1 (10) | 9 (56) | .04 |
| Loss of vision Do you ever have loss of vision? | 7 (27) | 3 (30) | 4 (25) | 1.0 |
| Seizure Have you ever had a seizure? | 6 (23) | 2 (20) | 4 (40) | 1.0 |
| Transient weakness Do you ever have sudden weakness in 1 part of the body, such as an arm or leg? | 5 (19) | 1 (10) | 4 (25) | .39 |
| Falls Have you fallen frequently? | 5 (19) | 1 (10) | 4 (25) | .62 |
| Dysphagia Do you ever have difficulty swallowing? | 3 (12) | 0 | 3 (19) | .26 |
| No symptoms‖ | 1 (4) | 0 | 1 (6) | NA |
NA, not applicable.
These neuropsychiatric symptoms were chosen based on the interviews with hTTP patients. All questions were followed up with: “How often? When did it start? Please describe.”
The 20-mo-old patient is not included in the table because these symptoms did not apply to him. His parents were only asked about seizures, which he did not have. He appears to have normal development.
χ2 analysis or Fisher’s exact test (if there were <5 subjects per category) was performed to assess the difference in frequency of symptoms in patients with or without stroke.
Patients were asked about auras as a follow-up question if they answered yes to having headaches. The auras were described as “sparkly eyes,” “flashes of lights,” “wavy lines,” or transient vision loss.
The asymptomatic patient is a 12-y-old girl who was diagnosed at birth after having cardiac arrest and stroke as a newborn. She currently reports no symptoms since the start of the plasma-derived factor VIII concentrate (pdFVIII) for prophylaxis.
As of July 2021, 45 US patients with confirmed hTTP had been enrolled into the International hTTP Registry. Twelve previously enrolled US patients had been lost to follow-up, 2 died, and 4 did not consent (supplemental Figure 1). Twenty-seven patients from 17 US states were interviewed between April 2020 and July 2021: 25 adults (ages 19-63 years, median age, 38 years) and 2 children (ages 20 months and 12 years). The participants were predominantly female (70%) and white (89%) and had a college or postgraduate education (72%) (supplemental Table 2). The patients’ sex, mutations, number of strokes, age of first symptoms, diagnosis, and follow-up are presented in supplemental Table 3. Twenty-five of 26 (96%) patients (excluding the infant) reported 2 to 14 (median, 6) of the 14 neuropsychiatric symptoms (Table 1). Symptom onset occurred across all ages. Common symptoms were recurrent headaches (88%), poor concentration (65%), and depression (62%). Headache with aura (presumed migraine) was reported by 15 (58%) patients. Only dysarthria was more common in patients with a history of stroke (Table 1). The symptoms were often described as beginning during “childhood,” during “high school,” or after a significant hTTP event, such as a hospitalization. These data suggest that patients of all ages are at risk for neuropsychiatric symptoms.
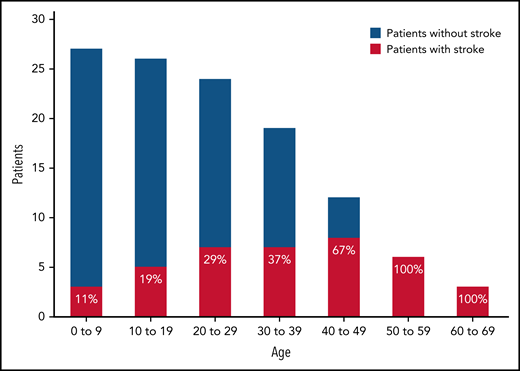
Strokes had occurred in 17 (63%; 95% confidence interval, 42%-81%) patients. The median age of the first stroke was 26 years (range, newborn to 58 years); the occurrence increased with age (Figure 1). All 6 patients whose age was ≥49 years had had a stroke. In 12 patients, stroke preceded the diagnosis of hTTP. Three of the 12 patients were diagnosed at the time of their first stroke; the other 9 patients were diagnosed with hTTP 2 months to 19 years after their first stroke. Eleven of the 17 patients had residual symptoms; 7 suffered recurrent strokes. Seven patients have received stroke-related disability benefits, beginning at a median age of 43 years (18-55 years) (supplemental Table 4).
Frequency of the initial occurrence of stroke related to decade of life. Each bar represents the total number of patients within each age group. For example, all 27 patients are represented in the 0 to 9 age group, of which 3 (11%) had a stroke before age 10 years. All 6 patients who were 50 years or older at the time of interview had a previous stroke.
Frequency of the initial occurrence of stroke related to decade of life. Each bar represents the total number of patients within each age group. For example, all 27 patients are represented in the 0 to 9 age group, of which 3 (11%) had a stroke before age 10 years. All 6 patients who were 50 years or older at the time of interview had a previous stroke.
Twenty-five (93%) patients had received plasma for treatment of symptoms or for prophylaxis; 21 patients described allergic reactions to plasma: rash/itching (14), dyspnea/chest pain (4), anaphylaxis (2), or infection (1 [hepatitis C]) (supplemental Table 5). Seventeen (63%) of the 27 patients were on prophylaxis at the time of interview: 12, plasma; 5, pdFVIII. Prophylaxis was begun at age 2 months to 63 years (median, 30 years) and continued for a duration of 1 to 53 years (median, 4.5 years). None of the 17 patients were on prophylaxis at the time of their first stroke. Of the 7 patients with recurrent strokes, none received prophylaxis at the time of their second stroke but were subsequently placed on prophylaxis. Four of those 7 had another stroke while on prophylaxis. Five patients who switched to pdFVIII from plasma reported improved convenience of self-administration.
Like patients in remission from iTTP, almost all patients with hTTP have neuropsychiatric symptoms. Our data emphasize that patients with hTTP have life-altering neuropsychiatric symptoms beginning at young ages that continue throughout their lives. The occurrence of neuropsychiatric symptoms must be confirmed in future studies using standardized assessments, such as the Repeatable Battery for the Assessment of Neuropsychological Status for cognitive decline and the Patient Health Questionnaire-9, for depression.4-7 The impact of the neuropsychiatric symptoms on the patients’ lives must be described by qualitative evaluation.16
Fifty-eight percent of patients had headaches with aura, presumed migraine. Previous studies have demonstrated that migraine with aura is an independent risk factor for stroke, especially cryptogenic ischemic stroke,17 and is associated with silent brain infarcts.18 The high frequency of strokes among patients with hTTP suggests that the neuropsychiatric symptoms may be caused by silent cerebral infarction, similar to patients with iTTP.11-14 The overall occurrence of stroke was 63%, more than twice the rate from prior reports, possibly because the median age of our patients is >10 years older than in prior reports,2,3 because patients with stroke may be more likely to be enrolled in the Registry and to participate in this study, or because of the small size of this study. Strokes often preceded the diagnosis of hTTP. Therefore, in patients with an embolic stroke of undetermined source,19,20 measurement of ADAMTS13 activity is important.
Our data emphasize the significance of beginning effective, lifetime prophylaxis at a young age. Current common practice is to begin plasma prophylaxis when a major complication has occurred. We recommend a baseline cerebral magnetic resonance imaging at the first occurrence of neuropsychiatric symptoms to identify silent strokes and to document the need for prophylactic therapy.11-14 However, the inconvenience of plasma administration and the frequent allergic reactions to plasma may cause hesitancy to begin prophylaxis and insufficient frequency once prophylaxis has begun. A report of the International hTTP Registry found no statistical difference in the frequency of acute episodes of hTTP in patients with or without prophylactic plasma.21 These observations highlight the limitations of current prophylaxis. Until recombinant ADAMTS1322 is available, an adequate amount of plasma (≥15 mL/kg) at frequent intervals (≤2 weeks) to maintain ADAMTS13 activity is appropriate.
Our data have significant limitations. The number of patients is small because hTTP is a rare disorder.1 In addition, there is potential for selection bias, as enrollment into the International hTTP Registry requires motivation of the patient and awareness of the Registry by the patient’s hematologist.
Despite these limitations, our data document that almost all patients have neuropsychiatric symptoms, suggesting the need for regular cognitive and depression screening of hTTP and effective prophylaxis when abnormalities are identified.
Acknowledgments
The authors acknowledge everyone who contributes to the International Hereditary TTP Registry, including patients, researchers, and the financial support from Takeda, Inc.
Authorship
Contribution: A.B. conducted all interviews, collected and synthesized the data, designed the tables and figures, and wrote the manuscript; E.T. and J.A.K.H. were key in the recruitment of patients and management of data through the International hTTP Registry; K.D.F. and J.A.K.H. performed the genetic testing; A.S.A., S.K.V., C.I.P., D.R.T., and J.N.G. are mentors for A.B. and provided valuable suggestions and insight throughout this study; J.N.G. created the concept for this study and helped with writing the initial versions of the manuscript; and all authors edited the manuscript and approved of the final version before submission.
Conflict-of-interest disclosure: J.A.K.H. has received a grant from Baxalta US Inc; serves as consultancy and on the speakers bureau for Shire and Ablynx; serves as consultancy for the Federal Office of Public Health; and is currently employed by Insel Gruppe AG. K.D.F. serves as consultancy for Bayer, Siemens, Genentech, Sanofi, and Instrumentation Laboratories and serves on the speakers bureau for Alexion. C.I.P. has received a grant from US Department of Veterans Affairs Merit award CX000340 and the Oklahoma Center for the Advancement of Science and Technology grant OCAST HR 19-111. The remaining authors declare no competing financial interests.
Correspondence: Azra Borogovac, University of Oklahoma Health Sciences Center, Stephenson Cancer Center, 800 NE 10th St, Room 5029, Oklahoma City, OK 73104; e-mail: azrab88@gmail.com.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal