Key Points
Impaired metabolic/functional fitness due to lower LKB1 level of myeloid-derived suppressor cells participates in the pathogenesis of ITP.
Decitabine enhances oxidative metabolism and suppressive functions of myeloid-derived suppressor cells via LKB1 in patients with ITP.
Abstract
Myeloid-derived suppressor cells (MDSCs) are heterogeneous immature cells and natural inhibitors of adaptive immunity. Metabolic fitness of MDSCs is fundamental for its suppressive activity toward effector T cells. Our previous studies showed that the number and inhibitory function of MDSCs were impaired in patients with immune thrombocytopenia (ITP) compared with healthy controls. In this study, we analyzed the effects of decitabine on MDSCs from patients with ITP, both in vitro and in vivo. We found that low-dose decitabine promoted the generation of MDSCs and enhanced their aerobic metabolism and immunosuppressive functions. Lower expression of liver kinase 1 (LKB1) was found in MDSCs from patients with ITP, which was corrected by decitabine therapy. LKB1 short hairpin RNA (shRNA) transfection effectively blocked the function of MDSCs and almost offset the enhanced effect of decitabine on impaired MDSCs. Subsequently, anti-CD61 immune-sensitized splenocytes were transferred into severe combined immunodeficient (SCID) mice to induce ITP in murine models. Passive transfer of decitabine-modulated MDSCs significantly raised platelet counts compared with that of phosphate buffered saline–modulated MDSCs. However, when LKB1 shRNA-transfected MDSCs were transferred into SCID mice, the therapeutic effect of decitabine in alleviating thrombocytopenia was quenched. In conclusion, our study suggests that the impaired aerobic metabolism of MDSCs is involved in the pathogenesis of ITP, and the modulatory effect of decitabine on MDSC metabolism contributes to the improvement of its immunosuppressive function. This provides a possible mechanism for sustained remission elicited by low-dose decitabine in patients with ITP.
Introduction
Immune thrombocytopenia (ITP) is a common and multifaceted autoimmune bleeding disorder characterized by persistent thrombocytopenia resulting from increased platelet destruction and impaired platelet production.1-4 In recent years, treatment has been targeted at individual patients and different stages of the disease, with increasing emphasis on optimizing the health-related quality of life.5 The loss of immune homeostasis,6 including overactivation of immune effector cells and impaired function of immune regulatory cells, is pivotal to its pathogenesis.7 T cells and dendritic cells are the driving cells leading to the initiation and perpetuation of ITP. Dysregulated T cells lead to a breakdown of the self-tolerance mechanisms and allows the production of antiplatelet autoantibodies.8
Myeloid-derived suppressor cells (MDSCs) are immunosuppressive immature innate immune cells, derived from myeloid progenitor cells.9 They inhibit T-cell proliferation and activation, promote T regulatory cell (Treg) recruitment and amplification, and inhibit B-cell proliferation and function,10,11 and their immunosuppressive function is based on normal fatty acid oxidation.12,13 In our previous study, high-dose dexamethasone (DXM) corrected MDSC dysfunction via Ets1 in ITP.14 However, the sustained response rate to corticosteroids in ITP appears to be relatively low,15 and some patients developed chronic/refractory ITP.
Decitabine is a hypomethylating agent that promotes cell differentiation at low doses and has a favorable effect on increased platelet production in myelodysplastic syndrome16 and posttransplantation thrombocytopenia.17 Disruption of methylation balance in immune cells leads to abnormal gene expression in ITP, contributing to the loss of immune regulation.18 A multicenter prospective clinical study led by our group confirmed that low-dose decitabine is well tolerated in the treatment of relapsed and refractory ITP patients, with a total effective rate of ∼50%, and some patients achieved long-term efficacy for more than 1 year.19 We have previously found that low-dose decitabine significantly increased the number of mature polyploidy megakaryocytes by reducing methylation of the TRAIL promoter region of megakaryocytes, thus promoting platelet production and exhibiting long-term clinical efficacy.19,20 Decitabine can regulate immune reaction in ITP by modulating Tregs and inhibiting STAT3 activation, enhancing their immunosuppressive function in ITP, as well as restricting the cytotoxicity of cytotoxic T lymphocytes (CTLs) to autologous platelets through the programmed cell death protein 1 (PD-1) pathway.21,22 However, it remains unknown whether decitabine can regulate the metabolic and suppressive activity of MDSCs in ITP.
Liver kinase B1 (LKB1), a tumor suppressor gene, encodes the serine STK11/LKB1, which plays an important role in regulating cell growth, polarity, and energy metabolism. It is located upstream of adenosine 5′-monophosphate–activated protein kinase (AMPK), an energy-sensing molecule that can promote oxidative metabolism.23 The LKB1 signaling pathway plays an important role linking metabolic balance and functional homeostasis in immune cells.24,25 LKB1 is a key regulator of lipid metabolism in T cells and participates in the optimal programming of suppressive activity, immune homeostasis, and modulation.26 LKB1 deficiency interferes with the normal survival of Tregs and mitochondrial metabolism and inhibits the tricarboxylic acid cycle and fatty acid oxidation, leading to insufficient intracellular adenosine triphosphate (ATP) synthesis27 and even the development of fatal early-onset autoimmune disease.28 LKB1 promoter-region hypermethylation can inhibit gene transcription and reduce LKB1 protein kinase expression. The DNA-demethylating drug 5-aza-29-deoxycytidine (5AZA-CdR) can correct LKB1 expression.29
The objective of this study was to elucidate the underlying molecular mechanism of low-dose decitabine that induce long-term response in the management of patients with ITP, especially through metabolic modulation of MDSCs. Here, we investigated the effect of low-dose decitabine on the metabolic and immunosuppressive function of MDSCs derived from patients with ITP. In addition, active ITP murine models were established to evaluate the effect of decitabine-modulate MDSCs in vivo.
Materials and methods
Patients and controls
For in vitro studies, 43 patients with ITP (Table 1) were enrolled in the Department of Hematology, Qilu Hospital, Shandong University, China, between February 2019 and December 2021. Twenty-seven age- and sex-matched healthy volunteers (Table 1) were recruited. For in vivo studies, an additional 13 patients with ITP (7 females and 6 males; 18-70 years old; median age, 45 years; baseline platelet count range, 2 × 109 to 28 × 109 platelets per L; median platelet count, 13.5 × 109 platelets per L; Table 2) were enrolled and treated with decitabine. All the patients had treatment (steroid)-refractory ITP and fulfilled the diagnostic criteria.5 This study was approved by the Medical Ethical Committee of the Qilu Hospital, Shandong University. The study was conducted in accordance with the principles of the Declaration of Helsinki.
Baseline characteristics of patients with ITP and healthy controls
| . | ITP patients (n = 43) . | Healthy controls (n = 27) . | P value . |
|---|---|---|---|
| Median age, (range), y | 48 (18-71) | 44 (20-68) | .5923 |
| Gender | 23 F | 14 F | .894 |
| Baseline platelet count, ×109/L | 13 (1-34) | 234 (153-294) | NA |
| Previous therapies, n | |||
| ≤2 | 14 | NA | NA |
| ≥3 | 29 |
| . | ITP patients (n = 43) . | Healthy controls (n = 27) . | P value . |
|---|---|---|---|
| Median age, (range), y | 48 (18-71) | 44 (20-68) | .5923 |
| Gender | 23 F | 14 F | .894 |
| Baseline platelet count, ×109/L | 13 (1-34) | 234 (153-294) | NA |
| Previous therapies, n | |||
| ≤2 | 14 | NA | NA |
| ≥3 | 29 |
Data are median (range) or n (%).
NA, not applicable.
Clinical characteristics of ITP patients receiving decitabine treatment
| Patients . | Age, y . | Sex . | Platelet count, ×109/L . | Antiplatelet antibodies . | Major previous drugs . | |
|---|---|---|---|---|---|---|
| Anti-GPIIb/IIIa . | Anti-GPIb/IX . | |||||
| 1 | 21 | M | 3 | − | − | DXM, Pred, rhTPO, TPO-RA |
| 2 | 46 | F | 19 | + | − | DXM, rhTPO, CA, |
| 3 | 51 | M | 25 | − | − | DXM, Pred, |
| 4 | 60 | M | 9 | − | + | DXM, Pred, RTX, SP |
| 5 | 26 | F | 13 | − | − | DXM, IVIG, rhTPO |
| 6 | 70 | F | 14 | − | − | DXM, Pred, IVIG, rhTPO |
| 7 | 34 | M | 6 | + | + | DXM, Pred, RTX |
| 8 | 42 | F | 28 | − | − | DXM, rhTPO |
| 9 | 31 | F | 11 | − | − | DXM, Pred, CA |
| 10 | 65 | M | 20 | − | + | DXM, Pred, rhTPO, Danazol |
| 11 | 48 | F | 12 | − | − | DXM, rhTPO, CA |
| 12 | 18 | M | 14 | − | − | Pred, rhTPO, CsA |
| 13 | 45 | F | 16 | − | − | DXM, IVIG, RTX |
| Patients . | Age, y . | Sex . | Platelet count, ×109/L . | Antiplatelet antibodies . | Major previous drugs . | |
|---|---|---|---|---|---|---|
| Anti-GPIIb/IIIa . | Anti-GPIb/IX . | |||||
| 1 | 21 | M | 3 | − | − | DXM, Pred, rhTPO, TPO-RA |
| 2 | 46 | F | 19 | + | − | DXM, rhTPO, CA, |
| 3 | 51 | M | 25 | − | − | DXM, Pred, |
| 4 | 60 | M | 9 | − | + | DXM, Pred, RTX, SP |
| 5 | 26 | F | 13 | − | − | DXM, IVIG, rhTPO |
| 6 | 70 | F | 14 | − | − | DXM, Pred, IVIG, rhTPO |
| 7 | 34 | M | 6 | + | + | DXM, Pred, RTX |
| 8 | 42 | F | 28 | − | − | DXM, rhTPO |
| 9 | 31 | F | 11 | − | − | DXM, Pred, CA |
| 10 | 65 | M | 20 | − | + | DXM, Pred, rhTPO, Danazol |
| 11 | 48 | F | 12 | − | − | DXM, rhTPO, CA |
| 12 | 18 | M | 14 | − | − | Pred, rhTPO, CsA |
| 13 | 45 | F | 16 | − | − | DXM, IVIG, RTX |
CA, caffeic acid; CsA, cyclosporine A; IVIg, intravenous gamma globulin; Pred, prednisone; rhTPO, recombinant human thrombopoietin; RTX, rituximab; SP, splenectomy, danazol; TPO-RA, thrombopoietin receptor agonist.
Decitabine
For in vitro and animal studies, decitabine (5-aza-2′-deoxycytidine; Millipore Sigma, St Louis, MO; US Food and Drug Administration approved) was dissolved in water and diluted with phosphate-buffered saline (PBS). The treatment options are detailed in the supplemental Methods on the Blood website.
Active ITP murine model
The active ITP murine model was established as previously reported.30,31 The strains and backgrounds of mice are detailed in the supplemental Methods. Briefly, platelets were obtained from the peripheral blood of wild-type C57BL/6J mice; their concentration was adjusted to 109 platelets per mL. CD61-knockout mice were infused with 108 platelets weekly via tail vein infusion for 4 consecutive weeks. Splenocyte suspensions were prepared from the spleens of CD61-knockout mice. On the day of splenocyte transfer, severe combined immunodeficiency (SCID) mice were subjected to 200 cGy total body irradiation to suppress their innate immune response and to enhance engraftment. Within 3 hours after irradiation, SCID mice were injected intraperitoneally with 2 × 104 splenocytes from CD61-knockout mice. Platelet counts and serum platelet CD61-specific antibodies (supplemental Methods) were monitored weekly for 5 weeks.
Isolation and culturing of PBMCs and MDSCs
Peripheral blood mononuclear cells (PBMCs) were obtained from patients with ITP and healthy controls, which were then cultured in vitro to harvest MDSCs and separated by magnetic beads, as described in the supplemental Methods.
Flow cytometry
Phenotypic markers of MDSCs, Tregs, T helper 1 (Th1), Th17, and Th22 cells as well as cell apoptosis were detected by flow cytometry, as described in the supplemental Methods.
Metabolic analysis
MDSC metabolism was analyzed using a Seahorse Extracellular Flux Analyzer (Agilent Technologies, Santa Clara, CA). MDSCs (4 × 104 cells) were subjected to various treatments to determine oxygen consumption rate (OCR), as detailed in the supplemental Methods.
ATP production
Cells were inoculated in opaque 96-well plates (Corning, New York, NY) and cultured overnight. ATP production was then measured with CellTiter-Glo reagent (Promega, Madison, WI).
Suppression capacity of MDSCs
CD4+CD25− T cells were isolated from PBMCs and labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE). They were then cocultured with decitabine/PBS/LKB1 short hairpin RNA (shRNA) or negative control (NC) shRNA-treated MDSCs. On day 5, effector-cell proliferation was measured via flow cytometry. Inhibition of MDSCs toward CTL-induced platelet apoptosis is described in the supplemental Methods.
Quantitative real-time PCR and western blotting
The messenger RNA (mRNA) expression of the LKB1 signaling pathway, inhibitory cytokine, and FAO/OXPHOS-related genes was measured by real-time reverse transcription polymerase chain reaction (RT-PCR). The primer sequences are detailed in Table 3. Gene expression was normalized to that of glyceraldehyde-3-phosphate dehydrogenase for relative quantification. The experimental protocols are described in the supplemental Methods.
Gene-specific primers for quantitative RT-PCR
| Gene . | Forward primer . | Reverse primer . |
|---|---|---|
| Human | ||
| GAPDH | 5′-GCACCGTCAAGGCTGAGAAC-3′ | 5′-TGGTGAAGACGCCAGTGGA-3′ |
| IL-10 | 5′-CTGAGAACCAAGACCCAGACA-3′ | 5′-AAAGGCATTCTTCACCTGCTCC-3′ |
| TGF-β | 5′-GCAACAATTCCTGGCGATACC-3′ | 5′-ATTTCCCCTCCACGGCTCAA-3′ |
| VEGF | 5′-GCAGAATCATCACGAAGTGGT-3′ | 5′-CCAGGGTCTCGATTGGATGG-3′ |
| LKB1 | 5′-GACCTGCTGAAAGGGATGCT-3′ | 5′-CAAGTACGGCACCACAGTCA-3′ |
| AMPKα1 | 5′-GGAGCCTTGATGTGGTAGGAA-3′ | 5′-TCAAATAGCTCTCCTCCTGAGAC-3′ |
| AMPKα2 | 5′-TCCTCAACACCTCAGCGTTC-3′ | 5′-CTTCCGGTCAAAGAGCCAGT-3′ |
| AMPKβ1 | 5′-TCCCAAAAGTGCTCCGATGT-3′ | 5′-ACGTAGGGCTCCTGATGGTA-3′ |
| AMPKβ2 | 5′-GCTGGTCTGAAGGAGGCAAG-3′ | 5′-TCCAGGATGGCAACAAAGTCA-3′ |
| AMPKγ1 | 5′-CTGAGTTCCCCAAGCCAGAG-3′ | 5′-AGTAGATGTCCACCACACGC-3′ |
| AMPKγ2 | 5′-CCTTCAGCACCGTTCACAGTA-3′ | 5′-ATTTACCACCACCAGCCGAT-3′ |
| AMPKγ3 | 5′-GTGTCAGGCAACGTACTCCA-3′ | 5′-GTCAGGATGGGTGCTGTCTC-3′ |
| ND-1 | 5′-CATGGCCAACCTCCTACTCCTC-3′ | 5′-TGGGGCCTTTGCGTAGTTGT-3′ |
| ND-3 | 5′-GTGCGGCTTCGACCCTAT-3′ | 5′-TGTTTGTAGGGCTCATGGTAGG-3′ |
| ND-6 | 5′-CACCAATCCTACCTCCATCGCTA-3′ | 5′-GGGAATGATGGTTGTCTTTGGAT-3′ |
| ATP-6 | 5′-CGTACGCCTAACCGCTAACA-3′ | 5′-AAGTGTAGAGGGAAGGTTAATGG-3′ |
| Mouse | ||
| β-actin | 5′-TGCGTGACATCAAAGAGAAG-3′ | 5′-TCCATACCCAAGAAGGAAGG-3′ |
| ACADM | 5′-AAAAGAGCCTGGGAACTCGG-3′ | 5′-CCATACGCCAACTCTTCGGT-3′ |
| PGC1β | 5′-ACTATCTCTCTGACACAGGGT-3′ | 5′-GAGTCAAAGTCACTGGCGTCC-3′ |
| HADHA | 5′-AGGCCGACATGGTGATTGAG-3′ | 5′-TCTGGAGTCACGCTTTCCAC-3′ |
| Gene . | Forward primer . | Reverse primer . |
|---|---|---|
| Human | ||
| GAPDH | 5′-GCACCGTCAAGGCTGAGAAC-3′ | 5′-TGGTGAAGACGCCAGTGGA-3′ |
| IL-10 | 5′-CTGAGAACCAAGACCCAGACA-3′ | 5′-AAAGGCATTCTTCACCTGCTCC-3′ |
| TGF-β | 5′-GCAACAATTCCTGGCGATACC-3′ | 5′-ATTTCCCCTCCACGGCTCAA-3′ |
| VEGF | 5′-GCAGAATCATCACGAAGTGGT-3′ | 5′-CCAGGGTCTCGATTGGATGG-3′ |
| LKB1 | 5′-GACCTGCTGAAAGGGATGCT-3′ | 5′-CAAGTACGGCACCACAGTCA-3′ |
| AMPKα1 | 5′-GGAGCCTTGATGTGGTAGGAA-3′ | 5′-TCAAATAGCTCTCCTCCTGAGAC-3′ |
| AMPKα2 | 5′-TCCTCAACACCTCAGCGTTC-3′ | 5′-CTTCCGGTCAAAGAGCCAGT-3′ |
| AMPKβ1 | 5′-TCCCAAAAGTGCTCCGATGT-3′ | 5′-ACGTAGGGCTCCTGATGGTA-3′ |
| AMPKβ2 | 5′-GCTGGTCTGAAGGAGGCAAG-3′ | 5′-TCCAGGATGGCAACAAAGTCA-3′ |
| AMPKγ1 | 5′-CTGAGTTCCCCAAGCCAGAG-3′ | 5′-AGTAGATGTCCACCACACGC-3′ |
| AMPKγ2 | 5′-CCTTCAGCACCGTTCACAGTA-3′ | 5′-ATTTACCACCACCAGCCGAT-3′ |
| AMPKγ3 | 5′-GTGTCAGGCAACGTACTCCA-3′ | 5′-GTCAGGATGGGTGCTGTCTC-3′ |
| ND-1 | 5′-CATGGCCAACCTCCTACTCCTC-3′ | 5′-TGGGGCCTTTGCGTAGTTGT-3′ |
| ND-3 | 5′-GTGCGGCTTCGACCCTAT-3′ | 5′-TGTTTGTAGGGCTCATGGTAGG-3′ |
| ND-6 | 5′-CACCAATCCTACCTCCATCGCTA-3′ | 5′-GGGAATGATGGTTGTCTTTGGAT-3′ |
| ATP-6 | 5′-CGTACGCCTAACCGCTAACA-3′ | 5′-AAGTGTAGAGGGAAGGTTAATGG-3′ |
| Mouse | ||
| β-actin | 5′-TGCGTGACATCAAAGAGAAG-3′ | 5′-TCCATACCCAAGAAGGAAGG-3′ |
| ACADM | 5′-AAAAGAGCCTGGGAACTCGG-3′ | 5′-CCATACGCCAACTCTTCGGT-3′ |
| PGC1β | 5′-ACTATCTCTCTGACACAGGGT-3′ | 5′-GAGTCAAAGTCACTGGCGTCC-3′ |
| HADHA | 5′-AGGCCGACATGGTGATTGAG-3′ | 5′-TCTGGAGTCACGCTTTCCAC-3′ |
Quantitative detection of MassARRAY targeted methylation sites
Total DNA was extracted from MDSCs using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany), as described in the supplemental Methods. We used Agena’s EpiDesigner software to design primers for the core DNA methylation region: the final fragment to be detected was at −488 to 11 bp.
shRNA transfection
A lentivirus (Genechem, Shanghai, China) encoding the human and murine LKB1 shRNA or murine Ets1 shRNA was transduced into MDSCs. After 48 hours, we added purinomycin (1 mg/mL) to the culture medium for 24 hours to screen the successfully transduced cells. LKB1 downregulation was measured by quantitative PCR.
Immunofluorescence
The femurs were harvested from the ITP-active model mice. Paraffin sections (6 μm) were incubated at 4°C overnight with primary anti-Ly-6G/Ly-6C and anti-LKB1 antibodies, followed by incubation with secondary antibodies for 2 hours. The nuclei were counterstained with 5 μg/mL 4′,6-diamidino-2-phenylindole (Beyotime Biotechnology). Whole-bone marrow immunofluorescence was performed using a panoramic scanner. Ten 80 × 80-μm areas were randomly selected from the MDSC-rich areas in the image.
Generation of MDSCs and cell therapy
MDSCs were derived from the femurs and tibias bone marrow of wild-type C57BL/6 mice, as described in the supplemental Methods.
SCID mice were divided into groups. The unimmunized control group received 2 × 104 nonimmunized CD61KO splenocytes, and the control group received only 2 × 104 splenocytes via intraperitoneal injection; the other groups received the same number of splenocytes, as well as 3 × 106 MDSCs treated with PBS, decitabine, LKB1 shRNA, LKB1 shRNA + decitabine, NC shRNA, or NC shRNA + decitabine.
Cytokine analysis
Serum samples of ITP mice were tested for 12 cytokines (interleukin-10 [IL-10], IL-4, transforming growth factor β [TGF-β], vascular endothelial growth factor [VEGF], TNF-α, IL-1β, IL-2, IL-6, IL-13, IL-17a, IFN-γ, IL-12 p70) using V-PLEX Human Proinflammatory Panel 1 Kits (Meso Scale Discovery, Rockville, MD) and the R&D Elisa Assay Kit (R&D Systems).
Statistical analysis
The results are expressed as median (with range or interquartile range), mean ± standard deviation, or mean ± standard error of the mean. Differences between the treatment and nontreatment groups were evaluated using paired Student t tests and multiple paired t tests. Differences between 2 independent groups were evaluated using the unpaired Student t test or Mann-Whitney U test for nonnormally distributed data. One-way analysis of variance (ANOVA) and 2-way ANOVA were performed for comparisons among the groups. Statistical significance was set at P < .05. All data were analyzed using SPSS 25.0.
Results
Low-dose decitabine expands CD11b+CD33+HLA-DRlow cell populations
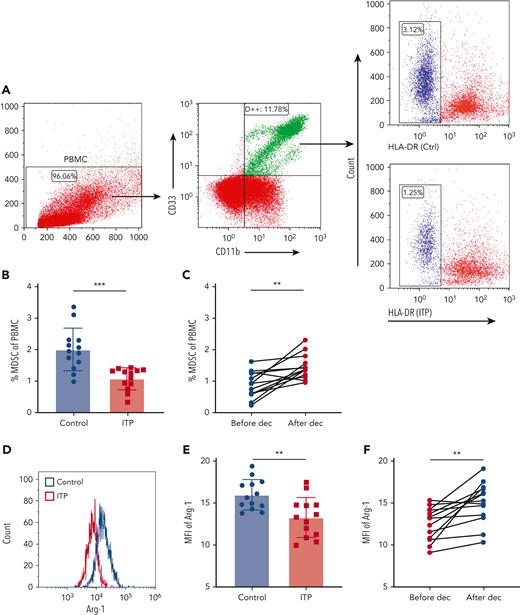
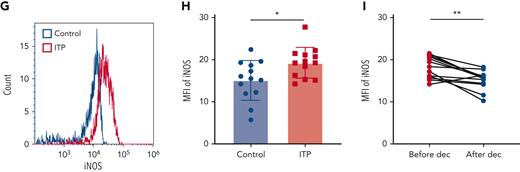
PBMCs from ITP patients and healthy control subjects were isolated, and MDSCs were evaluated via immunostaining of CD11b+CD33+ human leukocyte antigen-DRlow (HLA-DRlow) cells (Figure 1A). The proportion of MDSCs in circulation was significantly lower in ITP patients than in healthy controls (Figure 1B). After decitabine treatment, there was a significant increase in the percentage of MDSCs in PBMCs from the 13 patients with ITP (Figure 1C), although MDSC was reduced in 1 patient after therapy. Considering that the ITP population is heterogeneous and decitabine covers multiple molecular targets, a major therapeutic effect need not exert through correction of MDSC in that single patient, but rather through inhibition of CTL or augmentation of Tregs, etc. Arginase 1 (Arg1) expression and induced nitric oxide synthase (iNOS) expression in circulating MDSCs were represented by their mean fluorescence intensity in CD11b+CD33+HLA-DRlow cells (Figure 1D,G). Relative to levels in healthy controls, ITP patients had significantly lower Arg1 expression and significantly higher iNOS expression in MDSCs (Figure 1E,H). After decitabine treatment, Arg1 expression was significantly elevated and iNOS expression was significantly reduced (Figure 1F,I).
Decitabine improved the number and function of MDSCs. (A) Representative scattergram of PBMCs and CD11b+CD33+ cells within the PBMC gate. Histograms of CD11b+CD33+HLA-DRlow cells from healthy controls and ITP patients. (B) The proportion of CD11b+CD33+HLA-DRlow cells in PBMCs was lower in ITP patients than in healthy controls (unpaired Student t tests, ∗∗∗P = .0002) and (C) increased after decitabine treatment (paired Student t tests, ∗∗P = .0015). Histograms of (D) Arg1and (G) iNOS in CD11b+CD33+HLA-DRlow cells from healthy controls and ITP patients before treatment. (E-F) The expression (mean fluorescence intensity) of Arg1 in circulating MDSCs was lower (unpaired Student t tests, ∗∗P = .0032) in ITP patients than in healthy controls and was higher after decitabine treatment (paired Student t tests, ∗∗P = .0035). (H-I) The expression (mean fluorescence intensity) of iNOS in circulating MDSCs was higher (unpaired Student t tests, ∗P = .0182) in ITP patients than in healthy controls and was lower after decitabine treatment (paired Student t tests, ∗∗P = .0011). Bars represent mean ± standard deviation.
Decitabine improved the number and function of MDSCs. (A) Representative scattergram of PBMCs and CD11b+CD33+ cells within the PBMC gate. Histograms of CD11b+CD33+HLA-DRlow cells from healthy controls and ITP patients. (B) The proportion of CD11b+CD33+HLA-DRlow cells in PBMCs was lower in ITP patients than in healthy controls (unpaired Student t tests, ∗∗∗P = .0002) and (C) increased after decitabine treatment (paired Student t tests, ∗∗P = .0015). Histograms of (D) Arg1and (G) iNOS in CD11b+CD33+HLA-DRlow cells from healthy controls and ITP patients before treatment. (E-F) The expression (mean fluorescence intensity) of Arg1 in circulating MDSCs was lower (unpaired Student t tests, ∗∗P = .0032) in ITP patients than in healthy controls and was higher after decitabine treatment (paired Student t tests, ∗∗P = .0035). (H-I) The expression (mean fluorescence intensity) of iNOS in circulating MDSCs was higher (unpaired Student t tests, ∗P = .0182) in ITP patients than in healthy controls and was lower after decitabine treatment (paired Student t tests, ∗∗P = .0011). Bars represent mean ± standard deviation.
Low-dose decitabine enhanced aerobic metabolism of MDSCs in patients with ITP
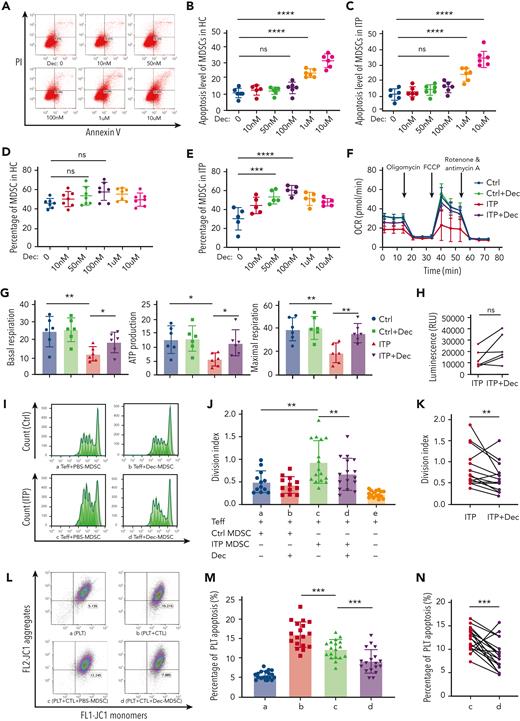
MDSCs from patients with ITP and healthy controls were cultured with various concentrations of decitabine (0-10 μmol/L) and obtained on day 7. Decitabine induced significantly higher percentage of Annexin V-positive and propidium iodide (PI)-negative cells at the higher doses of 1 μM and 10 μM (Figure 2A-C). A concentration gradient of 0 to 100 nmol/L stimulated MDSC amplification in a dose-dependent manner (Figure 2D,E). Therefore, 100 nmol/L was used for subsequent experiments.
Decitabine enhanced metabolic and immunosuppressive function in MDSCs. (A) The scatter plots showing apoptosis in adherent cells. The percentage of Annexin V–positive and PI-negative cells represented the cell apoptosis rate. (B-C) Decitabine induced significantly higher percentage of Annexin V–positive and PI-negative cells at the higher doses of 1 μM and 10 μM (healthy control, HC: ordinary one-way ANOVA, ∗∗∗∗P < .0001, multiple comparisons: P0 nM vs 100 nM = .8105, ∗∗∗∗P0 nM vs 1 μM < .0001, ∗∗∗∗P0 nM vs 10 μM < .0001; ITP patients: ordinary one-way ANOVA, ∗∗∗∗P < .0001, multiple comparisons: P0 nM vs 100 nM = .3248, ∗∗∗∗P0 nM vs 1 μM < .0001, ∗∗∗∗P0 nM vs 10 μM < .0001). ns, Not significant. (D) There was no significant difference between the control group and the decitabine-treated group in healthy controls (one-way ANOVA, n = 7, P = .0696; multiple comparisons: P0 nM vs 10 nM = .8793, P0 nM vs 50 nM = .4241, P0 nM vs 100 nM = .0521, P0 nM vs 1 μM = .2371, P0 nM vs 10 μM = .9251). (E) After 7 d of culture with decitabine (50 nmol/L, 100 nmol/L, or 1 μmol/L), PBMCs from patients with ITP had significantly increased MDSCs than before decitabine treatment (one-way ANOVA, n = 5, ∗P < .0001; multiple comparisons: P0 nM vs 10 nM = .0772, ∗∗∗P0 nM vs 50 nM = .0010, ∗∗∗∗P0 nM vs 100 nM < .0001, ∗∗P0 nM vs 1 μM = .0027). (F) MDSCs from ITP patients and healthy controls were treated with PBS and decitabine (100 nmol/L), respectively. OCR was measured following injections of oligomycin (1 mol/L), carbonyl cyanide-4 (trifluoromethoxy) phenylhydrazone (FCCP) (1 mol/L), and rotenone/antimycin A (0.5 mol/L). (G) Respective mitochondrial parameters basal respiration (unpaired t tests, ∗∗PCtrl vs ITP = .0087, ∗PITP vs ITP+Dec = .0484), ATP production (unpaired t tests, ∗PCtrl vs ITP = .0106, ∗PITP vs ITP+Dec = .0199) and maximal respiration (unpaired t tests, ∗∗PCtrl vs ITP = .0036, ∗∗PITP vs ITP+Dec = .0052) calculated via ATP-linked respiration/basal respiration. (H) Increased intracellular ATP levels in MDSCs after decitabine treatment (paired t tests, P = .0575). (I) Representative histogram of the proliferation of CD4+ Teffs (Ⅰ) Ctrl-PBS, (II) Ctrl-Dec, (III) ITP-PBS, (IV) ITP-Dec. (J) MDSC-mediated suppression of CD4+ Teff proliferation was measured using division index. MDSC inhibition in healthy controls was significantly higher than that in ITP patients (unpaired t tests, ∗∗PCtrl vs ITP = .0073). (K) Compared with PBS treatment, decitabine significantly enhanced MDSC inhibitory function in ITP (paired t tests, ∗∗PITP vs ITP+Dec = .0089). (L) The gated dot plots represent CTL-induced platelet apoptosis after coculture with PBS- or decitabine-treated MDSCs in ITP. (Ⅰ) Platelets only. (II) Platelets + CTLs. (III) Platelets + CTLs + PBS-MDSCs. (IV) Platelets + CTLs + Dec-MDSCs. (M-N) CTL-induced platelet apoptosis was significantly lower following coculture with decitabine-treated MDSCs (paired t tests, PLT + CTLs vs PLT + CTLs + PBS-MDSC, ∗∗∗P = .0004; PLT + CTLs + PBS-MDSC vs PLT + CTLs + Dec-MDSC, ∗∗∗P = .0010).
Decitabine enhanced metabolic and immunosuppressive function in MDSCs. (A) The scatter plots showing apoptosis in adherent cells. The percentage of Annexin V–positive and PI-negative cells represented the cell apoptosis rate. (B-C) Decitabine induced significantly higher percentage of Annexin V–positive and PI-negative cells at the higher doses of 1 μM and 10 μM (healthy control, HC: ordinary one-way ANOVA, ∗∗∗∗P < .0001, multiple comparisons: P0 nM vs 100 nM = .8105, ∗∗∗∗P0 nM vs 1 μM < .0001, ∗∗∗∗P0 nM vs 10 μM < .0001; ITP patients: ordinary one-way ANOVA, ∗∗∗∗P < .0001, multiple comparisons: P0 nM vs 100 nM = .3248, ∗∗∗∗P0 nM vs 1 μM < .0001, ∗∗∗∗P0 nM vs 10 μM < .0001). ns, Not significant. (D) There was no significant difference between the control group and the decitabine-treated group in healthy controls (one-way ANOVA, n = 7, P = .0696; multiple comparisons: P0 nM vs 10 nM = .8793, P0 nM vs 50 nM = .4241, P0 nM vs 100 nM = .0521, P0 nM vs 1 μM = .2371, P0 nM vs 10 μM = .9251). (E) After 7 d of culture with decitabine (50 nmol/L, 100 nmol/L, or 1 μmol/L), PBMCs from patients with ITP had significantly increased MDSCs than before decitabine treatment (one-way ANOVA, n = 5, ∗P < .0001; multiple comparisons: P0 nM vs 10 nM = .0772, ∗∗∗P0 nM vs 50 nM = .0010, ∗∗∗∗P0 nM vs 100 nM < .0001, ∗∗P0 nM vs 1 μM = .0027). (F) MDSCs from ITP patients and healthy controls were treated with PBS and decitabine (100 nmol/L), respectively. OCR was measured following injections of oligomycin (1 mol/L), carbonyl cyanide-4 (trifluoromethoxy) phenylhydrazone (FCCP) (1 mol/L), and rotenone/antimycin A (0.5 mol/L). (G) Respective mitochondrial parameters basal respiration (unpaired t tests, ∗∗PCtrl vs ITP = .0087, ∗PITP vs ITP+Dec = .0484), ATP production (unpaired t tests, ∗PCtrl vs ITP = .0106, ∗PITP vs ITP+Dec = .0199) and maximal respiration (unpaired t tests, ∗∗PCtrl vs ITP = .0036, ∗∗PITP vs ITP+Dec = .0052) calculated via ATP-linked respiration/basal respiration. (H) Increased intracellular ATP levels in MDSCs after decitabine treatment (paired t tests, P = .0575). (I) Representative histogram of the proliferation of CD4+ Teffs (Ⅰ) Ctrl-PBS, (II) Ctrl-Dec, (III) ITP-PBS, (IV) ITP-Dec. (J) MDSC-mediated suppression of CD4+ Teff proliferation was measured using division index. MDSC inhibition in healthy controls was significantly higher than that in ITP patients (unpaired t tests, ∗∗PCtrl vs ITP = .0073). (K) Compared with PBS treatment, decitabine significantly enhanced MDSC inhibitory function in ITP (paired t tests, ∗∗PITP vs ITP+Dec = .0089). (L) The gated dot plots represent CTL-induced platelet apoptosis after coculture with PBS- or decitabine-treated MDSCs in ITP. (Ⅰ) Platelets only. (II) Platelets + CTLs. (III) Platelets + CTLs + PBS-MDSCs. (IV) Platelets + CTLs + Dec-MDSCs. (M-N) CTL-induced platelet apoptosis was significantly lower following coculture with decitabine-treated MDSCs (paired t tests, PLT + CTLs vs PLT + CTLs + PBS-MDSC, ∗∗∗P = .0004; PLT + CTLs + PBS-MDSC vs PLT + CTLs + Dec-MDSC, ∗∗∗P = .0010).
We performed a mitochondrial stress test in MDSCs treated with or without decitabine to analyze their OCR and mitochondrial function. Relative to the healthy controls, OCR was lower in MDSCs from ITP patients (Figure 2F) but significantly improved after decitabine treatment. Similarly, basal respiration, ATP production, and maximal respiration (Figure 2G) were lower in MDSCs from ITP patients than in those from healthy controls but significantly improved after decitabine treatment. This suggests that, in refractory ITP, MDSCs have defective oxidative phosphorylation and reduced mitochondrial electron transport chain activity; decitabine may correct this. A trend of increased intracellular ATP levels after decitabine treatment was observed, albeit statistical difference was not reached (Figure 2H).
Low-dose decitabine augmented immunosuppressive functions of MDSCs in patients with ITP
In vitro, the MDSCs of both patients and controls were treated with PBS or decitabine and then cultured with CFSE-labeled CD4+CD25− effector T cells (Teffs) to evaluate their suppressive effects. MDSCs generated from ITP patients had significantly weaker inhibitory effects on Teffs than those generated from healthy controls (Figure 2I-J). In addition, the division index of Teffs was significantly lower when cocultured with decitabine-modulated MDSCs (Figure 2K), indicating that the immunosuppressive function of MDSCs was significantly enhanced by low-dose decitabine. In those that might have poor response to decitabine or with severely defective MDSCs null to almost any in vitro treatment, decitabine-modulated MDSC could possibly undergo apoptosis and induce less inhibitory effect on the proliferation of Teffs, as 2 opposite cases occurred.
We further investigated whether MDSCs treated with decitabine inhibited CTL cytotoxicity. CTL-induced platelet apoptosis was significantly suppressed after coculture with decitabine-treated MDSCs generated from patients with ITP. The apoptosis level of platelets in the Platelet (PLT) + Cytotoxic T lymphocyte (CTL) + PBS-MDSC group was significantly lower than that in the PLT + CTL group, and the apoptosis level of platelets in the PLT + CTL + Decitabine (Dec)-MDSC group was significantly lower than that in the PLT + CTL + PBS-MDSC group (Figure 2L-M). The percentage of apoptotic platelets were relatively stable when cocultured with healthy CTL, PBS-MDSC-modulated CTL, or Dec-MDSC-modulated CTL (supplemental Figure 2). Furthermore, the apoptosis level of platelets in the PLT + CTL + Dec group was significantly lower than that in the PLT + CTL group (supplemental Figure 3).
Decitabine-modulated MDSC treatment ameliorated thrombocytopenia in active ITP mice
We established an active ITP murine model to investigate the therapeutic effects of low-dose decitabine. The day of splenocyte engraftment was defined as day 0. Mice received immunized/nonimmunized CD61KO splenocytes. ITP mice received PBS (Control), PBS-MDSC, or Dec-MDSC, respectively. Platelet counts significantly decreased on day 7 after radiation and splenocyte transfer. On days 28 and 35, the decitabine-treated MDSC group had significantly higher platelet counts than the control and PBS-treated MDSC groups (Figure 3A), suggesting that amelioration of thrombocytopenia depends on decitabine-modulated MDSCs. The level of serum antiplatelet CD61-specific antibodies changed inversely with platelet counts (Figure 3B). Decitabine treatment significantly increased the proportion of MDSCs and Tregs and restored the balance of T-cell subsets in ITP mice (supplemental Figure 4A-E). Furthermore, autoantibody titer decreased and Treg proportion increased after decitabine treatment (supplemental Figure 5A,B). We also performed CD8+ T/CD19+ B cell-depletion in the murine models to address T cell–induced ITP vs antibody-mediated ITP (supplemental Figure 6A,B).
Low-dose decitabine-treated MDSCs ameliorated thrombocytopenia and enhanced bone marrow–derived MDSC metabolic function in active-ITP model mice. ITP models were established in irradiated SCID mice by engraftment with 2 × 104 splenocytes from CD61-knockout mice immunized against wild-type C57 mouse platelets. The mice were divided into 3 groups: the control group did not receive any treatment, the other 2 groups of active-ITP mice were given PBS- or decitabine-treated MDSC transfer, respectively, and platelet counts were monitored weekly for 5 weeks (mean ± standard error of the mean). (A) The lines represent the platelet counts of ITP mice (mean ± standard error of the mean). On days 28 and 35, the decitabine-treated MDSC group had significantly higher platelet counts than the control and PBS-treated MDSC groups. Significance among groups were determined by 2-way ANOVA (∗∗∗∗PTime < .0001, ∗∗∗Pplatelets = .0001, ∗Pinteraction = .0418; multiple comparisons on day 28: ∗∗∗PCtrl vs Dec-MDSC = .0005, ∗PPBS-MDSC vs Dec-MDSC = .0146; day 35: ∗∗∗PCtrl vs Dec-MDSC = .0001, ∗PPBS-MDSC vs Dec-MDSC = .0455). (B) The lines represent the anti-CD61 antibody level of ITP mice (mean ± standard error of the mean). The star indicates significant differences between groups emerged on day 14. (C) MDSCs were sorted from the bone marrow for OCR assessment. (D) Mitochondrial basal respiration (unpaired t tests, ∗∗∗PCtrl vs Dec-MDSC = .0001, ∗PPBS-MDSC vs Dec-MDS = .0327), ATP production (unpaired t tests,∗∗∗PCtrl vs Dec-MDSC = .0007, ∗PPBS-MDSC vs Dec-MDSC = .0436), and maximal respiration (unpaired t tests,∗∗PCtrl vs Dec-MDSC = .0042, ∗PPBS-MDSC vs Dec-MDSC = .0194) were higher in the decitabine-treated group than in the other 2 groups. (E) Intracellular ATP levels were significantly improved after decitabine-treated MDSC transfer (unpaired t tests,∗∗PCtrl vs Dec-MDSC = .0066, ∗PPBS-MDSC vs Dec-MDSC = .0445). (F-H) mRNA expression of ACADM, HADHA, and PGC1β was significantly lower in the control and PBS-treated groups than in the decitabine-treated group (unpaired t tests, ACADM: ∗∗PCtrl vs Dec-MDSC = .0033, ∗PPBS-MDSC vs Dec-MDSC = .0413; HADHA, ∗∗PCtrl vs Dec-MDSC = .0076, ∗PPBS-MDSC vs Dec-MDSC = .0313; PGC1β: ∗∗PCtrl vs Dec-MDSC = .0016, ∗PPBS-MDSC vs Dec-MDSC = .0252). (I) TGF-β, IL-10, IL-4, IL-2 serum levels were significantly higher in the decitabine-treated MDSC group than in the control or PBS-treated groups (unpaired t tests: IL-10: ∗PCtrl vs Dec-MDSC = .0225, ∗PPBS-MDSC vs Dec-MDSC = .0421; IL-4, ∗∗∗PCtrl vs Dec-MDSC = .0007, ∗PPBS-MDSC vs Dec-MDSC = .0488; TGF-β: ∗∗PCtrl vs Dec-MDSC = .0082, ∗PPBS-MDSC vs Dec-MDSC = .0409). TNF-α, IL-1β, IL-6, IL-17a, IFN-γ, IL-12 p70 serum levels were significantly lower in the decitabine-treated MDSC group than in the control or PBS-treated groups (unpaired t tests: TNF-α: ∗∗∗PCtrl vs Dec-MDSC = .0085, ∗∗PPBS-MDSC vs Dec-MDSC = .0005; IL-1β: ∗∗PCtrl vs Dec-MDSC = .0066, ∗PPBS-MDSC vs Dec-MDSC = .0356; IL-17a: ∗∗PCtrl vs Dec-MDSC = .0035, ∗PPBS-MDSC vs Dec-MDSC = .0470; IFN-γ: ∗∗PCtrl vs Dec-MDSC = .0096, ∗∗PPBS-MDSC vs Dec-MDSC = .0077; IL-12 p70: ∗∗PCtrl vs Dec-MDSC = .0035, ∗PPBS-MDSC vs Dec-MDSC = .0151. Mann-Whitney U tests: IL-6: ∗PCtrl vs Dec-MDSC = .0260, ∗PPBS-MDSC vs Dec-MDSC = .0152). The levels of VEGF, IL-2, and IL-13 did not vary significantly among the groups.
Low-dose decitabine-treated MDSCs ameliorated thrombocytopenia and enhanced bone marrow–derived MDSC metabolic function in active-ITP model mice. ITP models were established in irradiated SCID mice by engraftment with 2 × 104 splenocytes from CD61-knockout mice immunized against wild-type C57 mouse platelets. The mice were divided into 3 groups: the control group did not receive any treatment, the other 2 groups of active-ITP mice were given PBS- or decitabine-treated MDSC transfer, respectively, and platelet counts were monitored weekly for 5 weeks (mean ± standard error of the mean). (A) The lines represent the platelet counts of ITP mice (mean ± standard error of the mean). On days 28 and 35, the decitabine-treated MDSC group had significantly higher platelet counts than the control and PBS-treated MDSC groups. Significance among groups were determined by 2-way ANOVA (∗∗∗∗PTime < .0001, ∗∗∗Pplatelets = .0001, ∗Pinteraction = .0418; multiple comparisons on day 28: ∗∗∗PCtrl vs Dec-MDSC = .0005, ∗PPBS-MDSC vs Dec-MDSC = .0146; day 35: ∗∗∗PCtrl vs Dec-MDSC = .0001, ∗PPBS-MDSC vs Dec-MDSC = .0455). (B) The lines represent the anti-CD61 antibody level of ITP mice (mean ± standard error of the mean). The star indicates significant differences between groups emerged on day 14. (C) MDSCs were sorted from the bone marrow for OCR assessment. (D) Mitochondrial basal respiration (unpaired t tests, ∗∗∗PCtrl vs Dec-MDSC = .0001, ∗PPBS-MDSC vs Dec-MDS = .0327), ATP production (unpaired t tests,∗∗∗PCtrl vs Dec-MDSC = .0007, ∗PPBS-MDSC vs Dec-MDSC = .0436), and maximal respiration (unpaired t tests,∗∗PCtrl vs Dec-MDSC = .0042, ∗PPBS-MDSC vs Dec-MDSC = .0194) were higher in the decitabine-treated group than in the other 2 groups. (E) Intracellular ATP levels were significantly improved after decitabine-treated MDSC transfer (unpaired t tests,∗∗PCtrl vs Dec-MDSC = .0066, ∗PPBS-MDSC vs Dec-MDSC = .0445). (F-H) mRNA expression of ACADM, HADHA, and PGC1β was significantly lower in the control and PBS-treated groups than in the decitabine-treated group (unpaired t tests, ACADM: ∗∗PCtrl vs Dec-MDSC = .0033, ∗PPBS-MDSC vs Dec-MDSC = .0413; HADHA, ∗∗PCtrl vs Dec-MDSC = .0076, ∗PPBS-MDSC vs Dec-MDSC = .0313; PGC1β: ∗∗PCtrl vs Dec-MDSC = .0016, ∗PPBS-MDSC vs Dec-MDSC = .0252). (I) TGF-β, IL-10, IL-4, IL-2 serum levels were significantly higher in the decitabine-treated MDSC group than in the control or PBS-treated groups (unpaired t tests: IL-10: ∗PCtrl vs Dec-MDSC = .0225, ∗PPBS-MDSC vs Dec-MDSC = .0421; IL-4, ∗∗∗PCtrl vs Dec-MDSC = .0007, ∗PPBS-MDSC vs Dec-MDSC = .0488; TGF-β: ∗∗PCtrl vs Dec-MDSC = .0082, ∗PPBS-MDSC vs Dec-MDSC = .0409). TNF-α, IL-1β, IL-6, IL-17a, IFN-γ, IL-12 p70 serum levels were significantly lower in the decitabine-treated MDSC group than in the control or PBS-treated groups (unpaired t tests: TNF-α: ∗∗∗PCtrl vs Dec-MDSC = .0085, ∗∗PPBS-MDSC vs Dec-MDSC = .0005; IL-1β: ∗∗PCtrl vs Dec-MDSC = .0066, ∗PPBS-MDSC vs Dec-MDSC = .0356; IL-17a: ∗∗PCtrl vs Dec-MDSC = .0035, ∗PPBS-MDSC vs Dec-MDSC = .0470; IFN-γ: ∗∗PCtrl vs Dec-MDSC = .0096, ∗∗PPBS-MDSC vs Dec-MDSC = .0077; IL-12 p70: ∗∗PCtrl vs Dec-MDSC = .0035, ∗PPBS-MDSC vs Dec-MDSC = .0151. Mann-Whitney U tests: IL-6: ∗PCtrl vs Dec-MDSC = .0260, ∗PPBS-MDSC vs Dec-MDSC = .0152). The levels of VEGF, IL-2, and IL-13 did not vary significantly among the groups.
Five weeks after transfer, MDSCs of bone marrow were harvested from ITP mice. OCR analysis revealed a significant higher curve in the decitabine-treated MDSCs group compared with the other 2 groups (Figure 3C). We also identified a significant increase in basal respiration, ATP production, and maximal respiration in the decitabine-treated MDSCs group (Figure 3D). Intracellular ATP levels were significantly improved after decitabine-treated MDSCs transfer (Figure 3E). Moreover, we found that the mRNA expression level of ACADM, HADHA, and PGC1β in the control and PBS-treated MDSC groups were significantly lower than that in the decitabine-treated MDSCs group (Figure 3F-H). The levels of TNF-α, IL-1β, IL-6, IL-17a, IFN-γ, and IL-12 p70 in the Dec-MDSC group were significantly lower and the levels of IL-10, IL-4, and TGF-β in the Dec-MDSC group were significantly higher than those in the control group or PBS-MDSC group. However, there was no significant difference in serum levels of IL-2, VEGF, or IL-13 (Figure 3I).
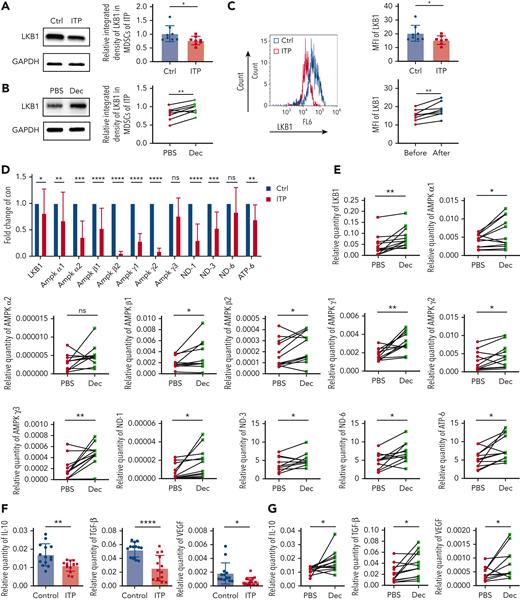
Low-dose decitabine upregulated the expression of the LKB1 signaling pathway and inhibitory cytokines in MDSCs
Western blot and flow cytometric analysis of MDSCs revealed significantly lower LKB1 protein levels and intracellular expression in patients with ITP than in healthy controls, which increased after decitabine treatment (Figure 4A-C).
Low-dose decitabine upregulated the expression of downstream regulators of the LKB1 signaling pathway and inhibitory cytokines in MDSCs. (A-B) Representative western blots of MDSC LKB1 and GADPH in MDSCs from healthy controls and ITP patients, with or without decitabine modulation in vitro. Relative LKB1 protein expression, obtained via densitometry (Ctrl vs ITP, unpaired t test, ∗P = .0232; PBS vs decitabine, paired t test, ∗∗P = .0051). (C) LKB1 expression in CD11b+CD33+HLA-DRlow cells from healthy controls and ITP patients before and after decitabine treatment in vivo. LKB1 expression was significantly lower in ITP patients than in healthy controls (Ctrl vs ITP, unpaired t test, ∗P = .0474; before decitabine vs after decitabine, paired t test, ∗∗P = .0059). (D) The mRNA expression of proteins involved in the LKB1–AMPK signaling pathway in MDSCs cultured in vitro from ITP patients and healthy controls (Ctrl vs ITP: multiple unpaired t test, ∗PLKB1 = .0229, ∗∗PAMPKα1 = .0024, ∗∗∗PAMPKα2 = .0002, ∗∗∗∗PAMPKβ1 < .0001, ∗∗∗∗PAMPKβ2 < .0001, ∗∗∗∗PAMPKγ1 < .0001, ∗∗∗∗PAMPKγ2 < .0001, PAMPKγ3 = .0967, ∗∗∗∗PND-1 < .0001, ∗∗∗PND-3 = .0004, PND-6 = .0533, ∗∗PATP-6 = .0037), which (E) significantly increased after in vitro decitabine modulation in ITP patients (PBS vs decitabine: multiple paired t test,∗∗PLKB1 = .0048, ∗PAMPKα1 = .0341, PAMPKα2 = .1034, ∗PAMPKβ1 = .0489, ∗PAMPKβ2 = .0399, ∗∗PAMPKγ1 = .0019, ∗PAMPKγ2 = .0387, ∗∗PAMPKγ3 = .0047, ∗PND-1 = .0279, ∗PND-3 = .0276, ∗PND-6 = .0199, ∗PATP-6 = .0116). Likewise, (F) The level of inhibitory cytokines in MDSCs cultured in vitro from ITP patients and healthy controls (healthy control vs ITP: Multiple unpaired t test, ∗∗PIL-10 = .0027, ∗∗∗∗PTGF-β < .0001, ∗PVEGF = .0147), which (G) significantly increased after in vitro decitabine modulation in ITP patents (PBS vs decitabine: multiple unpaired t test, ∗PIL-10 = .0165, ∗PTGF-β = .0210, ∗PVEGF = .0298).
Low-dose decitabine upregulated the expression of downstream regulators of the LKB1 signaling pathway and inhibitory cytokines in MDSCs. (A-B) Representative western blots of MDSC LKB1 and GADPH in MDSCs from healthy controls and ITP patients, with or without decitabine modulation in vitro. Relative LKB1 protein expression, obtained via densitometry (Ctrl vs ITP, unpaired t test, ∗P = .0232; PBS vs decitabine, paired t test, ∗∗P = .0051). (C) LKB1 expression in CD11b+CD33+HLA-DRlow cells from healthy controls and ITP patients before and after decitabine treatment in vivo. LKB1 expression was significantly lower in ITP patients than in healthy controls (Ctrl vs ITP, unpaired t test, ∗P = .0474; before decitabine vs after decitabine, paired t test, ∗∗P = .0059). (D) The mRNA expression of proteins involved in the LKB1–AMPK signaling pathway in MDSCs cultured in vitro from ITP patients and healthy controls (Ctrl vs ITP: multiple unpaired t test, ∗PLKB1 = .0229, ∗∗PAMPKα1 = .0024, ∗∗∗PAMPKα2 = .0002, ∗∗∗∗PAMPKβ1 < .0001, ∗∗∗∗PAMPKβ2 < .0001, ∗∗∗∗PAMPKγ1 < .0001, ∗∗∗∗PAMPKγ2 < .0001, PAMPKγ3 = .0967, ∗∗∗∗PND-1 < .0001, ∗∗∗PND-3 = .0004, PND-6 = .0533, ∗∗PATP-6 = .0037), which (E) significantly increased after in vitro decitabine modulation in ITP patients (PBS vs decitabine: multiple paired t test,∗∗PLKB1 = .0048, ∗PAMPKα1 = .0341, PAMPKα2 = .1034, ∗PAMPKβ1 = .0489, ∗PAMPKβ2 = .0399, ∗∗PAMPKγ1 = .0019, ∗PAMPKγ2 = .0387, ∗∗PAMPKγ3 = .0047, ∗PND-1 = .0279, ∗PND-3 = .0276, ∗PND-6 = .0199, ∗PATP-6 = .0116). Likewise, (F) The level of inhibitory cytokines in MDSCs cultured in vitro from ITP patients and healthy controls (healthy control vs ITP: Multiple unpaired t test, ∗∗PIL-10 = .0027, ∗∗∗∗PTGF-β < .0001, ∗PVEGF = .0147), which (G) significantly increased after in vitro decitabine modulation in ITP patents (PBS vs decitabine: multiple unpaired t test, ∗PIL-10 = .0165, ∗PTGF-β = .0210, ∗PVEGF = .0298).
The methylation level of the LKB1 promoter region was analyzed in MDSCs of patients before and after decitabine treatment. Thirty-four CpG residues in the CpG-rich area upstream of the transcription start site were amplified and sequenced (supplemental Figure 1A), and 21 continuous detection sites were identified (supplemental Figure 1B). Following decitabine treatment, most of the CpG residues in the LKB1 promoter region were demethylated to some extent in patients, but only 5 detectable CpG residues reached statistical difference (supplemental Figure 1C).
We explored whether decitabine could regulate the expression of the LKB1 signaling pathway regulators and inhibitory cytokines. mRNA expression of LKB1, AMPKα1, AMPKα2, AMPKβ1, AMPKβ2, AMPKγ1, AMPKγ2, ND-1, ND-3, and ATP-6 were significantly lower in ITP patients than in healthy control subjects (Figure 4D). Decitabine treatment significantly increased the mRNA expression of LKB1 signaling pathway regulators in the MDSCs of patients with ITP (Figure 4E). Furthermore, ITP patients had lower IL-10, VEGF, and TGFβ expression (Figure 4F), and the mRNA expression was significantly increased after decitabine treatment (Figure 4G).
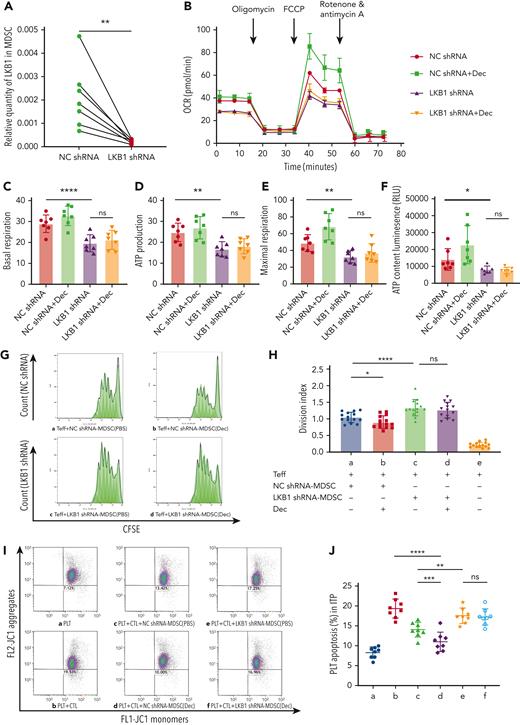
LKB1 shRNA interference offset the altered metabolic activity of MDSC induced by decitabine in vitro
shRNA transfection successfully silenced LKB1 in the MDSCs of patients with ITP. The LKB1-knockdown efficiency was ≥69.26%, as revealed by RT-PCR (Figure 5A). We used the following treatment groups: NC shRNA, LKB1 shRNA, NC shRNA + decitabine, and LKB1 shRNA + decitabine. The OCR of the LKB1 shRNA group was significantly lower than that of the NC shRNA group, with no significant difference in OCR between the LKB1 shRNA + decitabine group. OCR was significantly higher in the NC shRNA + decitabine group (Figure 5B). LKB1 knockdown significantly reduced basal respiration, ATP production, and maximum respiration, relative to the NC shRNA group (Figure 5C-E). The intracellular ATP level was significantly lower in the LKB1 shRNA group than in the NC shRNA group (Figure 5F).
LKB1 shRNA interference offset the altered metabolic activity and suppressed function augmentation of MDSC induced by decitabine in vitro. (A) LKB1 shRNA transfection for 3 days successfully silenced LKB1 in the MDSCs of ITP patients, achieving ≥ 69.26% LKB1 knockdown, determined via RT-PCR (n = 7, paired t tests, ∗∗P = .0091). (B) The MDSCs of patients with ITP were treated with NC shRNA, NC shRNA + Dec, LKB1 shRNA, or LKB1 shRNA + Dec, during in vitro culture, and the OCR was measured. (C-E) Respective mitochondrial parameters including basal respiration (paired t tests, ∗∗∗∗PNC shRNA vs LKB1 shRNA < .0001, PLKB1 shRNA vs LKB1 shRNA+Dec = .0909), ATP production (paired t tests, ∗∗PNC shRNA vs LKB1 shRNA = .0036, PLKB1 shRNA vs LKB1 shRNA+Dec = .2269), and maximal respiration (paired t tests, ∗∗PNC shRNA vs LKB1 shRNA = .0035, PLKB1 shRNA vs LKB1 shRNA+Dec = .0561). (F) In ITP patients, intercellular ATP level was lower in LKB1 shRNA-MDSCs than in NC shRNA-MDSCs, with no significant improvement after decitabine treatment (paired t tests, ∗PNC shRNA vs LKB1 shRNA= .0436, PLKB1 shRNA vs LKB1 shRNA+Dec = .1181). (G) Representative histograms of CD4+CFSE+ effector T-cell proliferation. The effector T-cell division index reflects cell proliferation after 5 days of coculture with MDSCs. (H) The inhibitory function of effector T cells cocultured with LKB1 shRNA-MDSCs was lower than that cocultured with NC shRNA-MDSCs (paired t tests, ∗∗∗∗PNC shRNA vs LKB1 shRNA < .0001); there was no significant statistical difference in the immunosuppression of MDSCs transfected with LKB1 shRNA whether treated with decitabine or not (paired t tests, PLKB1 shRNA vs LKB1 shRNA+Dec = .0729); after treatment with decitabine, the immunosuppression of MDSCs transfected with NC shRNA was significantly increased (paired t-test, ∗P NC shRNA vs NC shRNA+Dec =.0113). (I) The gated dot plots present CTL-induced platelet apoptosis after 3 days of coculture with NC shRNA or LKB1 shRNA MDSCs in ITP. (a) Platelets only. (b) Platelets + CTLs. (c) Platelets + CTLs + NC shRNA-MDSC(PBS). (d) Platelets + CTLs + NC shRNA-MDSC(Dec). (e) Platelets + CTLs + LKB1 shRNA-MDSC(PBS). (f) Platelets + CTLs + LKB1 shRNA-MDSC(Dec). (J) LKB1 blockade resulted in significantly weaker immunosuppressive capacity of MDSCs on the cytotoxicity of CTLs, compared with controls (paired t test, ∗∗PPLT+CTL+NC shRNA-MDSC(PBS) vs PLT+CTL+LKB1 shRNA-MDSC(PBS) = .0022). Moreover, LKB1 shRNA interference masked the effect of decitabine to enhance MDSC function (∗∗∗∗PPLT+CTL vs PLT+CTL+NC shRNA-MDSC(Dec) < .0001, paired t test, PPLT+CTL+LKB1 shRNA-MDSC(PBS) vs PLT+CTL+LKB1 shRNA-MDSC(Dec) = .3121, ∗∗∗PPLT+CTL+NC shRNA-MDSC(PBS) vs PLT+CTL+NC shRNA-MDSC(Dec) = .0007).
LKB1 shRNA interference offset the altered metabolic activity and suppressed function augmentation of MDSC induced by decitabine in vitro. (A) LKB1 shRNA transfection for 3 days successfully silenced LKB1 in the MDSCs of ITP patients, achieving ≥ 69.26% LKB1 knockdown, determined via RT-PCR (n = 7, paired t tests, ∗∗P = .0091). (B) The MDSCs of patients with ITP were treated with NC shRNA, NC shRNA + Dec, LKB1 shRNA, or LKB1 shRNA + Dec, during in vitro culture, and the OCR was measured. (C-E) Respective mitochondrial parameters including basal respiration (paired t tests, ∗∗∗∗PNC shRNA vs LKB1 shRNA < .0001, PLKB1 shRNA vs LKB1 shRNA+Dec = .0909), ATP production (paired t tests, ∗∗PNC shRNA vs LKB1 shRNA = .0036, PLKB1 shRNA vs LKB1 shRNA+Dec = .2269), and maximal respiration (paired t tests, ∗∗PNC shRNA vs LKB1 shRNA = .0035, PLKB1 shRNA vs LKB1 shRNA+Dec = .0561). (F) In ITP patients, intercellular ATP level was lower in LKB1 shRNA-MDSCs than in NC shRNA-MDSCs, with no significant improvement after decitabine treatment (paired t tests, ∗PNC shRNA vs LKB1 shRNA= .0436, PLKB1 shRNA vs LKB1 shRNA+Dec = .1181). (G) Representative histograms of CD4+CFSE+ effector T-cell proliferation. The effector T-cell division index reflects cell proliferation after 5 days of coculture with MDSCs. (H) The inhibitory function of effector T cells cocultured with LKB1 shRNA-MDSCs was lower than that cocultured with NC shRNA-MDSCs (paired t tests, ∗∗∗∗PNC shRNA vs LKB1 shRNA < .0001); there was no significant statistical difference in the immunosuppression of MDSCs transfected with LKB1 shRNA whether treated with decitabine or not (paired t tests, PLKB1 shRNA vs LKB1 shRNA+Dec = .0729); after treatment with decitabine, the immunosuppression of MDSCs transfected with NC shRNA was significantly increased (paired t-test, ∗P NC shRNA vs NC shRNA+Dec =.0113). (I) The gated dot plots present CTL-induced platelet apoptosis after 3 days of coculture with NC shRNA or LKB1 shRNA MDSCs in ITP. (a) Platelets only. (b) Platelets + CTLs. (c) Platelets + CTLs + NC shRNA-MDSC(PBS). (d) Platelets + CTLs + NC shRNA-MDSC(Dec). (e) Platelets + CTLs + LKB1 shRNA-MDSC(PBS). (f) Platelets + CTLs + LKB1 shRNA-MDSC(Dec). (J) LKB1 blockade resulted in significantly weaker immunosuppressive capacity of MDSCs on the cytotoxicity of CTLs, compared with controls (paired t test, ∗∗PPLT+CTL+NC shRNA-MDSC(PBS) vs PLT+CTL+LKB1 shRNA-MDSC(PBS) = .0022). Moreover, LKB1 shRNA interference masked the effect of decitabine to enhance MDSC function (∗∗∗∗PPLT+CTL vs PLT+CTL+NC shRNA-MDSC(Dec) < .0001, paired t test, PPLT+CTL+LKB1 shRNA-MDSC(PBS) vs PLT+CTL+LKB1 shRNA-MDSC(Dec) = .3121, ∗∗∗PPLT+CTL+NC shRNA-MDSC(PBS) vs PLT+CTL+NC shRNA-MDSC(Dec) = .0007).
LKB1 shRNA interference counteracted the functional augmentation of MDSC induced by decitabine in vitro
The suppression of CFSE-labeled CD4+CD25− Teff proliferation was significantly lower in the group cultured with LKB1 shRNA-transfected MDSCs than in the group cultured with NC shRNA (Figure 5G,H), indicating a reduction in the suppressive function of the MDSCs. Therefore, silencing LKB1 quenched the decitabine-induced enhancement of MDSC function observed in an earlier assay, whereas this enhancement persisted in the NC shRNA + decitabine group (Figure 5G,H). Likewise, it was demonstrated that LKB1 blockade resulted in significantly weaker immunosuppressive capacity of MDSCs on the cytotoxicity of CTLs, compared with controls (LKB1 shRNA-MDSCs vs NC shRNA-MDSCs group). Moreover, LKB1 shRNA interference masked the decitabine-induced enhancement of MDSC function (LKB1 shRNA + Dec-MDSCs vs LKB1 shRNA-MDSCs group) (Figure 5I-J).
LKB1 shRNA interference offset rescuing effect of decitabine-treated MDSC in a murine model of ITP
We further investigated whether LKB1 knockdown would eliminate the therapeutic effect of decitabine-treated MDSCs in an active ITP murine model. The day of splenocyte engraftment was defined as day 0. Meanwhile, mice received immunized or nonimmunized CD61KO splenocytes. We transferred PBS (Ctrl), NC shRNA + PBS-MDSCs (NC shRNA), NC shRNA + Dec-MDSCs (NC + Dec), LKB1 shRNA + PBS-MDSCs (LKB1 shRNA), LKB1 shRNA + Dec-MDSCs (LKB1 + Dec) into SCID mice. Platelet counts were monitored weekly (Figure 6A). On day 35, mice in the NC shRNA group had significantly higher platelet levels than those in the LKB1 shRNA group (Figure 6B), suggesting that amelioration of thrombocytopenia was dependent on LKB1. The transferred MDSCs were treated with decitabine in both NC + Dec and LKB1 + Dec groups. Nonetheless, decitabine did not ameliorate the LKB1 knockdown-induced reduction in platelet levels in the LKB1 + Dec group. In contrast, the platelet count in the NC + Dec group was significantly higher than in the LKB1 + Dec group (Figure 6B), suggesting that decitabine modulates LKB1 expression in MDSCs to ameliorate thrombocytopenia. We transferred NC shRNA + DXM-MDSCs, LKB1 shRNA + DXM-MDSCs, NC shRNA + Dec-MDSCs, and Ets1 shRNA + Dec-MDSCs to active ITP mouse models to verify that MDSC-LKB1 has an important role in the recovery of ITP due to treatment with decitabine and not due to treatment with steroids (supplemental Figure 7A,B).
LKB1 shRNA interference offset rescuing effect of decitabine-treated MDSC in a murine model of ITP. (A) A schematic diagram denoting different interventions for ITP mice. KO, knockout. (B) The dotted lines represent the platelet counts of SCID mice.The star indicates significant differences between groups emerged on day 28. On days 28 and 35, platelet counts were significantly higher in NC + Dec group. Significance among groups was determined by 2-way ANOVA (multiple comparisons on day 28: ∗∗∗PCtrl vs NC+Dec = .0005, ∗PNC+Dec vs LKB1+Dec = .0312; day 35: ∗∗∗PCtrl vs NC+Dec = .0005, ∗PNC shRNA vs NC+Dec = .0198, ∗∗PNC+Dec vs LKB1+Dec = .0033). (C) Immunofluorescence images of femurs stained on day 35 with fluorescein isothiocyanate (green; MDSCs), cy3 (red; LKB1), and 4′,6-diamidino-2-phenylindole (DNA). Representative staining of nuclei, Ly-6C, LKB1, and merged spleen images. Ten areas of 80 × 80 μm were randomly selected from the 6G/Ly-6C+ cell-rich areas of the film. MDSCs were counted per merged view, and MDSC-positive cell LKB1 expression was estimated from the mean fluorescence intensity, using ImageJ. Five trials were conducted for each group. Original magnification, scale bars: 20 μm. LKB1 fluorescence intensity (reflecting LKB1 expression) was significantly higher in the NC + Dec group than in Ctrl and NC shRNA group, and there was no statistically significant difference between the LKB1 shRNA group and LKB1 + Dec group (unpaired t test, ∗∗∗PCtrl vs NC+Dec = .0005, ∗PNC shRNA vs NC+Dec = .0349, PLKB1 shRNA vs LKB1+Dec = .9713). (D) ATP production was significantly higher in the NC + Dec group than Ctrl and NC shRNA group; however, there was no statistically significant difference between the LKB1 shRNA group and the LKB1 + Dec group (unpaired t test, ∗∗∗PCtrl vs NC+Dec = .0002, ∗PNC shRNA vs NC+Dec = .0152, PLKB1 shRNA vs LKB1+Dec = .9681). (E) MDSCs were sorted from bone marrow for OCR assessment. OCR was significantly higher in the NC + Dec group than in Ctrl and NC shRNA group. (F) basal respiration (unpaired t test, ∗∗PCtrl vs NC+Dec = .0010, ∗PNC shRNA vs NC+Dec = .0370, PLKB1 shRNA vs LKB1+Dec = .6933), ATP production (unpaired t test, ∗∗PCtrl vs NC+Dec = .0018, ∗PNC shRNA vs NC+Dec = .0355, PLKB1 shRNA vs LKB1+Dec = .9938), and maximal respiration (unpaired t test, ∗∗∗PCtrl vs NC+Dec = .0003, ∗∗PNC shRNA vs NC+Dec = .0098, PLKB1 shRNA vs LKB1+Dec = .8724). (G-I) ACADM and PGC1β mRNA expression were significantly higher in the NC + Dec group than in the Ctrl and NC shRNA group (Unpaired t test. ACADM: ∗∗∗P Ctrlvs. NC+Dec=0.0005, ∗P NC shRNAvs. NC+Dec=0.0331; PGC1β: ∗∗∗P Ctrlvs. NC+Dec=0.0008, ∗P NC shRNAvs. NC+Dec=0.0390). HADHA mRNA expression was significantly higher in the NC + Dec group than in the Ctrl group and was not statistically significantly different from that of NC shRNA (∗P Ctrlvs. NC+Dec=0.0212, P NC shRNAvs. NC+Dec=0.6881). The mRNA expressions of ACADM, PGC1β, and HADHA showed no significant difference between LKB1 shRNA and LKB1 + Dec.
LKB1 shRNA interference offset rescuing effect of decitabine-treated MDSC in a murine model of ITP. (A) A schematic diagram denoting different interventions for ITP mice. KO, knockout. (B) The dotted lines represent the platelet counts of SCID mice.The star indicates significant differences between groups emerged on day 28. On days 28 and 35, platelet counts were significantly higher in NC + Dec group. Significance among groups was determined by 2-way ANOVA (multiple comparisons on day 28: ∗∗∗PCtrl vs NC+Dec = .0005, ∗PNC+Dec vs LKB1+Dec = .0312; day 35: ∗∗∗PCtrl vs NC+Dec = .0005, ∗PNC shRNA vs NC+Dec = .0198, ∗∗PNC+Dec vs LKB1+Dec = .0033). (C) Immunofluorescence images of femurs stained on day 35 with fluorescein isothiocyanate (green; MDSCs), cy3 (red; LKB1), and 4′,6-diamidino-2-phenylindole (DNA). Representative staining of nuclei, Ly-6C, LKB1, and merged spleen images. Ten areas of 80 × 80 μm were randomly selected from the 6G/Ly-6C+ cell-rich areas of the film. MDSCs were counted per merged view, and MDSC-positive cell LKB1 expression was estimated from the mean fluorescence intensity, using ImageJ. Five trials were conducted for each group. Original magnification, scale bars: 20 μm. LKB1 fluorescence intensity (reflecting LKB1 expression) was significantly higher in the NC + Dec group than in Ctrl and NC shRNA group, and there was no statistically significant difference between the LKB1 shRNA group and LKB1 + Dec group (unpaired t test, ∗∗∗PCtrl vs NC+Dec = .0005, ∗PNC shRNA vs NC+Dec = .0349, PLKB1 shRNA vs LKB1+Dec = .9713). (D) ATP production was significantly higher in the NC + Dec group than Ctrl and NC shRNA group; however, there was no statistically significant difference between the LKB1 shRNA group and the LKB1 + Dec group (unpaired t test, ∗∗∗PCtrl vs NC+Dec = .0002, ∗PNC shRNA vs NC+Dec = .0152, PLKB1 shRNA vs LKB1+Dec = .9681). (E) MDSCs were sorted from bone marrow for OCR assessment. OCR was significantly higher in the NC + Dec group than in Ctrl and NC shRNA group. (F) basal respiration (unpaired t test, ∗∗PCtrl vs NC+Dec = .0010, ∗PNC shRNA vs NC+Dec = .0370, PLKB1 shRNA vs LKB1+Dec = .6933), ATP production (unpaired t test, ∗∗PCtrl vs NC+Dec = .0018, ∗PNC shRNA vs NC+Dec = .0355, PLKB1 shRNA vs LKB1+Dec = .9938), and maximal respiration (unpaired t test, ∗∗∗PCtrl vs NC+Dec = .0003, ∗∗PNC shRNA vs NC+Dec = .0098, PLKB1 shRNA vs LKB1+Dec = .8724). (G-I) ACADM and PGC1β mRNA expression were significantly higher in the NC + Dec group than in the Ctrl and NC shRNA group (Unpaired t test. ACADM: ∗∗∗P Ctrlvs. NC+Dec=0.0005, ∗P NC shRNAvs. NC+Dec=0.0331; PGC1β: ∗∗∗P Ctrlvs. NC+Dec=0.0008, ∗P NC shRNAvs. NC+Dec=0.0390). HADHA mRNA expression was significantly higher in the NC + Dec group than in the Ctrl group and was not statistically significantly different from that of NC shRNA (∗P Ctrlvs. NC+Dec=0.0212, P NC shRNAvs. NC+Dec=0.6881). The mRNA expressions of ACADM, PGC1β, and HADHA showed no significant difference between LKB1 shRNA and LKB1 + Dec.
LKB1 expression in MDSC-positive cells was analyzed via fluorescence intensity using Image (5 trials for each group): LKB1 expression was significantly higher in the NC + Dec group than in the NC shRNA group, indicating that decitabine significantly improves platelet count and increases MDSC LKB1 expression (Figure 6C). LKB1 expression was not significantly different between the LKB1 shRNA group and the LKB1 + Dec group, indicating that decitabine does not play a role in increasing platelets in the ITP murine model following LKB1 knockdown (Figure 6C).
OCR was significantly lower in the Ctrl group than in the NC + Dec group and was significantly higher in the NC + Dec group than in the NC shRNA group (Figure 6E). Similarly, following LKB1 knockdown, basal respiration, ATP production, and maximum respiration were significantly lower in the NC shRNA group than in the NC + Dec group. Following decitabine treatment, there were no significant differences between the LKB1 shRNA and LKB1 + Dec groups (Figure 6F). The same patterns were observed for intracellular ATP levels (Figure 6D).
ACADM and PGC1β mRNA expression was significantly lower in the NC shRNA group than in the NC + Dec group. HADHA mRNA expression also showed no significant differences among the groups (Figure 6G-I).
Discussion
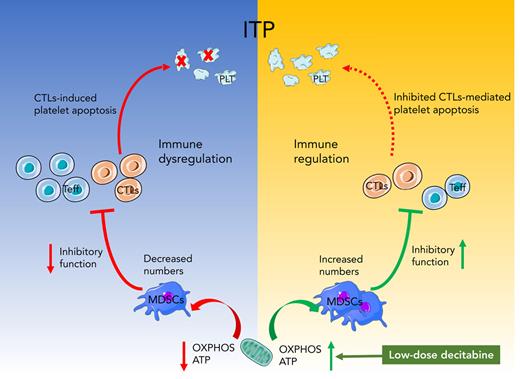
ITP is a multifaceted autoimmune disease initiated by the loss of immune tolerance.32 In this study, we explored the underlying mechanisms of decitabine action on MDSC in patients with ITP. We found that LKB1 disruption led to abnormal MDSC metabolism. By LKB1 deletion, using an ITP murine model, we identified upregulation of LKB1 signaling as the molecular target of low-dose decitabine in restoring normal aerobic metabolism of MDSCs. This in turn enhances the inhibitory function of MDSCs and alleviates thrombocytopenia.
MDSCs are essential for maintaining immune homeostasis,33 which is impaired in autoimmune diseases. For instance, impaired MDSC function in lupus-prone mice suggests its role in the development of systemic lupus erythematosus.34 The primary function of MDSCs is to inhibit various forms of immune responses. l-Arginine deprivation results in repressed expression of the T cell–signaling molecule, CD3ζ, and the arrest of T-cell cycle.35 Therefore, 2 enzymes that compete for l-arginine metabolism (Arg1) and iNOS are crucial for MDSC-induced T-cell dysfunction,36-38 as observed in lupus-prone mice, inflammatory bowel disease model, and colitis.11,39,40 Nitric oxide and Arg1 restrain exuberant or novel T-cell responses via a variety of different mechanisms,41,42 by interfering with direct Toll-like receptor signaling and MyD88-dependent activation of nuclear factor-кB.43,44 In our previous study, ITP patients had significantly lower proportions of peripheral blood MDSCs than healthy controls, with an impaired immunosuppressive function.14 High-dose DXM expanded the MDSC population and enhanced their suppressive function.14 In the current study, decitabine treatment resolved thrombocytopenia and restored the proportion as well as immunosuppressive function of MDSCs. The residual effect of immunosuppressive therapy on MDSCs cannot be ruled out. Nevertheless, MDSCs were aberrant at baseline, suggesting that the discontinued immunosuppressants did not induce or maintain therapeutic impact on MDSCs.
Mitochondrial aerobic metabolism is the most important metabolic mode in immunomodulatory cells.45 Immune cells, which regulate immune homeostasis, require oxidative and fatty acid β oxidation to generate energy.46,47 Due to the importance of oxygen in the electron transport chain, we measured OCR to evaluate mitochondrial function in MDSCs. The mitochondrial respiration was lower in ITP patients than in the healthy controls. Reduced cellular metabolism is associated with lower mitochondrial content and mitochondrial membrane potential.48 Oxidative phosphorylation is required for Treg/Th2 differentiation and the suppressive function of Tregs.49 Treg differentiation is promoted by neutrophil extracellular traps, which enhance oxidative phosphorylation in naïve CD4+ T cells.50 Here, we found that, for patients with ITP, decitabine corrected impaired mitochondrial respiration in MDSCs, enhancing their suppression of T-cell proliferation and CTL cytotoxicity. This suggests the association of aerobic metabolism and immunosuppressive function of MDSCs in patients with ITP.
We found that decitabine affects ITP by upregulating LKB1. The LKB1–AMPK signaling pathway primarily regulates cell metabolism, proliferation, survival, and responses to changes in nutrient and energy requirements. LKB1–AMPK signaling promotes ATP-producing catabolic pathways and contributes to T-cell differentiation and function by regulating metabolic reprogramming.51-53 In the current study, the expression of LKB1 and LKB1–AMPK signaling pathway-related molecules, and the inhibitory cytokines IL-10, TGF-β, and VEGF, were lower in patients with ITP compared with healthy controls. VEGF promotes the expansion of immature myeloid cells, and IL-10 effectively inhibits the antigen-presentation function of dendritic cells.54,55 Our findings show that, in patients with ITP, decitabine can rescue defective expression of LKB1–AMPK signaling pathway molecules and inhibitory cytokines. Importantly, our lentivirus-interference test revealed that LKB1 knockdown sacrificed the decitabine-induced improvements in MDSC metabolism and inhibitory function. LKB1 is therefore central in regulating MDSC metabolism and inhibitory function in patients with ITP, and decitabine plays an immunosuppressive role by regulating LKB1 expression in MDSCs.
The mechanism of ITP pathogenesis, as well as treatment response, may be different in patients with anti-glycoprotein Ibα (GPIbα) versus those with anti-β3 antibodies.56,57 In this study, 66.67% (2 of 3) of patients positive for GPIbα obtained platelet response (supplemental Table 1), suggesting that decitabine may be effective against anti-GPIbα-mediated ITP. Abnormal DNA methylation occurs in various autoimmune diseases.58,59 In ITP, imbalanced DNA methylation alters gene expression in immune cells and modulates immune regulation.18,60,61 Decitabine at a low dose was proved to restore the methylation level and expression of programmed cell death protein 1 (PD-1) on CD8+ T cells and reduce the cytotoxicity of CTLs of ITP patients.21 Low-dose decitabine restored T-cell homeostasis and downregulated phosphorylated STAT3 in patients with ITP.22 We speculate that abnormal methylation may reduce LKB1 expression in ITP. However, our findings revealed that, following decitabine treatment, only a limited part of CpG islands were demethylated, indicating that decitabine has an alternative therapeutic mechanism of action on MDSC. As a major suppressive component of innate immunity, the capability of MDSCs to induce T-cell arrest/apoptosis and to recruit Tregs is crucial for patients with ITP. In our current study, the immunomodulatory role of decitabine has now been expanded to the correction of immunosuppressive and metabolic activity of MDSCs, apart from inhibiting CTL cytotoxicity and augmenting Tregs.
Cellular therapy is currently important in managing autoimmune diseases. MDSC treatment potently inhibits graft-versus-host disease in mice.62 Adoptive transfer of MDSCs markedly ameliorated inflammatory arthritis and profoundly inhibited T-cell proliferation.63 Consistent with these observations, the adoptive transfer of MDSCs generated in vitro and treated with lactoferrin markedly reduced autoimmune inflammation in newborn mice with necrotizing enterocolitis, improving survival.64 Although naturally occurring MDSCs may not be sufficient to control autoimmune diseases, their therapeutic expansion, activation, or adoptive transfer can help to limit autoimmune pathology.65 We previously reported the adoptive transfer of DXM-modulated MDSCs as a promising cellular therapy for increasing ITP platelet count in murine ITP.14 In the current study, we further evaluated the effects of MDSC adoptive cell transfer in vivo, using ITP mice. Our animal studies reveal that decitabine-modulated MDSC transplantation alleviates thrombocytopenia, potentially via the regulation of mitochondrial DNA transcription and aerobic metabolism by decitabine. Consistent with our previous study,22 low-dose decitabine or Dec-MDSC significantly reduced serum levels of inflammatory cytokines and chemokines associated with T-cell differentiation and significantly increased serum TGF-β.
Compared with the increased platelet count in the NC-shRNA-transfected group, LKB1-knockdown MDSCs did not alleviate thrombocytopenia and led to higher mortality in our ITP murine model. Furthermore, treatment failure due to LKB1-knockdown MDSCs was not reversed by decitabine. LKB1-knockdown MDSCs did not have a therapeutic role and had a significantly reduced ability in regulating mitochondrial aerobic metabolism in ITP mice. This further confirms that MDSCs play an immunosuppressive role via LKB1, and their therapeutic effect can be enhanced by decitabine.
In conclusion, impaired MDSCs are involved in the immune pathogenesis of ITP. Low-dose decitabine increased the production of MDSCs in vitro and in vivo, rectified the abnormal expression of LKB1, and improved the metabolic and immunosuppressive function of MDSCs in ITP. Abnormal aerobic metabolism and LKB1 expression in MDSC may be involved in the relapse and refractoriness of ITP. Our findings reveal that an immunotherapeutic approach targeting MDSCs for ITP has the potential to achieve a sustained response to decitabine in adult patients with ITP.
Acknowledgments
The authors thank Editage (www.editage.com) for English language editing.
This work was supported by grants from National Natural Science Foundation of China (No. 81900121, No. 81973994, No. 81900123, No.81900124); Natural Science Foundation for Distinguished Young Scholar of Shandong Province (ZR2021JQ28); Major Research and Development Plan of Shandong Province (2021LCZX05); Young Taishan Scholar Foundation of Shandong Province (No. tsqn201909175, No. tsqn201812133); Clinical Research Center of Shandong University (No. 2020SDUCRCC009); Graduate Education Reform Project of Shandong University (No. XYJG2020141).
Authorship
Contribution: X.N., L.W., H.W., and T.Y. performed research and analyzed data; X.N. and Y.H. wrote the paper; M.H. and Y.H. designed and funded the research; J.X., G.L., and Y.L. assisted the research; and H.Z., M.X., and J.P. edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yu Hou, Department of Hematology, Qilu Hospital, Cheeloo College of Medicine, Shandong University, 107 Wenhuaxi Rd, 250012 Jinan, China; e-mail: houyu2009@sina.com.
References
Author notes
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal