In traditional Chinese medicine, 气 (Qi) represents energy or a vital force that is disrupted in disease. Restoring the balance or flow of Qi is a philosophical therapeutic approach. Although there is no Western scientific equivalent to this concept, in this issue of Blood, Ni et al1 use rigorous scientific methods to describe a rebalancing of energy metabolism using the hypomethylating agent decitabine, which increases platelet counts in immune thrombocytopenia (ITP) in part by restoring energy homeostasis through the liver kinase B1 and AMP-activated protein kinase (LKB1/AMPK) pathway and enhancing myeloid suppressor cell function.
ITP is an autoimmune disease resulting in low platelet counts and bleeding. The pathophysiology of ITP is complex and incompletely elucidated with increased platelet destruction as well as regulatory T cell dysfunction and a proinflammatory environment that disrupts regulation of thrombopoiesis. Treatment of ITP has revolved around immunosuppression with steroids remaining first line therapy with multiple other modalities including anti-CD20 antibodies, thrombopoietin agonists, kinase inhibitors and other immune modulators. Chronic ITP is common in adult patients, resulting in multiple treatment regimens, drug toxicity and diminished quality of life. Thus, a better understanding of the underlying pathophysiology of ITP, leading to targeted, effective and less toxic therapies is needed.
5-aza-2’-deoxycytidine (decitabine) is a hypomethylating agent typically used in the treatment of myelodysplastic syndrome. When used in low doses, decitabine promotes cellular differentiation and can ameliorate cytopenias, including thrombocytopenia, in MDS. Wang et al2 studied the effect of low dose decitabine in mouse models demonstrating an enhancement of megakaryocyte maturation and platelet release. Zhou et al3 extended these findings to in vitro studies of ITP patients, demonstrating a similar beneficial effect of decitabine. This group then extended their in vitro work with a multicenter safety and efficacy trial of low dose decitabine in patients with refractory ITP, with sustained responses in 44% of patients (20 of 45) at 6 months, and improved quality of life in responders.4
Given the complex dysfunctional immune environment of ITP, Han et al5 postulated that the effects of decitabine in ITP were likely to include beneficial immunoregulatory modulation in addition to simply increasing megakaryocyte maturation and platelet release. Using ITP patient samples in vitro and in a mouse model of ITP, they then demonstrated that low-dose decitabine restored immune tolerance in ITP with improved regulatory T -cell function, a decrease in proinflammatory cytokines and decreased STAT3 activation.
The current article by Ni et al extends these observations to a defect in metabolic regulation. LKB1 is a tumor suppressor and product of the serine-threonine kinase 11 (STK11) gene.
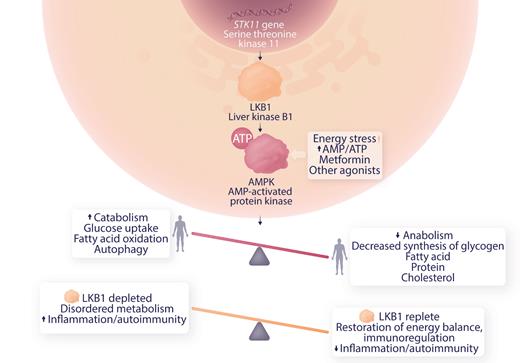
Germ line mutations in STK11 are associated with Peutz-Jeghers syndrome. These patients have gastrointestinal polyps in a setting of chronic inflammation, STAT3 activation, and increased expression of inflammatory factors associated with cancer progression. Somatic variants in STK11 are seen in skin, lung and other cancers. Deficiency of LKBP1 results in downregulation of MAPK, dysregulating metabolism toward an anabolic, proinflammatory state (see figure), and is implicated in the Warburg effect observed in cancer where anaerobic glycolysis is activated in normoxic conditions.6 Ni et al studied myeloid-derived suppressor cells (MDSC) which down-regulate immune responses in ITP patients and controls in vitro and in an animal model of severe ITP. This group has previously shown that MDSC were deficient in ITP patients and improved by dexamethasone therapy. They now present evidence for a role of LKB1 deficiency in MDSC in ITP patients and demonstrate that low dose decitabine therapy restores LKB1 levels, energy balance and MDSC function.
Role of LKB1 in AMPK regulation of metabolism and possible mechanism of modulation of autoimmunity in ITP. Professional illustration by Somersault18:24.
Role of LKB1 in AMPK regulation of metabolism and possible mechanism of modulation of autoimmunity in ITP. Professional illustration by Somersault18:24.
The body of work by these investigators demonstrates that low dose decitabine has multiple salutary effects on restoration of immune regulation and energy balance in ITP. In addition to broadening our understanding of the pathophysiology of ITP and illuminating additional therapeutic choices (eg, AMPK agonists) this work provides a compelling rationale for further clinical trials to define the use case for low dose decitabine therapy for ITP.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal