Key Points
Multiple doses result in high rates of seroconversion in CLL (94.2%) and MBL (100%), with progressively higher antispike antibody levels.
Neutralization against COVID-19 variants requires strong specific T-cell responses and higher antispike levels.
Abstract
Patients with chronic lymphocytic leukemia (CLL) or monoclonal B-lymphocytosis (MBL) have impaired response to COVID-19 vaccination. A total of 258 patients (215 with CLL and 43 with MBL) had antispike antibody levels evaluable for statistical analysis. The overall seroconversion rate in patients with CLL was 94.2% (antispike antibodies ≥50 AU/mL) and 100% in patients with MBL after multiple vaccine doses. After 3 doses (post-D3) in 167 patients with CLL, 73.7% were seropositive, 17.4% had antispike antibody levels between 50 and 999 AU/mL, and 56.3% had antispike antibody levels ≥1000 AU/mL, with a median rise from 144.6 to 1800.7 AU/mL. Of patients who were seronegative post-D2, 39.7% seroconverted post-D3. For those who then remained seronegative after their previous dose, seroconversion occurred in 40.6% post-D4, 46.2% post-D5, 16.7% post-D6, and 0% after D7 or D8. After seroconversion, most had a progressive increase in antispike antibody levels. Neutralization was associated with higher antispike antibody levels, more vaccine doses, and earlier severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants; neutralizing antibody against early clade D614G was detected in 65.3%, against Delta in 52.0%, and against Omicron in 36.5%. SARS-CoV-2–specific T-cell production of interferon γ and interleukin 2 occurred in 73.9% and 60.9%, respectively, of 23 patients tested. After multiple vaccine doses, by multivariate analysis, immunoglobulin M ≥0.53 g/L, immunoglobulin subclass G3 ≥0.22 g/L and absence of current CLL therapy were independent predictors of positive serological responses. Multiple sequential COVID-19 vaccination significantly increased seroconversion and antispike antibody levels in patients with CLL or MBL.
Introduction
The COVID-19 pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is well into its third year and emerging variants now account for most infections across the globe. Patients with chronic lymphocytic leukemia (CLL) have immune failure with hypogammaglobulinemia,1 higher infection risk,2-4 and higher risk of severe COVID-19.5-8 We9 and others10-15 have documented impaired response after 2 initial doses (post–dose 2 [D2]) of COVID-19 vaccination, with 44% of patients with CLL and 9.5% of patients with monoclonal B-lymphocytosis (MBL) failing seroconversion (Abbott Diagnostics assay level ≥50 AU/mL). Furthermore, post-D2, 75% and 50% of patients with CLL and MBL, respectively, have no or minimal neutralizing activity to early SARS-CoV-2 clades and the Delta variant.9
Patients with CLL are immunocompromised and those who are unable to generate their own antibody response remain vulnerable with a limited range of imperfect options. Continued social distancing equates to ongoing social isolation and for many is not possible, antiviral therapy targets only recent-onset COVID-19 infection, and the prophylactic antibody combination tixagevimab and cilgavimab (Evusheld, AstraZeneca) remains difficult to access for many patients with CLL globally and evidence of viral resistance is emerging, especially for SARS-CoV-2 variants BA.4 and BA.5.16-19 A study by the Leukemia and Lymphoma Society presented at the Scientific Symposium of the American Society of Hematology 2021 reported that many patients who were seronegative “took matters into their own hands” and sought additional vaccine doses.20 In our patient population, we also noted higher rates of seroconversion and higher antispike antibody levels in most patients who accessed third, fourth, and subsequent vaccine doses. Patients with MBL also have an immune defect.9,21-23 The prevalence of MBL in the community in those aged >60 years is extremely high at ∼10%,21,24 and many will have suboptimal vaccine response yet be unaware of their status and risk. Adequate protection against COVID-19 for the elderly population, therefore, remains a major public health issue.
Internationally, many governments and health systems have launched third, and sometimes fourth, vaccine dose programs in the general population or selected groups deemed immunocompromised or vulnerable. Recent Israeli, United Kingdom, French, and Danish data showed that a third dose in patients with CLL that were seronegative post-D2 resulted in seroconversion in 23.7%, ∼14%, 35%, and 30%, respectively.25-28 Generally, this supports the hypothesis that multiple doses boost the antispike antibody level and that additional doses may result in successful seroconversion.
In this prospective observational study, we evaluated responses in patients with CLL or MBL after 3, 4, and up to 8 doses of COVID-19 vaccine and report (1) seroconversion rates, (2) antispike antibody levels, (3) neutralizing antibody activity levels against the early SARS-CoV-2 clade D614G and the later variants Delta and Omicron, and (4) COVID-19–specific T-cell responses.
Methods
The study was approved by the Northern Sydney Local Health District Human Research Ethics Committee (approval number LNR/14/HAWKE/181). The diagnosis of CLL and MBL were according to International Workshop on Chronic Lymphocytic Leukemia guidelines.29 Vaccination occurred through the Australian Government program with Vaxzevria (ChAdOx1 nCoV-19, adenoviral vector–based vaccine; AstraZeneca), Cominarty (Pfizer-BioNTech, messenger RNA [mRNA]–based vaccine; Pfizer), Spikevax (mRNA-1273, mRNA-based vaccine; Moderna), or Nuvaxovid (NVX-CoV2373, spike protein subunit vaccine; Novavax).30 Most patients had an initial 2 doses with Vaxzevria, then subsequent doses mostly with Cominarty vaccine and some with Spikevax. Vaccine doses were accessed through vaccine hubs, pharmacies, or local medical practitioners, distributed by the Australian Government with Australian Technical Advisory Group on Immunization–issued guidelines regarding vaccine type, number, and schedule that evolved throughout the pandemic. Australia had very low COVID-19 case numbers until December 2021 (supplemental Figure 1, available on the Blood website). Although many patients were able to access additional doses, some were not, as detailed in supplemental Figure 2. The current study is an expansion of our 2-dose cohort,9 but focuses on outcomes after 3, and up to 8 multiple sequential vaccine doses. The reported ultimate positivity rate was calculated based on the latest antispike antibody level from post-D2 onward. Blood samples were collected ∼4 weeks after each sequential vaccine dose. The SARS-CoV-2 IgG II Quant assay (Abbott Diagnostics, Macquarie Park, NSW) was performed in accordance with the manufacturer’s instructions. An antispike antibody level of ≥50 AU/mL was defined as positive, and the maximum reported level was >25 000 AU/mL.
SARS-CoV-2 live virus neutralization assay
IFN-γ/IL-2 fluorospot assay
Peripheral blood mononuclear cells were seeded in T-cell interferon γ (IFN-γ)/interleukin 2 (IL-2) dual color FluoroSpot plates (Mabtech) and incubated with overlapping SARS-CoV-2 peptide pool spanning the complete S-protein (1 μg/mL; Miltenyi), as previously described.9,32 Reported data are represented as the mean of the duplicates expressed as spot-forming units per 106 cells. The threshold for a positive response was set based on the average number of spot-forming units per 106 cells across all negative controls plus 3 standard deviations. The mean number of responding cells in negative controls (no stimulation controls, that is, cells incubated in media with no peptides) were subtracted from stimulated samples to account for background responses.
Statistical analysis
Statistical analyses were conducted using SAS version 9.4. A generalized mixed model was fitted (using SAS PROC GLIMMIX) where neutralization antibody response (positive or negative) was the outcome. COVID-19 variants and antispike antibody levels (≥1000 or <1000) were included as factors, together with an interaction term. Number of doses were also included as a factor with values 2, 3, 4, or ≥5. To acknowledge the dependence in the data (ie, each patient provided results for up to 3 variants), variants were included as a random term with subject as the repeating term using a compound symmetry variance/covariance matrix. Univariate logistic regression models, modeling the odds of adequate/positive response (antispike antibodies ≥1000 AU/mL), fitted with conditional exact methods to obtain exact P values and 95% confidence interval. Exact methods were employed owing to the small patient numbers in some categories. Multivariate logistic regression models were subsequently fitted including multiple clinical variables found to be significant in univariate models. If 2 clinical variables were highly correlated, only 1 variable was included. Any analyses of immunoglobulin G (IgG) levels (or IgG subclasses) excluded patients on IgG replacement therapy (IgRT).
Results
Patient characteristics
From 1 March 2021 to 24 June 2022, a total of 258 patients (215 with CLL and 43 with MBL) with antispike antibodies measured after at least 2 doses of vaccination were included in the analysis. The median age for patients with CLL and patients with MBL was 72 years. The proportion of male patients with CLL or MBL was 55.8% and 32.6%, respectively. Patient characteristics and baseline demographics are summarized in Table 1. In this post-D2 cohort, there were 229 patients whose antispike antibody level was assessed and seroconversion rates were 55.5% in CLL (106/191) and 92.1% in MBL (35/38) (supplemental Table 1; supplemental Figure 2). At the time of D2, there was no reported COVID-19 infection and all had negative antinucleocapsid assays. Since December 2021, 32 patients (28 CLL and 4 MBL) have reported COVID-19 infection, of whom there have been 30 patients with relatively mild disease and 2 that became severely ill with 1 fatal outcome. Both patients with severe COVID-19 were hospitalized and had CLL, and their latest antispike antibody levels before infection were 1.0 AU/mL and 2462.3 AU/mL (supplemental Figure 3). After COVID-19 infection, no further analysis is reported on these patients as this will reflect response to infection and not only vaccination.
General patient information
| Total number of patients (n = 258) | CLL (n = 215) | MBL (n = 43) |
| Males, n (%) | 120 (55.8) | 14 (32.6) |
| Median age (range), y | 72 (22-95) | 72 (49-88) |
| Median age at diagnosis (range), y | 60 (18-85) | 67.5 (27-84) |
| Seroconversion (at the time of analysis) | ||
| Positive, n (%) | 180 (94.2) | 43 (100) |
| Negative, n (%) | 11 (5.8) | 0 (0) |
| Lost contact, n | 24 | 0 |
| Cytogenetics | ||
| Del 13q, n (%) | 92/145 (63.4) | 6/10 (60) |
| Del 11q, n (%) | 16/145 (11) | 0/10 (0) |
| Del 17p, n (%) | 10/145 (6.9) | 0/10 (0) |
| Trisomy 12, n (%) | 23/145 (15.9) | 1/10 (10) |
| Unknown, n | 70 | 36 |
| IGHV, n | ||
| Mutated | 27 | — |
| Unmutated | 7 | — |
| Unknown | 181 | 43 |
| Treatment status at vaccination, n (%) | ||
| Treatment naïve | 119 (55.3) | 43 (100) |
| Off therapy in remission (complete or partial remission) | 46 (21.4) | 0 (0) |
| Off therapy in relapse | 6 (2.8) | 0 (0) |
| On therapy | 44 (20.5) | 0 (0) |
| Immunoglobulin replacement therapy, n (%) | ||
| Currently on | 27 (12.6) | 0 (0) |
| Prior but not current | 10 (4.7) | 0 (0) |
| Never on immunoglobulin replacement therapy | 149 (69.3) | 43 (100) |
| Unknown | 29 (13.5) | — |
| Anti-CD20 inhibitor in the last 12 mo, n (%) | ||
| Yes | 14 (6.5) | 0 (0) |
| No | 201 (93.5) | 43 (100) |
| Total number of patients (n = 258) | CLL (n = 215) | MBL (n = 43) |
| Males, n (%) | 120 (55.8) | 14 (32.6) |
| Median age (range), y | 72 (22-95) | 72 (49-88) |
| Median age at diagnosis (range), y | 60 (18-85) | 67.5 (27-84) |
| Seroconversion (at the time of analysis) | ||
| Positive, n (%) | 180 (94.2) | 43 (100) |
| Negative, n (%) | 11 (5.8) | 0 (0) |
| Lost contact, n | 24 | 0 |
| Cytogenetics | ||
| Del 13q, n (%) | 92/145 (63.4) | 6/10 (60) |
| Del 11q, n (%) | 16/145 (11) | 0/10 (0) |
| Del 17p, n (%) | 10/145 (6.9) | 0/10 (0) |
| Trisomy 12, n (%) | 23/145 (15.9) | 1/10 (10) |
| Unknown, n | 70 | 36 |
| IGHV, n | ||
| Mutated | 27 | — |
| Unmutated | 7 | — |
| Unknown | 181 | 43 |
| Treatment status at vaccination, n (%) | ||
| Treatment naïve | 119 (55.3) | 43 (100) |
| Off therapy in remission (complete or partial remission) | 46 (21.4) | 0 (0) |
| Off therapy in relapse | 6 (2.8) | 0 (0) |
| On therapy | 44 (20.5) | 0 (0) |
| Immunoglobulin replacement therapy, n (%) | ||
| Currently on | 27 (12.6) | 0 (0) |
| Prior but not current | 10 (4.7) | 0 (0) |
| Never on immunoglobulin replacement therapy | 149 (69.3) | 43 (100) |
| Unknown | 29 (13.5) | — |
| Anti-CD20 inhibitor in the last 12 mo, n (%) | ||
| Yes | 14 (6.5) | 0 (0) |
| No | 201 (93.5) | 43 (100) |
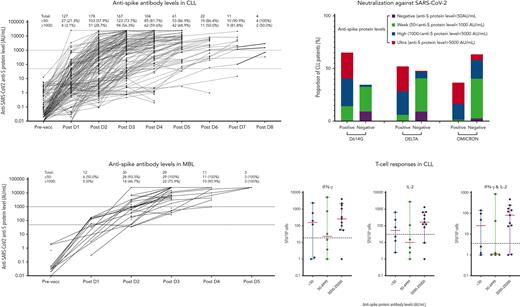
Serological response of antispike seroconversion rate and antibody levels in CLL
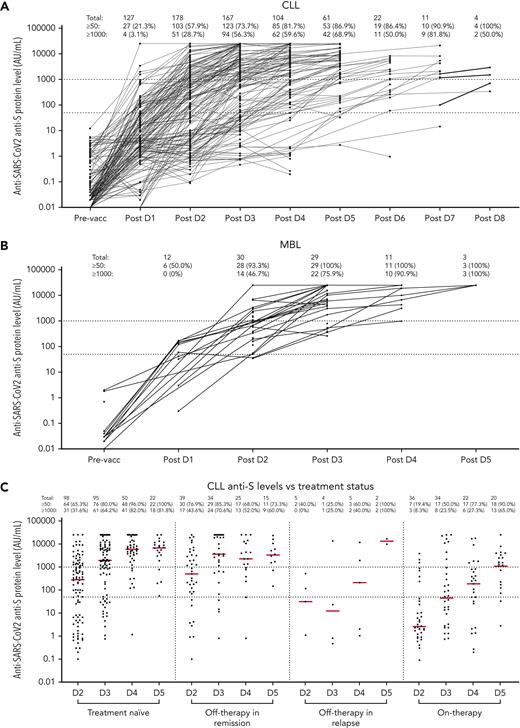
The ultimate seroconversion rate for CLL was 94.2% (180/191; ie, total of 215 patients with CLL, excluding 13 and 11 patients missing post-D2 or post-D3 data, respectively, but with later time points recorded), defined as an antispike antibody concentration ≥50 AU/mL (Abbott Diagnostics). No patient seroconverted if they remained seronegative after 6 doses.
After 3 doses, the seroconversion rate was 73.7% (123/167) (Figures 1 and 2). For patients who were seronegative after D2 (85/191), 68 received a third dose, 27 of which (39.7%) seroconverted. The median antispike antibody level post-D3 rose from 144.6 to 1800.7 AU/mL. To assess heterologous vs homologous vaccine doses, we analyzed a subset of 75 patients: 64 with Vaxzevria as D1+2 followed by an mRNA vaccine for D3 (heterologous) vs 11 patients who received mRNA for D1, D2, and D3 (all Cominarty, homologous). The antispike antibody increase in the heterologous cohort was 1.6 times higher than in the homologous cohort (95% confidence interval, 0.5-5.28), adjusted for D2 response, but was not statistically significant (P = .42) (supplemental Figure 4A).
Sequentialpostvaccination antispike protein IgG levels. Changes in antispike protein IgG levels are shown in patients with CLL (A) or MBL (B) and treatment history (C). An antispike antibody level >50 AU/mL is classified as a positive response and an antispike antibody level ≥1000 AU/mL is classified as a strong positive response. Red bars in panel C indicate the median antispike antibody level in each group. An antispike antibody level of 0 could not be displayed in this figure. Although Australia had low COVID-19 case numbers, consensus guidelines developed at the beginning of the pandemic recommended deferral of CLL therapy where possible during COVID-19 outbreaks.33,34 Hence, the number of patients on therapy (panel C box 4) is relatively low. pre-vacc, prevaccine.
Sequentialpostvaccination antispike protein IgG levels. Changes in antispike protein IgG levels are shown in patients with CLL (A) or MBL (B) and treatment history (C). An antispike antibody level >50 AU/mL is classified as a positive response and an antispike antibody level ≥1000 AU/mL is classified as a strong positive response. Red bars in panel C indicate the median antispike antibody level in each group. An antispike antibody level of 0 could not be displayed in this figure. Although Australia had low COVID-19 case numbers, consensus guidelines developed at the beginning of the pandemic recommended deferral of CLL therapy where possible during COVID-19 outbreaks.33,34 Hence, the number of patients on therapy (panel C box 4) is relatively low. pre-vacc, prevaccine.
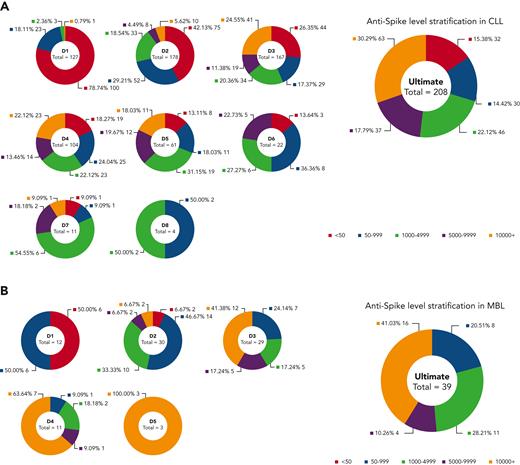
Anti–SARS-CoV-2 spike antibody level stratification in patients with CLL or MBL postvaccination. Antispike level stratification after sequential vaccination were shown in patients with CLL (A) and MBL (B). Only patients with known quantitative antispike antibody levels were included.
Anti–SARS-CoV-2 spike antibody level stratification in patients with CLL or MBL postvaccination. Antispike level stratification after sequential vaccination were shown in patients with CLL (A) and MBL (B). Only patients with known quantitative antispike antibody levels were included.
In patients with CLL after D4, 81.7% seroconverted (>50 AU/mL), but only 35.6% achieved a level >5000 AU/mL capable of Omicron neutralization, that is, 64.4% of patients with CLL are inadequately protected with 4 doses (Figure 2). After ≥4 doses, the number and proportion of patients with CLL who seroconverted or achieved a higher antispike antibody level increased, and therefore, fewer patients proceeded with additional doses; hence, the denominator of patients at risk progressively decreased with multiple sequential doses (supplemental Table 1; supplemental Figure 2). Sequentially for those who remained seronegative after their most recent dose, seroconversion occurred in 40.6% (13/32) after D4, 46.2% (6/13) after D5, 16.7% (1/6) after D6, and none (0/1) after D7 or D8 (supplemental Table 1).
However, importantly, and in contrast to those with no detectable antispike antibody, patients with CLL with detectable but low-level antispike antibodies frequently achieved a higher level of antibody with subsequent doses and the level typically progressively increased with each subsequent vaccine dose (Figure 1A,C). Hence, the ultimate seroconversion rate for CLL was 94.2%, of whom 79.1% achieved antispike antibody levels ≥1000 AU/mL; 54.1%, ≥5000 AU/mL; and 34.1%, ≥10 000 AU/mL. The later and higher antispike antibody levels were commonly associated with neutralization activity against all major COVID-19 variants, including Delta and Omicron. Seroconversion rates after each dose are summarized in supplemental Table 1. Patients with only qualitative (ie, positive or negative) results were not included in Figure 2.
Antispike protein seroconversion and antibody levels after 3 to 5 vaccine doses in patients with MBL
In MBL, the seroconversion rate after D3 was 100% (29/29), including the 3 patients who failed to seroconvert after D2 (Table 1). There were 11 patients with MBL that received a fourth dose and 3 that received a fifth dose (Figure 2). After D4, although all achieved ≥50 AU/mL, only 72.7% achieved ≥5000 AU/mL; that is, 27.3% of patients with MBL remain vulnerable. The median antispike antibody level in patients with MBL increased from 904.9 AU/mL post-D2, to 6188.2 AU/mL post-D3, 19 016.2 AU/mL post-D4, and >25 000 AU/mL post-D5 (Figure 1B). Hence, in MBL, multiple sequential vaccine doses progressively increment to higher antispike antibody levels. No significant vaccine adverse events were reported in patients with MBL.
Adverse events
Adverse events were mainly mild and related to localized injection site or short-term systemic inflammatory responses. There were no serious adverse events reported with the Vaxzevria vaccine, and specifically no episodes of vaccine-induced thrombosis thrombocytopenia syndrome. One patient required hospital admission for bradycardia following the patient’s first dose of Cominarty (mRNA), which was administered as the patient’s third total dose after 2 Vaxzevria doses; this did not recur with the subsequent fourth total vaccine dose, which was Nuvaxovid.
Neutralization titers against SARS-CoV-2 D614G, Delta, and Omicron variants following 2 to 8 vaccination doses
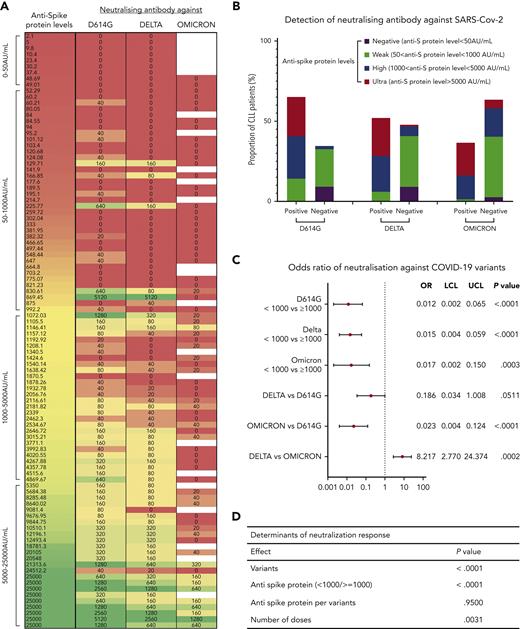
Detection of neutralizing antibody against the D614G, Delta, and Omicron variants was performed on 98 CLL and 13 MBL samples, including sequential samples collected ∼4 weeks after each dose from the same patient, in 50 patients with CLL and 12 patients with MBL. There were 27 samples collected post-D2, 34 post-D3, 21 post-D4, 10 post-D5, 5 post-D6, and 1 post-D7. Detection of neutralizing antibody against the 3 SARS-CoV-2 variants is summarized in Figure 3A-B.
Detection of neutralization antibodies against SARS-CoV-2 variants D614G, Delta, and Omicron. (A) Heatmap summarizes the distribution of antispike antibody levels of each sample tested and their corresponding neutralizing activities against D614G, Delta, and Omicron antibody. (B) Bar chart showing the proportion of samples with positive or negative neutralization, split into 4 stratifications of antispike (anti-S) protein levels. (C) Odds ratio (OR) indicates the differences among patients with antispike levels between <1000 and ≥1000 AU/mL (top), or the differences between strains for those with an antispike protein level >1000 AU/mL (bottom), that is, the odds of a positive neutralizing antibody response against Delta was only 0.186 times the odds of that against D614G. The OR was adjusted for number of doses. (D) Determinants of neutralization response (positive/negative). The overall P values accounted for different variants, number of doses, serological responses (positive/negative), and antispike antibody levels. Antispike antibody levels per variant were not significant; that is, for all 3 variants, the patients with antispike antibody levels ≥1000 AU/mL were always consistently better than those with antispike antibody levels <1000 AU/mL. All other terms are statistically significant (having adjusted for the others). LCL, lower confidence limit; UCL, upper confidence limit.
Detection of neutralization antibodies against SARS-CoV-2 variants D614G, Delta, and Omicron. (A) Heatmap summarizes the distribution of antispike antibody levels of each sample tested and their corresponding neutralizing activities against D614G, Delta, and Omicron antibody. (B) Bar chart showing the proportion of samples with positive or negative neutralization, split into 4 stratifications of antispike (anti-S) protein levels. (C) Odds ratio (OR) indicates the differences among patients with antispike levels between <1000 and ≥1000 AU/mL (top), or the differences between strains for those with an antispike protein level >1000 AU/mL (bottom), that is, the odds of a positive neutralizing antibody response against Delta was only 0.186 times the odds of that against D614G. The OR was adjusted for number of doses. (D) Determinants of neutralization response (positive/negative). The overall P values accounted for different variants, number of doses, serological responses (positive/negative), and antispike antibody levels. Antispike antibody levels per variant were not significant; that is, for all 3 variants, the patients with antispike antibody levels ≥1000 AU/mL were always consistently better than those with antispike antibody levels <1000 AU/mL. All other terms are statistically significant (having adjusted for the others). LCL, lower confidence limit; UCL, upper confidence limit.
Of the 98 tested samples from patients with CLL, antispike antibody levels did not reach the positive/negative threshold of ≥50 AU/mL in 9 patients, and no neutralizing antibodies against any variant were detected in these 9 patients. In the remaining patients, 37 had levels between 50 and 1000 AU/mL, and 52 had levels ≥1000 AU/mL. Of the 98 samples post-D2, 24 predated emergence of the Omicron variant and have been included in a previous report9 but were retained in this study to enable comparison with, and statistical evaluation of, subsequent vaccine doses.
Of all 98 samples from patients with CLL, 65.3% had neutralizing antibody against the early SARS-CoV-2 clade D614G, 52.0% against Delta, and 36.5% against Omicron (excluding the 24 samples that predated Omicron). Detection of neutralizing antibodies against all 3 SARS-CoV-2 variants demonstrated a moderate, nonlinear, but statistically significant correlation (Spearman definition) with antispike antibody levels (supplemental Figure 5). Correlation coefficients to the 3 variants are D614G, 0.65 (P < .0001); Delta, 0.53 (P = .0029); and Omicron, 0.78 (P < .0001).
For all variants, the odds of a positive neutralizing response with antispike antibody <1000 AU/mL is markedly reduced compared with patients with antispike antibodies ≥1000 AU/mL. The OR for all variants are similar from 0.012 to 0.017 (Figure 3C). We then evaluated differences between variants for patients with an antispike antibody level ≥1000 AU/mL, accounting for the number of doses received by the patients when calculating the odds of positive neutralization. The adjusted ORs ranked the likelihood of positive neutralization for a patient with an antispike antibody level ≥1000 AU/mL as D614G>Delta>Omicron (Figure 3C).
SARS-CoV-2–specific T-cell responses following sequential doses
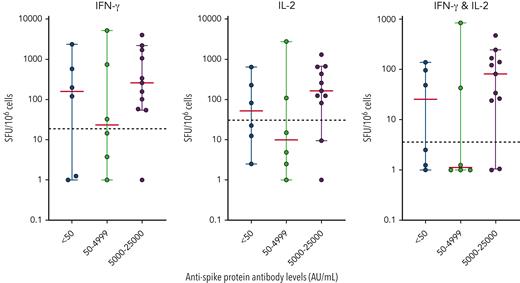
IFN-γ and IL-2 responses specific to the SARS-CoV-2 peptide pool (Miltenyi) were measured in 23 patients by the FluoroSpot assay. Patients were selected to evaluate a broad spectrum of antispike antibody levels and different number of doses. Of the 23 selected patients, 17 (73.9%) were positive for IFN-γ, 14 (60.9%) were positive for IL-2, and 14 (60.9%) were positive for both IFN-γ and IL-2. Based on the latest antispike antibody levels of these 23 patients, we observed strong correlation between positive T-cell responses and high antispike levels (>5000 AU/mL) (Figure 4A). All 7 patients who had an antispike antibody level >25 000 AU/mL showed consistent positive T-cell responses against the stimulatory SARS-CoV-2 peptide pool. Conversely, no correlation was found in patients with an antispike antibody level <5000 AU/mL. Furthermore, no correlation was found between T-cell responses and number of doses administered (supplemental Figure 5) in any of the 23 patients.
T-cell function analysis in patients with CLL after multiple vaccination doses. The IFN-γ and IL-2 responses were tested against the Miltenyi spike peptide pool. The mean number of responding cells in negative controls were subtracted from stimulated samples to account for background responses and results were expressed as spot-forming units (SFU) per 106 cells. The positivity threshold (dotted line) was set based on the average number of responding cells across all negative controls in the cohort plus 3× the standard deviation. Median (red) and 95% confidence intervals were plotted for each antispike protein antibody level strata.
T-cell function analysis in patients with CLL after multiple vaccination doses. The IFN-γ and IL-2 responses were tested against the Miltenyi spike peptide pool. The mean number of responding cells in negative controls were subtracted from stimulated samples to account for background responses and results were expressed as spot-forming units (SFU) per 106 cells. The positivity threshold (dotted line) was set based on the average number of responding cells across all negative controls in the cohort plus 3× the standard deviation. Median (red) and 95% confidence intervals were plotted for each antispike protein antibody level strata.
Neutralizing activities were measured in 14 of the 23 patients assessed for T-cell responsiveness. Strong neutralizing activities against all 3 variants, D614G, Delta, and Omicron, were observed in 7 patients and were highly consistent with their positive T-cell responses in 85.7% (6/7) of these patients. The 1 patient with positive neutralizing activities but inadequate T-cell responses had an antispike antibody level of 1072 AU/mL.
Statistical analysis for persistent vaccine failure after multiple doses
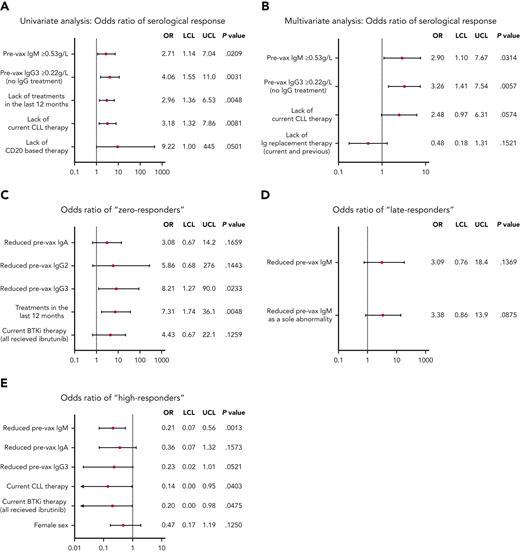
The association between best serological response after multiple doses and clinical variables was estimated using univariate logistic regression models. Immunoglobulin levels and treatments for CLL are shown in supplemental Figures 6 and 7 and supplemental Table 2, respectively. Statistically significant association was identified between positive/adequate serological responses (antispike antibodies ≥1000 AU/mL) and IgM ≥0.53 g/L (OR, 2.71; P = .0209), IgG3 ≥0.22 g/L (OR, 4.06; P = .0031; only assessed in patients without IgRT), absence of CLL treatment in the last 12 months (OR, 2.96; P = .0048), and absence of current CLL therapy (OR, 3.18; P = .0081), including CD20-based therapy (OR, 9.22; P = .0501). Ibrutinib and venetoclax were associated with negative response (OR, 2.33 and 1.73, respectively), but did not achieve a low P value owing to the small number of patients.
We then fitted a multivariate logistic regression model, modeling the odds of a positive/adequate response (antispike antibodies ≥1000 AU/mL) based on clinical and biological variables identified in the aforementioned univariate analysis. Variables statistically associated with an adequate antispike antibody response include IgM ≥0.53 g/L (OR, 2.90; P = .0314), IgG3 ≥0.22 g/L (OR, 3.26; P = .0057), and the absence of any current CLL therapy (OR, 2.48; P = .0574). Interestingly, a trend of a better antispike antibody response was observed in patients who received IgRT at any time (currently or previously) (Figure 5B), possibly owing to the emergence of antispike antibodies in the vaccinated IgRT source population.35
Univariate and multivariate analysis of serological responses. OR for positive serological response (antispike antibodies ≥1000 AU/mL) to COVID-19 vaccine calculated by univariate (A) and multivariate (B) logistic regression models. Biological and clinical factors, using univariate models, associated with zero responders (>4 doses but remain antispike antibodies <50 AU/mL) (C), late responders (antispike antibodies <1000 AU/mL after 3 doses but increased to >1000 AU/mL after the fourth dose) (D), high responders (antispike antibodies ≥20 000 AU/mL at any point after their first dose) (E). Any analysis of IgG levels (or IgG subclasses) excluded patients on IgRT. All patients were treated with Bruton tyrosine kinase inhibitor (BTKi) received ibrutinib. P < .2 was used as the cutoff value for the above models. Pre-vax, prevaccination.
Univariate and multivariate analysis of serological responses. OR for positive serological response (antispike antibodies ≥1000 AU/mL) to COVID-19 vaccine calculated by univariate (A) and multivariate (B) logistic regression models. Biological and clinical factors, using univariate models, associated with zero responders (>4 doses but remain antispike antibodies <50 AU/mL) (C), late responders (antispike antibodies <1000 AU/mL after 3 doses but increased to >1000 AU/mL after the fourth dose) (D), high responders (antispike antibodies ≥20 000 AU/mL at any point after their first dose) (E). Any analysis of IgG levels (or IgG subclasses) excluded patients on IgRT. All patients were treated with Bruton tyrosine kinase inhibitor (BTKi) received ibrutinib. P < .2 was used as the cutoff value for the above models. Pre-vax, prevaccination.
Statistical predictors and associations with vaccine responses
Vaccine responses after multiple doses were highly variable, ranging from 0 to >25 000 AU/mL (upper reported limit of the Abbott assay). To enable evaluation of biological or clinical predictors of antispike antibody levels, we categorized patients with CLL into 3 cohorts based on their serological responses and considering the number of doses administered. Univariate models were constructed for (1) patients who had >4 doses, but maintained antispike antibodies <50 AU/mL (zero responders); (2) patients with antispike antibodies <1000 AU/mL post-D3, but then increased to ≥1000 AU/mL after ≥4 doses (late responders); and (3) patients who reached ≥20 000 AU/mL at any point post-D1 (high responders). Using these definitions, 11 patients were identified as zero responders, 28 patients were defined as late responders and 36 patients were high responders.
There were large sample size discrepancies between the comparator groups, hence, we focused on large or small OR values and used a P value cutoff of P < .2 for this analysis. Using the aforementioned approach, zero response vs any response was highly associated with reduced IgA (OR, 3.08; P = .1659), reduced IgG2 (OR, 5.86; P = .1443), reduced IgG3 (OR, 8.21; P = .0233), treatment in the last 12 months (OR, 7.31; P = .0048), and current ibrutinib treatment (OR, 4.43; P = .1259). Factors associated with a late response vs other response patterns included reduced IgM (OR, 3.09; P = .1396) and reduced IgM as a sole immunoglobulin abnormality (OR, 3.38; P = .0875). Current ibrutinib treatment (OR, 2.41; P = .2149) was not statistically significant but may be clinically relevant. Failure to become a high responder was associated with reduced IgM (OR, 0.21; P = .0013), reduced IgA (OR, 0.36; P = .1573), reduced IgG3 (OR, 0.23; P = .0521), treatment in the last 12 months (OR, 0.14; P = .04), current ibrutinib treatment (OR, 0.20; P = .0475), and female sex (OR, 0.47; P = .1250) (Figure 5). All patients treated with BTKi received ibrutinib.
Discussion
Immune impairment in CLL and also MBL, leads to a significantly impaired response to COVID-19 vaccination with lower and slower rates of both seroconversion and antispike antibody levels generated compared with normal individuals.9-12,36-38 An Israeli group recently documented that a third vaccine dose in patients with CLL, seroconverted 23.8% of those who had been negative after D2.25 We found a somewhat higher rate of 39.7% for D2 to D3 seroconversion, which is very similar to the 35% observed in a French study, in which the same Abbott assay for antispike antibody levels was used, but in which all 3 doses were mRNA-based vaccines.27 These differences may reflect the CLL population under study (higher or lower numbers with advanced disease), different vaccines used (all Comirnaty in Israel vs mainly Vaxzevria followed by Comirnaty in Australia), and possibly the assay used to measure antispike antibody levels (Roche vs Abbott) that have different noninterchangeable numerical values and assay kinetics.39,40 In any event, it highlights the principal that additional doses provide seroconversion in a significant proportion of patients.
The key messages from this study are that multiple sequential COVID-19 vaccine doses ultimately seroconvert all patients with MBL and a very high proportion, 94.2% of patients with CLL This is more than double the seroconversion after 2 vaccine doses, and generates progressive increases in the antispike antibody level in most patients, thereby providing, in some individuals, adequately antispike antibody levels necessary for neutralization of the SARS-CoV-2 Delta and Omicron variants. Induction of high antispike antibody levels achieved by multiple vaccine doses are associated with stronger SARS-CoV-2–specific T-cell responses.
There are compelling arguments to support strategies to achieve an endogenous immune response to COVID-19 vaccination. Access to prophylactic monoclonal antibody therapies such as tixagevimab and cilgavimab, which had high neutralization activity against early variants, remains difficult in many areas of the world, including Australia at the time of the study in patients with CLL (supplemental Table 3); this despite CLL being the hematologic malignancy with the highest rate of vaccine failure.37,41 Furthermore, there is already evidence that the Omicron family of variants, BA.1, BA.2, BA.4, and BA.5, are less sensitive to tixagevimab and cilgavimab17,19,42 and sotrovimab17 than previous SARS-CoV-2 variants. Hence, there is an ongoing risk that future SARS-CoV-2 mutations, strains, and variants may further escape the effect of prophylactic monoclonal antibody therapies, rendering them potentially ineffective. For example, although tixagevimab and cilgavimab had high neutralizing activity against early variants (20 ng/mL), its activity is now 14-fold lower at ∼300 ng/mL and, effectively, a monotherapy as tixagevimab has no activity against BA.2 and BA.5.17 BA.5 reduces the potency for tixagevimab and cilgavimab and sotrovimab by 14.3-fold and 16.8-fold, respectively.17 Therefore, patients who are immunocompromised are left with Paxlovid, molnupiravir, or the less potent remdesivir as their remaining effective options. Vaccination also produces a SARS-CoV-2–specific T-cell response that may be very important in long-term protection from severe COVID-19 and mortality,43 and a T-cell response cannot develop from passive antibody therapy such as tixagevimab and cilgavimab or from pooled convalescent immunoglobulin.
The number of individuals at risk is considerable. CLL is the most common hematologic malignancy with a high prevalence arising from prolonged survival with current treatment options. By comparison, however, MBL is extraordinarily common with a clone detectable in ∼10% of the adult population aged >60 years;44-46 we recently postulated that unrecognized MBL with its attendant immune impairment may be a factor leading to higher risk of severe COVID-19 in those aged >60 years.21 Vaccination strategies of 2 to 4 doses based on data from individuals that are healthy and immunocompetent leave many patients with CLL or MBL (including unrecognized MBL) that are immunocompromised, inadequately protected. After D4, only 35.6% of patients with CLL and 73.7% of patients with MBL had antispike antibody levels ≥5000 AU/mL, the lowest threshold for Omicron-neutralizing antibodies. If provided with adequate vaccine access, >94% of patients with CLL will seroconvert, and most will achieve higher antispike antibody levels. Hence, strategies for achieving seroconversion, with higher endogenous antibody production remain an important unmet need. The results of this and other recent studies showing improved humoral responses after a third vaccine dose in patients with CLL provide support for tailored vaccine strategies that incorporate serological testing and multiple sequential vaccine dosing.20,25,26,47
It remains unclear whether the progressive seroconversion or the progressive rise in antispike antibody levels result primarily from the total vaccine dosage, the sequence of vaccine type administered, interval spacing between doses, or a combination of these factors. Multiple vaccine options are universally available and relatively inexpensive in most countries. Furthermore, at the time preparation of this manuscript, Pfizer48 and Moderna49 have announced bivalent mRNA vaccines combining high viral neutralizing activity against earlier SARS-CoV-2 clade and Omicron BA.4 and BA.5.
Neutralization data from this study demonstrate that seroconversion with a low antispike antibody level (ie, ≥50 but <1000 AU/mL) is insufficient to provide viral neutralization protection against wild-type and early variants of SARS-CoV-29,50 and less so against later variants (Figure 3). Indeed, neutralization against Omicron was not observed until the antispike antibody level surpassed 5000 AU/mL, and consistent neutralization of Omicron was only observed with antispike antibody levels ≥20 000 AU/mL. Similar data were reported in the literature,13 in particular, Parry et al51 demonstrated a 28% reduction in neutralization capacity against Delta compared with the Wuhan variant. We fitted a generalized mixed model (using SAS PROC GLIMMIX) to identify determinants of the neutralization antibody response against D614G, Delta, and Omicron (positive/negative as the outcome). Our analysis, consistent with others,42,51 showed that novel variants, antispike antibody levels <1000 AU/mL, and fewer doses are contributing factors for poor neutralization with statistical significance (Figure 3D). Antispike antibody levels interpreted together with neutralizing data show that most patients with CLL lack sufficient neutralizing activity, especially against the latest SARS-CoV-2 variants.52
SARS-CoV-2–specific T-cell function also showed a strong association with higher serological responses (antispike antibodies ≥5000 AU/mL) after multiple doses. Less than half (5/12, 41.7%) of the patients with antispike antibody levels <5000 AU/mL had normal levels of both IFN-γ and IL-2, compared with 81.8% (9/11) with antispike antibody levels ≥5000 AU/mL. Although a strong T-cell response was almost always associated with a high antispike antibody level, good T-cell responses were not necessarily accompanied by high antispike antibody levels with neutralizing activity. Similar results were shown in the literature, demonstrating a heterogeneous mix of T-cell responses among all patients with CLL,53 whereas a weak correlation between antispike antibody level and IFN-γ was observed.54 It remains unclear to what extent the B-cell vs T-cell response contributes to protection against severe COVID-19, especially pneumonitis.43
Univariate analysis for parameters associated with positive/adequate (antispike antibodies ≥1000 AU/mL) serological responses showed correlations that included IgM ≥0.53 g/L, IgG3 ≥0.22 g/L, absence of treatment in the last 12 months, and absence of current CLL therapy (including CD20-containing therapy), all consistent with our previous study,9 except for IgG2 (OR, 1.85; P = .1763). Although the ORs for ibrutinib and venetoclax of 2.33 and 1.73, respectively, did not achieve a low P value owing to the small number of patients, it is possible that these data reflect clinically significant risks for failure to seroconvert. The association between vaccine response and IgG3 ≥0.22 g/L again suggests that the IgG3 subclass is important in COVID-19 vaccine efficacy.55
Reduced IgM is again identified as a major contributing factor to poor serological responses and to being a late responder. IgM deficiency is extremely common in early-stage CLL,3 72.2% in the current cohort, and almost half of MBL (49.3%) in our center (unpublished data). The importance of IgM in the primary immune response was apparent in serological responses after the initial 2 doses, and this study reinforces this finding with multiple vaccine doses. Total IgG levels, as opposed to adequate IgG2 or IgG3 subclass levels, were not associated with positive vaccine response, in keeping with IgG1 not being an important determinant of COVID-19 vaccine response.
Multivariate analysis confirmed IgM ≥0.53 g/L (OR, 2.90; P = .0314), IgG3 ≥0.22 g/L (OR, 3.26; P = .0057), and the absence of current CLL therapy (OR, 2.48; P = .0574) as key statistical associations with adequate serological responses (antispike antibodies ≥1000 AU/mL), consistent with our previous report and the literature.9-12,25-27 Unlike others,10,11,25 reduced IgA was associated with either complete vaccine failure or failure to achieve antispike antibodies ≥20 000 AU/mL after multiple doses, in univariate analyses. Differences in the diagnostic antispike antibody assay performance56,57 (Abbott9,12 vs Roche10,11) may contribute to discrepancies in the identification of statistically significant predictors of responses.
In summary, multiple sequential COVID-19 vaccine doses significantly increase serological responses, both seroconversion and higher antispike antibody levels, together with neutralizing activities, and resulted in stronger SARS-CoV-2–specific T-cell response in a high proportion of patients with CLL and in virtually all patients with MBL. These data are consequential and globally applicable for patients with CLL and MBL that are immunocompromised. COVID-19 vaccination policies must address the specific needs of these patients and afford them an optimal level of protection as the pandemic proceeds.
Authorship
Contribution: Y.S., J.A.F., J.H., K.N., P.D., I.K., L.W., N.V.B., and S.P.M. collected the data; S.P.M., J.A.F., P.D., and I.K. confirmed the accuracy of the clinical data; V.M., A. Akerman, A. Aggarwal, G.M.C.G., C.M.D., K.J.S., S.T., and A.L.C. performed neutralization and ELISpot experiments and interpreted the data; Y.S. compiled the data for statistical analysis; A.S. performed the statistical analysis; Y.S. and S.P.M. prepared the final manuscript; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stephen Mulligan, Department of Haematology, Royal North Shore Hospital, Reserve Road, Sydney, NSW 2065, Australia; e-mail: stephen.mulligan@sydney.edu.au.
References
Author notes
Data are available on request from the corresponding author, Stephen Mulligan (stephen.mulligan@sydney.edu.au).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal