Key Points
Pola-R-CHP is provisionally cost-effective compared with R-CHOP for the frontline treatment of DLBCL at a WTP of $150 000/QALY.
The cost-effectiveness of pola-R-CHP depends on its long-term outcomes (5-year PFS of at least 66.1% needed to remain cost-effective).
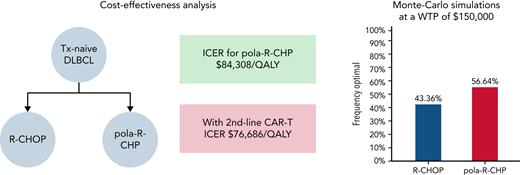
Visual Abstract
In patients with treatment-naive diffuse large B-cell lymphoma (DLBCL), the POLARIX study (A Study Comparing the Efficacy and Safety of Polatuzumab Vedotin With Rituximab-Cyclophosphamide, Doxorubicin, and Prednisone [R-CHP] Versus Rituximab-Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone [R-CHOP] in Participants With Diffuse Large B-Cell Lymphoma) reported a 6.5% improvement in the 2-year progression-free survival (PFS), with no difference in overall survival (OS) or safety using polatuzumab vedotin, rituximab, cyclophosphamide, doxorubicin, and prednisone (pola-R-CHP) compared with standard rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP). We evaluated the cost-effectiveness of pola-R-CHP for DLBCL. We modeled a hypothetical cohort of US adults (mean age, 65 years) with treatment-naive DLBCL by developing a Markov model (lifetime horizon) to model the cost-effectiveness of pola-R-CHP and R-CHOP using a range of plausible long-term outcomes. Progression rates and OS were estimated from POLARIX. Outcome measures were reported in incremental cost-effectiveness ratios, with a willingness-to-pay (WTP) threshold of $150 000 per quality-adjusted life-year (QALY). Assuming a 5-year PFS of 69.6% with pola-R-CHP and 62.7% with R-CHOP, pola-R-CHP was cost-effective at a WTP of $150 000 (incremental cost-effectiveness ratio, $84 308/QALY). pola-R-CHP was no longer cost-effective if its 5-year PFS was 66.1% or lower. One-way sensitivity analysis revealed that pola-R-CHP is cost-effective up to a cost of $276 312 at a WTP of $150 000. pola-R-CHP was the cost-effective strategy in 56.6% of the 10 000 Monte Carlo iterations at a WTP of $150 000. If the absolute benefit in PFS is maintained over time, pola-R-CHP is cost-effective compared with R-CHOP at a WTP of $150 000/QALY. However, its cost-effectiveness is highly dependent on its long-term outcomes and costs of chimeric antigen receptor T-cell therapy. Routine usage of pola-R-CHP would add significantly to health care expenditures. Price reductions or identification of subgroups that have maximal benefit would improve cost-effectiveness.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common type of non-Hodgkin lymphoma, and rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) has remained the standard-of-care treatment for DLBCL.1 In more recent years, several randomized clinical trials have been conducted in an attempt to improve the efficacy of R-CHOP by adding novel targeted agents such as bortezomib,2 lenalidomide,3 or ibrutinib.4 However, none of these targeted agents has significantly improved outcomes, and R-CHOP remains the standard treatment for DLBCL.
Polatuzumab vedotin (pola) is an antibody-drug conjugate composed of an anti-CD79b monoclonal antibody conjugated by a protease-cleavable linker to monomethyl auristatin E, a potent microtubule inhibitor.5 Pola is approved in combination with bendamustine and rituximab (BR) in relapsed/refractory (RR)-DLBCL and has shown a significantly higher complete response rate and risk reduction of death by 58% compared with BR alone in a phase 2 study.6 The recent POLARIX study (A Study Comparing the Efficacy and Safety of Polatuzumab Vedotin With Rituximab-Cyclophosphamide, Doxorubicin, and Prednisone [R-CHP] Versus Rituximab-Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone [R-CHOP] in Participants With Diffuse Large B-Cell Lymphoma) was a phase 3 trial evaluating the efficacy and safety of pola, rituximab, cyclophosphamide, doxorubicin, and prednisone (pola-R-CHP) compared with standard-of-care R-CHOP in patients with intermediate- or high-risk previously untreated DLBCL. With a median follow-up of 28.2 months, the study found a significant progression-free survival (PFS) benefit at 2 years (76.7% vs 70.2%; P = .02) with the use of pola-R-CHP but no improvement in 2-year overall survival (OS), with a similar safety profile.7
There have been a variety of cost-effective analyses previously conducted evaluating treatments for DLBCL. Prior cost-effectiveness analyses have shown that the addition of rituximab to CHOP not only significantly increases OS but also has a favorable incremental cost-effectiveness ratio compared with other oncology treatments in widespread use.8,9 Another study showed that the addition of rituximab to standard CHOP chemotherapy was associated with improved survival but at a higher cost and was potentially cost-effective by standard thresholds for patients <60 years old, although cost-effectiveness decreased significantly with age.10 The cost-effectiveness of chimeric antigen receptor T-cell therapy (CAR-T) in multiply RR-DLBCL indicates that it may be cost-effective at a willingness to pay (WTP) threshold of $150 000 per quality-adjusted life-year (QALY).11
The current study evaluated the cost-effectiveness of pola-R-CHP compared with R-CHOP in treatment-naive patients with DLBCL.
Methods
Model structure
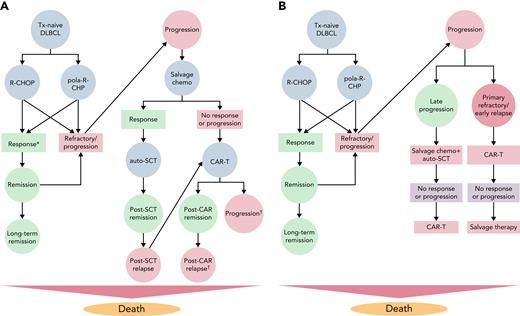
We developed a state-transition Markov model using TreeAge Pro 2021 release 1.2 (TreeAge, Williamstown, MA), simulating a cohort of US adults (mean age, 65 years) with treatment-naive DLBCL who are presenting for initial therapy. The model adhered to the guidelines from the Second Panel on Cost-Effectiveness in Health and Medicine.12 We used this model first to examine the impact of the 2 treatment regimens studied in the POLARIX trial: (1) R-CHOP, defined as 6 cycles of rituximab, cyclophosphamide, doxorubicin, vincristine, and oral prednisone, followed by 2 cycles of rituximab for a total of 8 cycles; and (2) pola-R-CHP, which consists of pola, rituximab, cyclophosphamide, doxorubicin, and oral prednisone for 6 cycles followed by 2 cycles of rituximab. For each treatment strategy, persons in the model transition between the following health states after completing initial therapy: remission, progression to RR-DLBCL, remission after autologous stem cell transplant (auto-SCT), remission after CAR-T therapy, and death (Figure 1A). The model used 1-month cycles and a lifetime horizon. Age-specific probability of all-cause mortality was estimated from the 2018 Centers for Disease Control and Prevention US Life Tables.13 We estimated the effects of grade 3 or 4 adverse events associated with initial therapy, including febrile neutropenia, anemia requiring blood transfusion, and peripheral neuropathy.
Model diagrams for analysis comparing pola-R-CHP vs R-CHOP in untreated DLBCL. (A) For all states, patients remain in the same state if they are not transitioning to another state in the model. ∗Patients are considered to enter remission if they have achieved a complete remission at the end of initial therapy. Patients with any residual disease after the completion of initial therapy are considered primary refractory and proceed to salvage chemoimmunotherapy (salvage chemo) along with patients who progress during or after initial therapy. Patients with RR DLBCL receive salvage chemo; if they respond, they proceed to auto-SCT. If they do not respond, they proceed with third-line CAR T. †Patients who progress after third-line CAR-T therapy are considered to have a poor prognosis, low quality of life, and high costs. (B) Model diagram for analysis comparing pola-R-CHP vs R-CHOP in untreated DLBCL when CAR-T therapy is used in the second-line setting for early progression/primary nonresponse. In this model, patients who are primary nonresponders after initial therapy or progress within 12 months of initial therapy receive CAR-T therapy (axi-cel) in the second-line setting using the ZUMA-7 data. Patients who are late progressors (>12 months) receive salvage chemo as a bridge to auto-SCT and can receive third-line CAR-T therapy if they do not respond or relapse. Patients who receive second-line CAR-T therapy and progress can receive salvage chemo, with low overall response rates.
Model diagrams for analysis comparing pola-R-CHP vs R-CHOP in untreated DLBCL. (A) For all states, patients remain in the same state if they are not transitioning to another state in the model. ∗Patients are considered to enter remission if they have achieved a complete remission at the end of initial therapy. Patients with any residual disease after the completion of initial therapy are considered primary refractory and proceed to salvage chemoimmunotherapy (salvage chemo) along with patients who progress during or after initial therapy. Patients with RR DLBCL receive salvage chemo; if they respond, they proceed to auto-SCT. If they do not respond, they proceed with third-line CAR T. †Patients who progress after third-line CAR-T therapy are considered to have a poor prognosis, low quality of life, and high costs. (B) Model diagram for analysis comparing pola-R-CHP vs R-CHOP in untreated DLBCL when CAR-T therapy is used in the second-line setting for early progression/primary nonresponse. In this model, patients who are primary nonresponders after initial therapy or progress within 12 months of initial therapy receive CAR-T therapy (axi-cel) in the second-line setting using the ZUMA-7 data. Patients who are late progressors (>12 months) receive salvage chemo as a bridge to auto-SCT and can receive third-line CAR-T therapy if they do not respond or relapse. Patients who receive second-line CAR-T therapy and progress can receive salvage chemo, with low overall response rates.
We modeled the management of RR-DLBCL in a pragmatic fashion reflecting real-world practice.14,15 Patients with RR-DLBCL first received salvage chemoimmunotherapy as a bridge to auto-SCT based on results from the long-term follow-up of the SCHOLAR-1 (Retrospective Non-Hodgkin Lymphoma Research) trial.16 Patients who achieved a complete or partial response after salvage chemoimmunotherapy proceeded to auto-SCT in the next month. Patients without a response proceeded to CAR-T therapy. CAR-T therapy was modeled by using a similar model as that from a previously published cost-effectiveness analysis by Lin et al.11 We modeled CAR-T therapy as axicabtagene ciloleucel (axi-cel) using the 4-year outcomes from the ZUMA-1 (Long-Term Safety and Activity of Axicabtagene Ciloleucel in Refractory Large B-cell Lymphoma) trial.17-19 We modeled patients whose disease is refractory to CAR-T therapy or relapsed after CAR-T therapy as having poor survival with low quality of life and high monthly costs as they have poor clinical outcomes with widely disparate treatment strategies.20 We also modeled patients who achieved remission for >5 years as having a very low risk of subsequent progression with a slightly higher risk of all-cause mortality.21,22 We also modeled that even after patients were assigned to a certain therapy (eg, CAR-T therapy or salvage chemoimmunotherapy followed by auto-SCT), they may not be able to receive it or discontinue it due to worsening performance status or other reasons reflecting real-world practice.
Modeling without long-term outcomes data
Ideally, the durability of remission is assessed at 5 or 10 years, but these data are often unavailable, and thus short-term outcomes are extrapolated to estimate long-term outcomes.23 However, this approach can lead to inaccurate results.24 In the current study, to inform the long-term outcomes of R-CHOP, we had the long-term results of the LHN-98.5 trial, which compared R-CHOP vs CHOP.25 In addition, several studies have shown that an additional 6.9% to 8% of patients treated with R-CHOP progress between 2 and 5 years, which informed our 5-year PFS estimate for R-CHOP. However, for pola-R-CHP, the median follow-up of POLARIX was 28.2 months, and thus only the 2-year OS and PFS were available. The uncertainty in long-term outcomes with pola-R-CHP was addressed by modeling several different scenarios. For the base case scenario, we modeled the same rates of progression after 2 years with pola-R-CHP and R-CHOP, meaning that the improved PFS with pola-R-CHP persisted at 5 years. Based on expert opinion, we also varied the 5-year PFS at 75%, 70%, 65%, 60%, and 55% with pola-R-CHP. Also modeled were scenarios in which a reduction in patients progressing with pola-R-CHP between 2 and 5 years was seen (corresponding to a 5-year PFS of >70%), best-case scenario, and another scenario in which the patients who avoided progression with pola-R-CHP, progress after 2 years (5-year PFS of 62.5%), worst-case scenario.
Model inputs
Transition probabilities and costs were identified from published literature and public data sources as detailed in Table 1.7,11,16,17,19,20,25-35 Rates of remission, survival, and relapse for pola-R-CHP and R-CHOP were obtained from the POLARIX trial.7 We included costs related to drug costs, administration costs, costs related to adverse events, transplant and cellular therapy costs, and follow-up costs. The costs of pola-R-CHP and R-CHOP were determined by using the average wholesale price from Micromedex (supplemental index IV, available on the Blood Web site). Costs were inflation-adjusted to 2021 US dollars by using the medical care component of the Consumer Price Index. Base case point estimates of cost were varied by at least ±50% for the sensitivity analysis.
Model input parameters, including transition probabilities, costs, and utilities
| Parameter . | Base case . | Sensitivity analysis range . | Monte Carlo distribution . | References . |
|---|---|---|---|---|
| Inputs: outcome probabilities | ||||
| pola-R-CHP | ||||
| CR after initial therapy | 0.78 | 0.7 to 0.85 | β | 7 |
| PR after initial therapy | 0.075 | 0.02 to 0.17 | β | 7 |
| OS rate at 2 y | 0.887 | 0.857 to 0.916 | β | 7 |
| PFS rate at 2 y | 0.767 | 0.727 to 0.808 | β | 7 |
| AEs | ||||
| AE leading to therapy discontinuation | 0.062 | 0.02 to 0.09 | β | 7 |
| Febrile neutropenia | 0.13 | 0.05 to 0.21 | β | 7 |
| Anemia requiring blood transfusion | 0.12 | 0.05 to 0.20 | β | 7 |
| Grade II or higher peripheral neuropathy | 0.138 | 0.06 to 0.18 | β | 7 |
| Other AEs requiring hospitalization | 0.133 | 0.07 to 0.20 | β | 7 |
| R-CHOP | ||||
| CR after initial therapy | 0.74 | 0.67 to 0.81 | β | 7 |
| PR after initial therapy | 0.098 | 0.05 to 0.15 | β | 7 |
| OS rate at 2 y | 0.886 | 0.856 to 0.916 | β | 7 |
| PFS rate at 2 y | 0.702 | 0.658 to 0.746 | β | 7 |
| AEs | ||||
| AE leading to therapy discontinuation | 0.066 | 0.02 to 0.11 | β | 7 |
| Febrile neutropenia | 0.08 | 0.02 to 0.21 | β | 7 |
| Anemia requiring blood transfusion | 0.084 | 0.04 to 0.14 | β | 7 |
| Grade III or IV peripheral neuropathy | 0.167 | 0.09 to 0.22 | β | 7 |
| Other AEs requiring hospitalization | 0.143 | 0.09 to 0.21 | β | 7 |
| Salvage chemoimmunotherapy | ||||
| CR after initial therapy | 0.07 | 0.03 to 0.15 | β | 16 |
| PR after initial therapy | 0.18 | 0.13 to 0.23 | β | 16 |
| OS rate after 1 y | 0.29 | 0.23 to 0.32 | β | 16 |
| Auto-SCT | ||||
| Receipt of transplantation after CR | 0.84 | 0.70 to 0.94 | β | 16 |
| Receipt of transplantation after PR | 0.68 | 0.58 to 0.78 | β | 16 |
| OS rate after CR + SCT after 1 y | 0.83 | 0.70 to 0.97 | β | 16 |
| OS rate after PR + SCT after 1 y | 0.59 | 0.45 to 0.72 | β | 16 |
| CAR-T therapy (axi-cel) | ||||
| Probability of death before infusion | 0.02 | 0.002 to 0.05 | β | 19 |
| CR after initial therapy | 0.58 | 0.49 to 0.67 | β | 19 |
| PR after initial therapy | 0.24 | 0.17 to 0.33 | β | 19 |
| OS rate after 1 y | 0.61 | 0.52 to 0.70 | β | 19 |
| PFS after 1 y | 0.44 | 0.34 to 0.53 | β | 19 |
| OS rate after 4 y | 0.44 | 0.34 to 0.54 | β | 17 |
| Event-free survival after 2 y | 0.38 | 0.31 to 0.41 | β | 17 |
| Inputs: costs, 2021 US dollars | ||||
| Pharmaceutical | ||||
| Pola-R-CHP | 208 803 | 100 000 to 310 000 | γ | 26 |
| R-CHOP | 78 911 | 40 000 to 160 000 | γ | 26 |
| Salvage chemoimmunotherapy | 25 175 | 20 000 to 100 000 | γ | 26 |
| Axi-cel | 414 248 | 200 000 to 700 000 | γ | 11,26 |
| Administration and AEs | ||||
| Pola-R-CHP (administration cost only) | 4938 | 2000 to 8000 | γ | 27,28 |
| R-CHOP (administration cost only) | 4938 | 2000 to 8000 | γ | 27,28 |
| Salvage chemoimmunotherapy | 26 008 | 14 000 to 44 000 | γ | 27,28 |
| Axi-cel | 60 687 | 35 000 to 70 000 | γ | 28,29 |
| Auto-SCT | 139 194 | 70 000 to 190 000 | γ | 28,29 |
| Monthly cost of care post–CAR-T therapy progression | 11 271 | 5000 to 16 000 | γ | 20 |
| Long-term follow-up monthly cost | 150 | 50 to 250 | γ | 30 |
| Inputs: utilities, QALYs | ||||
| Treatment-naive DLBCL | 0.83 | 0.4 to 0.95 | β | 31 |
| RR-DLBCL | 0.63 | 0.3 to 0.8 | β | 32 |
| Auto-SCT therapy (2 mo) | 0.43 | 0.2 to 0.6 | β | 11 |
| CAR-T therapy (2 mo) | 0.50 | 0.3 to 0.7 | β | 11 |
| Remission after CAR-T therapy | 0.70 | 0.6 to 0.9 | β | 11 |
| Long-term remission (after 5 y) | 1 | 0.9 to 1 | β | 11 |
| Progression after CAR-T therapy | 0.45 | 0.1 to 0.6 | β | 11 |
| Febrile neutropenia | −0.09 | −0.01 to −0.2 | β | 33 |
| Anemia requiring transfusion | −0.04 | −0.01 to −0.08 | β | 34 |
| Peripheral neuropathy | −0.0689 | −0.04 to – 0.14 | β | 35 |
| Parameter . | Base case . | Sensitivity analysis range . | Monte Carlo distribution . | References . |
|---|---|---|---|---|
| Inputs: outcome probabilities | ||||
| pola-R-CHP | ||||
| CR after initial therapy | 0.78 | 0.7 to 0.85 | β | 7 |
| PR after initial therapy | 0.075 | 0.02 to 0.17 | β | 7 |
| OS rate at 2 y | 0.887 | 0.857 to 0.916 | β | 7 |
| PFS rate at 2 y | 0.767 | 0.727 to 0.808 | β | 7 |
| AEs | ||||
| AE leading to therapy discontinuation | 0.062 | 0.02 to 0.09 | β | 7 |
| Febrile neutropenia | 0.13 | 0.05 to 0.21 | β | 7 |
| Anemia requiring blood transfusion | 0.12 | 0.05 to 0.20 | β | 7 |
| Grade II or higher peripheral neuropathy | 0.138 | 0.06 to 0.18 | β | 7 |
| Other AEs requiring hospitalization | 0.133 | 0.07 to 0.20 | β | 7 |
| R-CHOP | ||||
| CR after initial therapy | 0.74 | 0.67 to 0.81 | β | 7 |
| PR after initial therapy | 0.098 | 0.05 to 0.15 | β | 7 |
| OS rate at 2 y | 0.886 | 0.856 to 0.916 | β | 7 |
| PFS rate at 2 y | 0.702 | 0.658 to 0.746 | β | 7 |
| AEs | ||||
| AE leading to therapy discontinuation | 0.066 | 0.02 to 0.11 | β | 7 |
| Febrile neutropenia | 0.08 | 0.02 to 0.21 | β | 7 |
| Anemia requiring blood transfusion | 0.084 | 0.04 to 0.14 | β | 7 |
| Grade III or IV peripheral neuropathy | 0.167 | 0.09 to 0.22 | β | 7 |
| Other AEs requiring hospitalization | 0.143 | 0.09 to 0.21 | β | 7 |
| Salvage chemoimmunotherapy | ||||
| CR after initial therapy | 0.07 | 0.03 to 0.15 | β | 16 |
| PR after initial therapy | 0.18 | 0.13 to 0.23 | β | 16 |
| OS rate after 1 y | 0.29 | 0.23 to 0.32 | β | 16 |
| Auto-SCT | ||||
| Receipt of transplantation after CR | 0.84 | 0.70 to 0.94 | β | 16 |
| Receipt of transplantation after PR | 0.68 | 0.58 to 0.78 | β | 16 |
| OS rate after CR + SCT after 1 y | 0.83 | 0.70 to 0.97 | β | 16 |
| OS rate after PR + SCT after 1 y | 0.59 | 0.45 to 0.72 | β | 16 |
| CAR-T therapy (axi-cel) | ||||
| Probability of death before infusion | 0.02 | 0.002 to 0.05 | β | 19 |
| CR after initial therapy | 0.58 | 0.49 to 0.67 | β | 19 |
| PR after initial therapy | 0.24 | 0.17 to 0.33 | β | 19 |
| OS rate after 1 y | 0.61 | 0.52 to 0.70 | β | 19 |
| PFS after 1 y | 0.44 | 0.34 to 0.53 | β | 19 |
| OS rate after 4 y | 0.44 | 0.34 to 0.54 | β | 17 |
| Event-free survival after 2 y | 0.38 | 0.31 to 0.41 | β | 17 |
| Inputs: costs, 2021 US dollars | ||||
| Pharmaceutical | ||||
| Pola-R-CHP | 208 803 | 100 000 to 310 000 | γ | 26 |
| R-CHOP | 78 911 | 40 000 to 160 000 | γ | 26 |
| Salvage chemoimmunotherapy | 25 175 | 20 000 to 100 000 | γ | 26 |
| Axi-cel | 414 248 | 200 000 to 700 000 | γ | 11,26 |
| Administration and AEs | ||||
| Pola-R-CHP (administration cost only) | 4938 | 2000 to 8000 | γ | 27,28 |
| R-CHOP (administration cost only) | 4938 | 2000 to 8000 | γ | 27,28 |
| Salvage chemoimmunotherapy | 26 008 | 14 000 to 44 000 | γ | 27,28 |
| Axi-cel | 60 687 | 35 000 to 70 000 | γ | 28,29 |
| Auto-SCT | 139 194 | 70 000 to 190 000 | γ | 28,29 |
| Monthly cost of care post–CAR-T therapy progression | 11 271 | 5000 to 16 000 | γ | 20 |
| Long-term follow-up monthly cost | 150 | 50 to 250 | γ | 30 |
| Inputs: utilities, QALYs | ||||
| Treatment-naive DLBCL | 0.83 | 0.4 to 0.95 | β | 31 |
| RR-DLBCL | 0.63 | 0.3 to 0.8 | β | 32 |
| Auto-SCT therapy (2 mo) | 0.43 | 0.2 to 0.6 | β | 11 |
| CAR-T therapy (2 mo) | 0.50 | 0.3 to 0.7 | β | 11 |
| Remission after CAR-T therapy | 0.70 | 0.6 to 0.9 | β | 11 |
| Long-term remission (after 5 y) | 1 | 0.9 to 1 | β | 11 |
| Progression after CAR-T therapy | 0.45 | 0.1 to 0.6 | β | 11 |
| Febrile neutropenia | −0.09 | −0.01 to −0.2 | β | 33 |
| Anemia requiring transfusion | −0.04 | −0.01 to −0.08 | β | 34 |
| Peripheral neuropathy | −0.0689 | −0.04 to – 0.14 | β | 35 |
AE, adverse event; CR, complete response; PR, partial response.
Estimates of utilities with lymphoma health states, as well as adverse events associated with initial chemotherapy, were obtained from the literature and prior cost-effectiveness analyses that estimated utilities from oncology-specific quality of life scores.
Assumptions
As with any cost-effectiveness analysis, certain model assumptions were made a priori. The first assumption was that the effectiveness of second- and third-line treatment for DLBCL is the same for pola-R-CHP and R-CHOP. We do not have any data at this time on the efficacy of subsequent therapies in patients who received frontline pola-R-CHP. This assumption was not tested in sensitivity analyses. Other key assumptions are addressed in the following sections and were tested through one- and two-way sensitivity analyses.
Model calibration
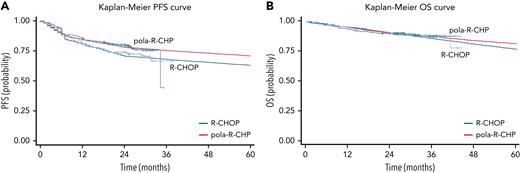
The current model was calibrated by using a constrained optimization algorithm that identifies model inputs to match survival curves. We used a previously validated algorithm that calibrated parameters in our model using a limited-memory BFGS-B optimization algorithm, defining goodness of fit as the sum of squared distance between the published survival curves and simulated ones from our model. The OS and PFS were specifically calibrated at different time points for each treatment arm as well as the treatment strategies for second- and third-line therapy (Figure 2; supplemental index I).
Modeled Kaplan-Meier curves. (A) Modeled PFS for pola-R-CHP and R-CHOP in untreated DLBCL. (B) Modeled OS for pola-R-CHP and R-CHOP in untreated DLBCL.
Modeled Kaplan-Meier curves. (A) Modeled PFS for pola-R-CHP and R-CHOP in untreated DLBCL. (B) Modeled OS for pola-R-CHP and R-CHOP in untreated DLBCL.
Analysis
The cost-effectiveness of each of the treatment strategies as described here was reported from a health care perspective. Outcome measures were reported as life-years gained and incremental cost-effectiveness ratios (ICERs; 2021 US dollars per QALY) with a WTP threshold of $150 000/QALY as well as the OS and PFS at 2 and 5 years.36 Costs and utilities were discounted by 3% annually.
Sensitivity analyses
Extensive one-way sensitivity analyses were performed to evaluate the effect of all defined variables according to each of the strategies, including age, life expectancy after long-term remission, and costs and outcomes with cellular therapy for RR-DLBCL. We also modeled an alternative scenario in which CAR-T therapy is the second-line therapy for primary refractory/early relapsed DLBCL as this is an emerging paradigm in clinical practice given the recent US Food and Drug Administration approval of axi-cel in this setting (Figure 1B).37,38 For the Monte Carlo probabilistic sensitivity analysis, 10 000 iterations were performed by using γ distributions for cost and β distributions for transition probabilities.
Results
Base case analysis (scenario 1: pola-R-CHP and R-CHOP have same 2- to 5-year relapse rates)
In the base case analysis, we assumed a same rate of progression after 2 years with both R-CHOP and pola-R-CHP. Assuming a 5-year PFS of 69.6% with pola-R-CHP and a 5-year PFS of 62.7% with R-CHOP, initial therapy with pola-R-CHP results in an additional 0.62 life-year and an incremental 0.91 QALY (Figure 3). However, pola-R-CHP had a total lifetime cost of $419 769 compared with $342 833 for R-CHOP ($76 936 additional cost with pola-R-CHP). Based on this finding, pola-R-CHP was cost-effective ($84 308/QALY) at WTPs of $100 000/QALY and $150 000/QALY (Table 2).
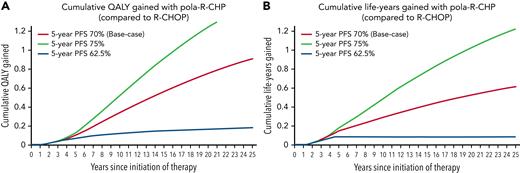
Graphs of cumulative QALYs gained and life-years gained. (A) Graph of cumulative QALYs gained over a lifetime horizon in pola-R-CHP compared with R-CHOP. The red curve represents the base case scenario in which R-CHOP and pola-R-CHP have similar rates of progression between 2 and 5 years, and additional QALYs are gained throughout the cohort’s lifetime. The green curve represents the optimistic scenario in which pola-R-CHP has a 75.1% 5-year PFS, and the blue curve represents the pessimistic scenario in which pola-R-CHP has a 5-year PFS of 62.6%. (B) Graph of cumulative life-years gained over a lifetime horizon in pola-R-CHP compared with R-CHOP. The red curve represents the base case scenario in which R-CHOP and pola-R-CHP have similar rates of progression between 2 and 5 years and show that patients gain life-years or have a lower mortality with pola-R-CHP. The green curve represents the optimistic scenario in which pola-R-CHP has a 75.1% 5-year PFS, and the blue curve represents the pessimistic scenario in which pola-R-CHP has a 5-year PFS of 62.6%.
Graphs of cumulative QALYs gained and life-years gained. (A) Graph of cumulative QALYs gained over a lifetime horizon in pola-R-CHP compared with R-CHOP. The red curve represents the base case scenario in which R-CHOP and pola-R-CHP have similar rates of progression between 2 and 5 years, and additional QALYs are gained throughout the cohort’s lifetime. The green curve represents the optimistic scenario in which pola-R-CHP has a 75.1% 5-year PFS, and the blue curve represents the pessimistic scenario in which pola-R-CHP has a 5-year PFS of 62.6%. (B) Graph of cumulative life-years gained over a lifetime horizon in pola-R-CHP compared with R-CHOP. The red curve represents the base case scenario in which R-CHOP and pola-R-CHP have similar rates of progression between 2 and 5 years and show that patients gain life-years or have a lower mortality with pola-R-CHP. The green curve represents the optimistic scenario in which pola-R-CHP has a 75.1% 5-year PFS, and the blue curve represents the pessimistic scenario in which pola-R-CHP has a 5-year PFS of 62.6%.
Detailed analysis of outcomes and cost-effectiveness of R-CHOP and pola-R-CHP
| Treatment . | 2- and 5-Year outcomes . | Cost-effectiveness . | Incremental cost . | ICER ($/QALY) . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2-y OS . | 2-y PFS . | 5-y OS . | 5-y PFS . | Life-years . | Effectiveness (QALYs) . | Incremental effectiveness . | Cost (2021 US dollars) . | |||
| R-CHOP | 88.4% | 70.2% | 77.4% | 62.7% | 11.37 | 10.89 | – | 342 833 | – | |
| Base-case (pola-R-CHP and R-CHOP have same 2- to 5-year relapse rates) | ||||||||||
| pola-R-CHP | 88.6% | 76.6% | 81.1% | 69.6% | 11.89 | 11.80 | 0.91 | 419 769 | 76 936 | 84 308 |
| Scenario 2 (pola-R-CHP decreases risk of relapse between 2 and 5 y) | ||||||||||
| pola-R-CHP | 88.6% | 76.6% | 84.3% | 75.1% | 12.60 | 12.40 | 1.51 | 377 192 | 45 784 | 30 321 |
| Scenario 3: pola-R-CHP merely delays inevitable relapses after 2 y | ||||||||||
| pola-R-CHP | 88.6% | 76.6% | 78.2% | 62.6% | 11.47 | 11.06 | 0.17 | 514 833 | 172 000 | 1 011 765 |
| Treatment . | 2- and 5-Year outcomes . | Cost-effectiveness . | Incremental cost . | ICER ($/QALY) . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2-y OS . | 2-y PFS . | 5-y OS . | 5-y PFS . | Life-years . | Effectiveness (QALYs) . | Incremental effectiveness . | Cost (2021 US dollars) . | |||
| R-CHOP | 88.4% | 70.2% | 77.4% | 62.7% | 11.37 | 10.89 | – | 342 833 | – | |
| Base-case (pola-R-CHP and R-CHOP have same 2- to 5-year relapse rates) | ||||||||||
| pola-R-CHP | 88.6% | 76.6% | 81.1% | 69.6% | 11.89 | 11.80 | 0.91 | 419 769 | 76 936 | 84 308 |
| Scenario 2 (pola-R-CHP decreases risk of relapse between 2 and 5 y) | ||||||||||
| pola-R-CHP | 88.6% | 76.6% | 84.3% | 75.1% | 12.60 | 12.40 | 1.51 | 377 192 | 45 784 | 30 321 |
| Scenario 3: pola-R-CHP merely delays inevitable relapses after 2 y | ||||||||||
| pola-R-CHP | 88.6% | 76.6% | 78.2% | 62.6% | 11.47 | 11.06 | 0.17 | 514 833 | 172 000 | 1 011 765 |
First, the clinical outcomes (2- and 5-year OS and PFS) are displayed. Then effectiveness is displayed as life-years as well as QALYs. The costs displayed are the total lifetime costs related to DLBCL treatment for each strategy.
Sensitivity analyses
One-way sensitivity analyses were performed on all model inputs for the Markov decision process model (supplemental Sections V and VI). At a WTP of $150 000/QALY, the model was sensitive to the cost of pola-R-CHP as well as the 5-year PFS for both pola-R-CHP and R-CHOP (supplemental Figure 12). At a WTP of $100 000/QALY, the model was additionally sensitive to the cost of R-CHOP and CAR-T therapy, as well as the probability of progression after CAR-T therapy (supplemental Figure 13).
Sensitivity analyses of 5-year PFS
Scenario 2: pola-R-CHP decreases risk of relapse between 2 and 5 years
In this scenario, pola-R-CHP had a lower risk of progression between 2 and 5 years compared with R-CHOP, which corresponded with a 5-year PFS of 75% with pola-R-CHP. This scenario models that the PFS curves of pola-R-CHP and R-CHOP from the POLARIX trial continue to separate, and the benefit of pola-R-CHP in the 2-year PFS reported in the POLARIX study7 would be increased at 5 years. In this scenario, pola-R-CHP has an incremental effectiveness of 1.23 life-years and 1.51 QALYs with an incremental cost of $45 784. pola-R-CHP is cost-effective at WTPs of $50 000/QALY, $100 000/QALY, and $150 000/QALY (ICER $30 321/QALY) (Table 3).
Sensitivity analyses of the 5-y PFS with pola-R-CHP in DLBCL (compared with R-CHOP)
| Cost-effectiveness . | Proportion of simulations that pola-R-CHP is cost effective at various WTP thresholds . | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 5-y PFS with pola-R-CHP . | Life-years gained . | Effectiveness (QALYs) . | Incremental effectiveness . | Cost (2021 US dollars) . | Incremental cost . | ICER . | $50 000 . | $100 000 . | $150 000 . |
| 60% | −0.21 | 9.40 | −1.49 | 446 006 | $103 173 | Dominated | 21% | 26% | 33% |
| 65% | 0.15 | 11.25 | 0.36 | 432 982 | $90 149 | 250 413 | 36% | 44% | 49% |
| 70% | 0.69 | 11.82 | 0.93 | 417 600 | $70 682 | 80 394 | 49% | 57% | 61% |
| 75% | 1.23 | 12.40 | 1.51 | 377 192 | $45 784 | 30 321 | 57% | 67% | 72% |
| Cost-effectiveness . | Proportion of simulations that pola-R-CHP is cost effective at various WTP thresholds . | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 5-y PFS with pola-R-CHP . | Life-years gained . | Effectiveness (QALYs) . | Incremental effectiveness . | Cost (2021 US dollars) . | Incremental cost . | ICER . | $50 000 . | $100 000 . | $150 000 . |
| 60% | −0.21 | 9.40 | −1.49 | 446 006 | $103 173 | Dominated | 21% | 26% | 33% |
| 65% | 0.15 | 11.25 | 0.36 | 432 982 | $90 149 | 250 413 | 36% | 44% | 49% |
| 70% | 0.69 | 11.82 | 0.93 | 417 600 | $70 682 | 80 394 | 49% | 57% | 61% |
| 75% | 1.23 | 12.40 | 1.51 | 377 192 | $45 784 | 30 321 | 57% | 67% | 72% |
In this table, the 5-year PFS is varied from 60% to 75%, and the clinical outcomes (OS), effectiveness (life-years gained and QALY), and total cost related to DLBCL are displayed.
Scenario 3: pola-R-CHP merely delays inevitable relapses after 2 years
In this scenario, all the patients who did not progress with pola-R-CHP at 2 years progress between 2 and 5 years after diagnosis, leading to a similar PFS between R-CHOP and pola-R-CHP at 5 years (62.7% and 62.6% respectively). This scenario models that the 2-year PFS curves of pola-R-CHP and R-CHOP from the POLARIX study7 converge when extending to 5 years. In this scenario, pola-R-CHP has an incremental effectiveness of 0.10 life-year gained and 0.17 QALY with an incremental cost of $172 000. pola-R-CHP is not cost-effective at a WTP of $150 000/QALY (ICER $1 011 765/QALY).
One- and two-way sensitivity analyses
Cost of pola-R-CHP
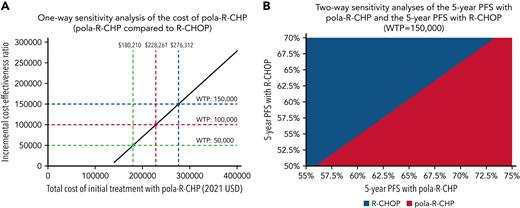
A one-way sensitivity threshold analysis was performed (Figure 4A). We varied the cost of pola-R-CHP and identified that if pola-R-CHP costs less than $276 312, it is cost-effective at a WTP of $150 000/QALY.
One- and two-way sensitivity analyses. (A) One-way sensitivity analysis of the cost of pola-R-CHP. In this analysis, we vary the cost of pola-R-CHP while keeping other parameters constant and show that pola-R-CHP is cost-effective if it costs less than $276 312 (blue dotted lines) at a WTP of $150 000. (B) Two-way sensitivity analysis of the 5-year PFS of pola-R-CHP and 5-year PFS of R-CHOP. In this analysis, we vary the 5-year PFS of pola-R-CHP and the 5-year PFS of R-CHOP simultaneously while keeping other parameters constant. The area shaded red represents the scenarios in which pola-R-CHP is the cost-effective strategy at a WTP of $150 000; the area shaded blue represents the scenarios in which pola-R-CHP is no longer the cost-effective strategy.
One- and two-way sensitivity analyses. (A) One-way sensitivity analysis of the cost of pola-R-CHP. In this analysis, we vary the cost of pola-R-CHP while keeping other parameters constant and show that pola-R-CHP is cost-effective if it costs less than $276 312 (blue dotted lines) at a WTP of $150 000. (B) Two-way sensitivity analysis of the 5-year PFS of pola-R-CHP and 5-year PFS of R-CHOP. In this analysis, we vary the 5-year PFS of pola-R-CHP and the 5-year PFS of R-CHOP simultaneously while keeping other parameters constant. The area shaded red represents the scenarios in which pola-R-CHP is the cost-effective strategy at a WTP of $150 000; the area shaded blue represents the scenarios in which pola-R-CHP is no longer the cost-effective strategy.
5-Year PFS
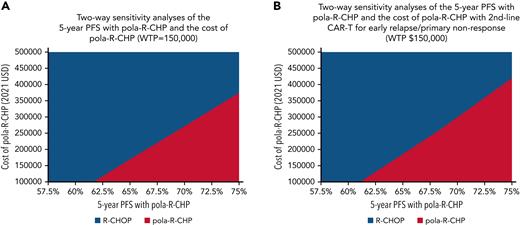
A two-way sensitivity analysis varying the 5-year PFS with pola-R-CHP (from 55% to 75%) and the 5-year PFS with R-CHOP (from 50% to 70%) was performed (Figure 4B). We showed that as long as pola-R-CHP had a 5-year PFS of at least 4.7% higher than that of R-CHOP, it was cost-effective at $150 000/QALY. We varied the cost of pola-R-CHP and the 5-year PFS with pola-R-CHP (Figure 5A) and showed that if the 5-year PFS is 65%, pola-R-CHP would have to cost less than $163 000 to be cost-effective, whereas if the 5-year PFS is 72.5%, it can cost up to $320 000 to be cost-effective.
Two-way sensitivity analyses of the cost of pola-R-CHP and the 5-year PFS of pola-R-CHP. (A) Two-way sensitivity analysis of the 5-year PFS of pola-R-CHP and cost of pola-R-CHP when CAR-T therapy is used in the third-line setting for all patients. In this analysis, we vary the cost of pola-R-CHP and the 5-year PFS of pola-R-CHP simultaneously while keeping other parameters constant. The area shaded red represents the scenarios in which pola-R-CHP is the cost-effective strategy at a WTP of $150 000; the areas shaded blue represent the scenarios in which pola-R-CHP is no longer the cost-effective strategy. (B) Two-way sensitivity analysis of the 5-year PFS of pola-R-CHP and cost of pola-R-CHP when CAR-T therapy is used in the second-line setting for primary refractory/early progression (<12 months). Patients who progress after 12 months receive salvage chemoimmunotherapy as a bridge to auto-SCT. In this analysis, we vary the cost of pola-R-CHP and the 5-year PFS of pola-R-CHP simultaneously while keeping other parameters constant. The area shaded red represents the scenarios in which pola-R-CHP is the cost-effective strategy at a WTP of $150 000; the area shaded blue represents the scenarios in which pola-R-CHP is no longer the cost-effective strategy.
Two-way sensitivity analyses of the cost of pola-R-CHP and the 5-year PFS of pola-R-CHP. (A) Two-way sensitivity analysis of the 5-year PFS of pola-R-CHP and cost of pola-R-CHP when CAR-T therapy is used in the third-line setting for all patients. In this analysis, we vary the cost of pola-R-CHP and the 5-year PFS of pola-R-CHP simultaneously while keeping other parameters constant. The area shaded red represents the scenarios in which pola-R-CHP is the cost-effective strategy at a WTP of $150 000; the areas shaded blue represent the scenarios in which pola-R-CHP is no longer the cost-effective strategy. (B) Two-way sensitivity analysis of the 5-year PFS of pola-R-CHP and cost of pola-R-CHP when CAR-T therapy is used in the second-line setting for primary refractory/early progression (<12 months). Patients who progress after 12 months receive salvage chemoimmunotherapy as a bridge to auto-SCT. In this analysis, we vary the cost of pola-R-CHP and the 5-year PFS of pola-R-CHP simultaneously while keeping other parameters constant. The area shaded red represents the scenarios in which pola-R-CHP is the cost-effective strategy at a WTP of $150 000; the area shaded blue represents the scenarios in which pola-R-CHP is no longer the cost-effective strategy.
Other sensitivity analyses
When we varied the cost of CAR-T therapy, it did not influence the cost-effectiveness of pola-R-CHP at a WTP of $150 000. However, as shown in supplemental Figure 14, if CAR-T therapy costs less than $281 663, pola-R-CHP would no longer be the cost-effective strategy at a WTP of $100 000/QALY.
CAR-T as second-line therapy
As a sensitivity analysis, we changed the model to reflect CAR-T treatment as the second-line therapy for patients with primary refractory or early relapsed (within 12 months of initial therapy) DLBCL, using results from ZUMA-737 to inform our model. Patients with late relapse (>12 months after initial therapy) received salvage chemoimmunotherapy as a bridge to auto-SCT as second-line therapy, with CAR-T as third-line therapy if they progress. In this scenario, pola-R-CHP had a cost of $463 076 compared with a cost of $394 966 with R-CHOP (incremental cost of $72 085). Initial therapy with pola-R-CHP resulted in an additional 0.65 life-year and an incremental 0.94 QALY and was cost-effective at WTPs of $100 000 and $150 000 ($76 686/QALY). In Figure 5B, two-way sensitivity analyses were performed in which we simultaneously varied the costs of pola-R-CHP and the 5-year PFS with pola-R-CHP when CAR-T therapy is used in the second-line setting for primary refractory/early relapse DLBCL. It shows that compared with the primary analysis (in which CAR-T therapy is limited to the third-line setting) at any given 5-year PFS, pola-R-CHP can cost more but still be cost-effective. For example, when CAR-T therapy is limited to the third-line setting, pola-R-CHP can cost up to $163 000 to be cost-effective at a 5-year PFS of 65%. In comparison, when CAR-T therapy is used in the second-line setting, pola-R-CHP can cost up to $185 600 in this scenario to remain cost-effective.
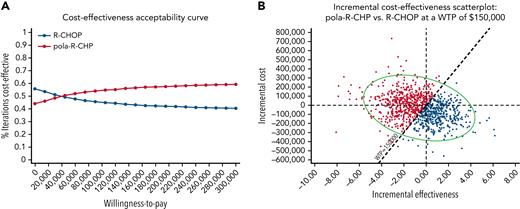
Probability sensitivity analyses
Probabilistic sensitivity analysis was derived by performing 10 000 Monte Carlo model iterations for each strategy. Cost-effectiveness acceptability curves were generated for the model (Figure 6A). At the WTP threshold of $150 000, pola-R-CHP was the cost-effective strategy in 57% of iterations (Figure 6B). At the WTP threshold of $100 000, pola-R-CHP was the cost-effective strategy in 55% of iterations.
Monte-Carlo simulation results. (A) Cost-effectiveness acceptability curves using WTP thresholds for each strategy in untreated DLBCL. The red curve represents the percent iterations pola-R-CHP is the cost-effective strategy at each WTP threshold; the blue curve represents the percent iterations that pola-R-CHP is no longer the cost-effective strategy. (B) Monte Carlo probabilistic sensitivity analysis results in untreated DLBCL at a WTP threshold of $150 000. This figure displays the incremental cost-effectiveness scatter plot comparing pola-R-CHP and R-CHOP, showing the iterations occurring either below the WTP threshold that represent when pola-R-CHP is the cost-effective strategy and the iterations above the WTP threshold that represent when pola-R-CHP is no longer the cost-effective strategy; the oval demonstrates the 95% confidence interval. This visually represents what was found in the cost-effectiveness acceptability curve.
Monte-Carlo simulation results. (A) Cost-effectiveness acceptability curves using WTP thresholds for each strategy in untreated DLBCL. The red curve represents the percent iterations pola-R-CHP is the cost-effective strategy at each WTP threshold; the blue curve represents the percent iterations that pola-R-CHP is no longer the cost-effective strategy. (B) Monte Carlo probabilistic sensitivity analysis results in untreated DLBCL at a WTP threshold of $150 000. This figure displays the incremental cost-effectiveness scatter plot comparing pola-R-CHP and R-CHOP, showing the iterations occurring either below the WTP threshold that represent when pola-R-CHP is the cost-effective strategy and the iterations above the WTP threshold that represent when pola-R-CHP is no longer the cost-effective strategy; the oval demonstrates the 95% confidence interval. This visually represents what was found in the cost-effectiveness acceptability curve.
Discussion
This study is the first, to our knowledge, to evaluate the cost-effectiveness of pola in combination with frontline chemoimmunotherapy in DLBCL. Assuming that PFS improvement with pola-R-CHP is maintained long-term (70% 5-year PFS), it was the cost-effective treatment at a WTP of $150 000/QALY compared with R-CHOP. Its cost-effectiveness was highly dependent on the 5-year PFS and the reduction in the number of patients who need subsequent CAR-T therapy that is associated with substantial costs. Its cost-effectiveness was also dependent on a modeled long-term mortality-benefit with pola-R-CHP compared with R-CHOP, which has not yet been seen clinically due to the short follow-up of POLARIX. pola-R-CHP was not cost-effective in a substantial minority of Monte Carlo iterations, indicating there are clinical scenarios and health care settings in which it may not be the cost-effective strategy.
It is important to note that the cost-effectiveness of pola-R-CHP is highly sensitive to the long-term PFS of pola-R-CHP and R-CHOP. Assuming the same rate of progression between 2 years and 5 years with the 2 strategies (5-year PFS of 69.6% with pola-R-CHP and 62.7% with R-CHOP), we showed that pola-R-CHP is cost-effective at WTPs of $100 000 and $150 000. There are several reasons why this assumption is likely to reflect real-world experience with pola-R-CHP. The majority of disease relapses for DLBCL after frontline chemoimmunotherapy occur within the first 2 years after diagnosis.39 Prior studies have reported that the incidence of relapse between 2 and 5 years after initial treatment for DLBCL ranges between 6.9% and 8%.40,41 These studies show that patients with DLBCL who do not relapse within 2 years of diagnosis will have excellent clinical outcomes and a life expectancy similar to that of the age- and sex-matched general population at 5 years.40 We thus anticipate that the absolute difference in the 5-year PFS of both pola-R-CHP and R-CHOP will be largely similar to that of the 2-year PFS reported in the recent POLARIX trial, in which case the use of frontline pola-R-CHP will be the cost-effective strategy. We did model both best-case and worst-case scenarios to provide the range of cost-effectiveness scenarios for pola-R-CHP as it can vary widely in different settings. In the best-case scenario in which there is a possibility that the pola-R-CHP has a lower rate of progression between 2 and 5 years compared with R-CHOP, pola-R-CHP would be cost-effective at WTPs of $50 000, $100 000, and $150 000. In the worst-case scenario, it is possible that rather than preventing relapse, pola-R-CHP simply delays relapse, and more patients treated with pola-R-CHP will have progression of disease between 2 and 5 years. In this setting, pola-R-CHP would no longer be cost-effective as we showed that it has to have at least a 4.7% improvement in the 5-year PFS to remain cost-effective at a WTP up to $150 000. This highlights the importance of understanding long-term outcomes with pola-R-CHP to truly determine its cost-effectiveness.
The reason for frontline pola-R-CHP being cost-effective despite its substantially higher upfront cost is due to the high costs of subsequent therapy for patients with RR-DLBCL, especially CAR-T therapy. Indeed, we show that pola-R-CHP would not be cost-effective at a WTP of $100 000 if the cost of CAR-T therapy is lower than $281 663. We also show that pola-R-CHP would be more cost-effective at a WTP of $150 000 when CAR-T therapy is used in the second-line setting. Two recent randomized phase 3 studies evaluating second-line CAR-T therapy compared with standard of care have shown that second line CAR-T therapy has improved PFS compared with standard-of-care therapy for DLBCL patients with primary refractory or early relapsed disease.37,38 This led to the recent US Food and Drug Administration approval of second-line axi-cel for primary refractory or early relapsed DLBCL.37 Thus, we anticipate that the use of frontline pola-R-CHP will become even more cost-effective as second-line CAR-T therapy is adopted clinically (ICER of $76 686/QALY compared with $84 308/QALY when CAR-T therapy is the third-line option). Our results also suggest that in settings in which CAR-T therapy is less expensive or not widely available, pola-R-CHP would no longer be cost-effective. Finally, if the costs of CAR-T therapy can be decreased through alternative mechanisms of payment such as reimbursement only for successful infusion and increased competition with the advent of more products such as allogeneic off-the-shelf CAR-T therapy, pola-R-CHP may no longer be the cost-effective strategy at a more conservative WTP threshold such as $100 000/QALY.
There are ∼29 108 new cases of DLBCL per year in the United States.42 A previous study showed that the direct health care costs for the average patient with DLBCL was $213 589 in the first year after diagnosis, which corresponds to $6.2 billion in total health care expenditures.43 If pola-R-CHP is adopted as frontline therapy for all patients with DLBCL in the United States, this will lead to an increase of $1.8 billion in health care expenditures. This highlights that interventions to potentially decrease the cost of pola-R-CHP or identify subpopulations that derive the highest benefit would improve its budget impact. Although the POLARIX trial was not powered to identify differences between subgroups, an exploratory analysis found that there were higher response rates with pola-R-CHP in patients aged >60 years, no bulky disease, an International Prognostic Index score of 3 to 5, and in ABC subtype, all subpopulations in which pola-R-CHP may be more cost-effective.
Prior cost-effectiveness and decision analyses have been performed to help guide payers, clinicians, and patients regarding the choice of treatment strategies for DLBCL. In the frontline setting, multiple cost-effective analyses have shown that R-CHOP is cost-effective when compared with CHOP alone at a WTP of $50 000 to $100 000 per life-year gained.8,9,44 A subsequent cost-effectiveness analysis showed that a precision medicine–based approach with the use of novel agents added to R-CHOP for ABC subtype and R-CHOP for GCB subtype of DLBCL was cost-effective compared with R-CHOP alone for all patients.45 In the relapsed refractory setting, a prior cost-effectiveness analysis found that third-line CAR-T therapy might be cost-effective at a WTP of $150 000 depending on its long-term outcomes.11 More recently, a study reported that the combination of pola with BR in transplant-ineligible RR-DLBCL patients in the third-line setting was cost-effective compared with BR alone at a WTP of $100 000.31 Our study is novel given that it examines the cost-effectiveness of pola combined with chemoimmunotherapy in the frontline setting and also models several different treatment options in the RR setting (ie, third- and second-line CAR-T therapy) and how the treatment in the relapsed setting affects the cost-effectiveness of pola-R-CHP in the frontline setting.
Cost-effectiveness analyses provide useful model-based simulations and a framework for predicting the cost-to-benefit ratio of different therapeutic modalities for a certain disease state by balancing the cost of the intervention against the benefit; however, they are also accompanied by some limitations. As with any oncologic decision model process, the rates of response, remission, and relapse within each treatment group influence the model outputs. We estimated these rates by using the POLARIX trial and historical data for DLBCL. However, we lack long-term outcome data for pola-R-CHP, which is the main limitation of this analysis. To address this limitation, we modeled a variety of 5-year PFS estimates and scenarios. In addition, our base case analysis assumed the same rate of progression after 2 years in the R-CHOP and pola-R-CHP arms. This assumption is both biologically plausible46 and consistent with prior clinical studies in DLBCL showing low rates of relapse after 2 years and that there was no excess mortality associated with lymphoma in patients who were disease-free at 2 years.40,41
Another limitation is that we only included direct health care costs, which may not account for indirect costs such as absenteeism or productivity loss. We also looked at the cost only from the health care perspective. The cost of pola can vary with different payers and in different health care settings, which may influence the cost-effectiveness. It is also possible that some of these costs may be transmitted to patients who will likely have a different WTP threshold and cost-benefit ratio from their standpoint. Another limitation is that we used data from a prospective clinical trial. As pola-R-CHP is used in real-world practice, certain inputs such as the rate of adverse events or treatment discontinuation may be substantially different given that the patient population represented in trials is typically younger and healthier than the real-world population. Furthermore, patients aged >80 years were not studied in the POLARIX study, and thus the cost-effectiveness of pola-R-CHP in these patients who comprise a substantial proportion of the DLBCL population is unknown. Lastly, we were unable to model evolving therapies that may be used for RR-DLBCL such as bispecific T-cell engagers or other novel antibody-drug conjugates.
In conclusion, although pola-R-CHP showed an improvement in PFS but not in OS with short follow-up, this cost-effective analysis shows that the addition of pola to chemoimmunotherapy for frontline management of DLBCL may be cost-effective at a WTP of $150 000 if the efficacy benefit of pola-R-CHP is sustained long term. These analyses can help provide support for clinicians’ utilization of pola-R-CHP and identification of patient populations in which it may be the most cost-effective. Widespread adoption of pola-R-CHP in the frontline setting will lead to substantial increased direct health care costs. Further efforts are needed to reduce the cost of pola and other novel therapies in the first- and second-line setting for DLBCL.
Authorship
Contribution: S.K.T. and N.R.T. designed the study; S.K.T. and N.R.T. acquired the data; S.K.T., N.R.T., and M.S. analyzed the data; S.K.T., N.R.T., M.S., A.F.H., and Y.S. interpreted the data and performed the statistical analysis; S.K.T., N.R.T., and A.F.H. wrote the manuscript; and S.P., L.E.B., A.V.D., M.G.M., L.L.P., Y.-P.W., T.S., J.Z., S.J.F., L.W.K., and S.T.R. helped revise the manuscript. All authors discussed the data and the analysis methods and contributed to the manuscript.
Conflict-of-interest disclosure: L.E.B. reports research funding from Merck, Inc., Amgen, AstraZeneca, and Mustang Bio; and consultancy for Novartis, Gilead, F. Hoffmann–La Roche Ltd., BeiGene, and Genentech, Inc. A.V.D. reports consultancy, honoraria, and research funding from Genentech, Bayer Oncology, and AstraZeneca; research funding from Takeda Oncology, Gilead Sciences, and Secura Bio; consultancy and research funding from TG Therapeutics; consultancy and honoraria from AbbVie and Beigene Pharmacyclics; honoraria and research funding from Bristol Myers Squibb; and honoraria from Rigel Pharm. M.G.M. reports honoraria from Janssen and EUSA; and research funding from TG Therapeutics, Epizyme, BMS, MorphoSys, and Beigene. T.S. reports research funding from Kite, a Gilead Company, TG Therapeutics, Celgene, and BeiGene; speakers bureau for Janssen and Seattle Genetics; consultancy, research funding, and speakers bureau from AstraZeneca and PCYC; and consultancy and other (travel support, research funding) from Juno Therapeutics. L.L.P. reports other (food) from Hoffmann–La Roche; and other (travel) from Novartis and Pfizer. J.Z. reports research funding from Secura Bio, Daiichi Sankyo, and AbbVie; honoraria from Kyowa Kirin, Secura Bio, and Seattle Genetics; and consultancy for Secura Bio, Ono, Legend, Kyowa Kirin, Myeloid Therapeutics Verastem, and Daiichi Sankyo. S.J.F. reports consultancy and current holder of individual stocks in a privately held company for Mustang Bio and Lixte Biotechnology; and consultancy for Allogene. L.W.K. reports consultancy and current equity holder in publicly traded company for PeproMene Bio, Inc. A.F.H. reports consultancy and research funding from AstraZeneca, ADC Therapeutics, Merck, Genentech, Bristol Myers Squibb, and Seagen; consultancy for Tubulis, Takeda, and Karyopharm; and research funding from Kite, a Gilead Company and Gilead Sciences. The remaining authors have no conflicts to disclose.
Correspondence: Nikhil R. Thiruvengadam, 11234 Anderson St, MC 1516, Loma Linda, CA 92354; email: NThiruvengadam@llu.edu.
References
Author notes
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement”in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal