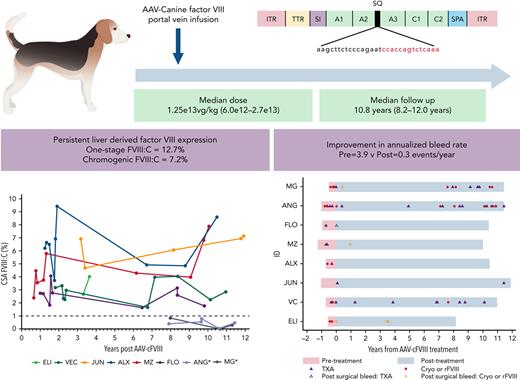
Key Points
Liver-derived FVIII expression with correction of the bleeding phenotype was seen for >10 years after a single AAV-BDD-cFVIII infusion.
No evidence of liver malignancy or chronic liver disease was seen at postmortem.
Abstract
Questions remain concerning the long-term efficacy, safety, and site(s) of transgene expression following adeno-associated vector (AAV) therapy. We report a long-term follow-up of 8 (male = 4, hemizygous, and female = 4, homozygous) dogs with severe hemophilia A treated with a single portal vein infusion of a B-domain–deleted (BDD)-canine FVIII (cFVIII) AAV vector (median dose = 1.25 × 1013 vg/kg, AAV2 = 4, AAV6 = 3, and AAV8 = 1). After a median follow-up of 10.8 years (8.2-12.0 years), persistent FVIII:C (median one-stage = 12.7%, chromogenic = 7.2%) was seen in all responding dogs (n = 6), with improvement in annualized bleed rates (pre = 3.9 vs post = 0.3 event per year; P = .003). Anti-AAV capsid neutralizing antibodies (nAbs) toward the dosed capsid were detected throughout the study, with limited cross-reactivity to other capsids. nAb titers for all capsid serotypes declined with time, although they remained at levels precluding redosing with the same capsid. AAV-BDD-cFVIII DNA was detected in the liver of all dogs (median = 0.15 vg per diploid genome), with lower levels in the spleen in 4 dogs (median = 0.005 vg per diploid genome). Consistent with the liver-specific promoter, BDD-cFVIII mRNA was only detected in the liver. Postmortem examination demonstrated no evidence of chronic liver disease or liver malignancy. Persistent FVIII expression and an improved bleeding phenotype was seen for more than a decade after vector delivery. This is the longest follow-up reported in a preclinical model supporting long-term efficacy and safety of AAV-mediated gene therapy.
Introduction
Hemophilia A is an X-linked inherited bleeding disorder resulting in deficiency of coagulation factor VIII (FVIII). Individuals with a complete lack (<1%) of functional FVIII protein (severe hemophilia A) suffer recurrent bleeding events affecting the joints. To prevent serious bleeding and long-term bleed-related consequences, regular IV infusion using exogenous FVIII concentrates as prophylaxis is the current standard of care. Despite the benefits of this approach, prophylaxis is associated with significant impact on patients’ daily life, alloimmunity, and societal cost.
Since the sequencing of the FVIII and FIX genes in the 1980s,1,2 there has been a hope that gene replacement therapy could offer a long-term curative treatment for hemophilia. Adeno-associated viral (AAV) vectors have become the most studied approach for in vivo gene delivery in preclinical and clinical studies, owing to lack of pathogenicity of the wild-type virus and proposed extrachromosomal (episomal) mechanism of persistence. Despite increasing numbers of ongoing phase 3 hemophilia AAV studies,3 there remain questions surrounding the long-term durability and safety of this therapeutic approach. The hemophilia A dog model offers an important preclinical resource to evaluate some of these questions concerning long-term efficacy and safety. The hemophilia A dog models maintained at Queen’s University and the University of North Carolina display genotypic and phenotypic similarities to severe hemophilia A in humans. These animals possess a naturally occurring intron-22 inversion-like mutation in the canine F8 gene,4 exhibit a spontaneous bleeding phenotype, and demonstrate alloimmunity toward infused FVIII.5-7 In the Queen’s University dogs, we have previously reported evidence of early efficacy of treatment with an AAV-B–domain-deleted (BDD) cFVIII vector, in a cohort of 8 of these animals for between 0.5 and 3.3 years.8,9 In this report, we describe the long-term outcomes of this treatment after a decade of follow-up focusing on clinical outcomes, safety, immune responses, and FVIII expression.
Materials and methods
AAV vector structure and construction
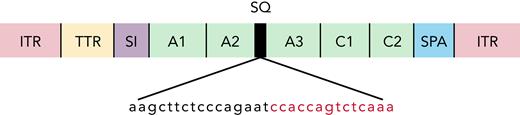
AAV vectors were constructed as has been described previously.9 The AAV-cFVIII construct consists of a 202 nucleotide (nt) transthyretin promoter, synthetic chimeric intron (106 nt, EF1 splice donor, and immunoglobulin G splice acceptor), noncodon optimized canine B-domain deleted SQ-FVIII cDNA (4367 nt), and 46 nt synthetic polyadenylation sequence (Figure 1; supplemental data, available on the Blood Web site). Vector genomes (VGs) were packaged into either AAV2, AAV6, or AAV8 capsids by triple transfection into 293 cells. VG titration was performed using quantitative real-time polymerase chain reaction (qRT-PCR) using a linearized plasmid DNA standard.
Noncodon optimized AAV-cFVIII-BDD vector structure. ITR, inverted tandem repeat; SI, synthetic chimeric intron; SPA, synthetic polyadenylation sequence; TTR, transthyretin promoter.
Noncodon optimized AAV-cFVIII-BDD vector structure. ITR, inverted tandem repeat; SI, synthetic chimeric intron; SPA, synthetic polyadenylation sequence; TTR, transthyretin promoter.
Animal procedures and testing
All animal procedures were performed in accordance with the “Guide for the Care and Use of Laboratory Animals” and Canadian Council for Animal Care and reviewed by the University Animal Care Committee. AAV-cFVIII vector was administered into the portal vein at laparotomy under general anesthesia as previously described (supplemental data).8 All animals received canine cryoprecipitate before and following surgery.10 Testing for blood counts and serum chemistry was performed throughout the study. Humane study endpoints were selected based on a range of chronic age-related pathologies (arthritis = 5, prostatitis = 2, pyometra = 2). Postmortem examinations were performed on all animals by a registered veterinary pathologist with tissue samples formalin fixed/paraffin embedded and/or flash frozen in liquid nitrogen and stored at −80°C.
Bleed definition
Bleeds were defined based on the recommendation of the International Society of Thrombosis and Haemostasis/Scientific and Standardization Committee and classified based on location and treatment received.11 New bleeding events were defined as any bleed occurring either in a new location or within the same location ≥72 hours after cessation of initial treatment. Clinically significant bleeding events included those in which specific hemostatic treatment was given (tranexamic acid [TXA] or canine FVIII [cFVIII]).
WBCT, FVIII, and inhibitor testing
Whole blood clot times (WBCTs) were performed immediately after collection into 2 glass tubes and incubated in a 37°C water bath. One-stage FVIII activity (OSA) was measured on an automated coagulometer (Siemens BCS-XP; Siemens Healthcare GmbH, Germany) using a canine normal reference curve prepared using canine pooled normal plasma sourced from local plasma donations (12 to 20 normal dogs) diluted in Owren veronal buffer and 2.5% cFVIII-deficient plasma. The limit of sensitivity of this assay is 2%. Longitudinal measurement of chromogenic FVIII assay was performed using the Biophen (HYPHEN BioMed, France) chromogenic FVIII substrate assay (CSA). For CSA assays, study samples were diluted 1:10 in assay buffer as per kit protocol and run against a canine pooled plasma standard curve generated by serial dilution with in-house pooled cFVIII-deficient plasma. The standard curve range was 0.004 to 1.0 U/mL, with the linear range being 0.03 to 0.25 U/mL. Inhibitor testing was performed using the Bethesda assay, with an assay cutoff of >0.6 BU/mL.12
AAV antibody testing
HEK293T cells were seeded at 4e4 cells per well in a 96-well plate and incubated overnight. Cesium chloride density gradient purified AAV-luciferase vectors were diluted in phosphate-buffered saline (PBS) + 1% bovine serum albumin (BSA) to 4e6 vg/uL and mixed 1:1 with serial dilutions of plasma in PBS + 1% BSA for 1 hour. The recombinant AAV/plasma mixture was added to the HEK293T cells at a multiplicity of infection of 1000 with 10 μM Etoposide and incubated at 37°C. Transduction percentage was assessed by luciferase activity at 72 hours and measured in relative luminescence units relative to control transductions (vector and PBS + 1% BSA). Transductions were performed in duplicate on separate plates. Titer is the calculated plasma dilution where average transduction equals 50%, plotted on a line between serial dilutions. Minimum required dilutions were 1/4 for AAV2, AAV6, and AAV8 and 1/16 for AAV5 owing to sample limitations.
In situ hybridization and immunohistochemistry
Formalin-fixed paraffin-embedded canine liver sections (5 μm) were collected on Superfrost Plus slides using RNAse-free conditions. A singleplex in situ hybridization protocol was performed on serial sections using a Ventana Discovery Ultra Autostainer (Tucson, AZ) and an RNAscope (Newark, CA), Universal 2.5 Reagent Kit, with custom-generated probes to detect cFVIII DNA and RNA. Hepatocytes staining positive for cFVIII DNA or RNA were quantified using Visiopharm image analysis software (Hoersholm, Denmark). For immunohistochemistry (IHC), sections were stained using an anti-cFVIII primary (1:1000, SAC8C-IG; Affinity Biologics) and donkey anti-sheep AF-647 conjugated secondary antibody (1:1000, A21448; Invitrogen). Autofluorescence was captured using an excitation filter of 488 nm. Slides were imaged on an Axio Scan.Z1 (Zeiss) slide scanner using a Plan-Apochromat 40×/0.8 objective equipped with a Hamamatsu Orca Flash camera.
RT-qPCR
Genomic DNA was purified using the DNeasy Blood and Tissue kit (Qiagen, Germany), following the kit protocol with addition of 4 μL RNase A (100 mg/mL) from ≥3 tissue areas from the same region and pooled. RNA isolation was performed using the RNeasy mini kit (Qiagen). TaqMan quantitative PCR (qPCR) and qRT-PCR were performed using custom primers and probes (Thermo Fisher, Wilmington, DE) targeting the SQ-linker region of BDD-cFVIII (supplemental Table 6). The standard curve was constructed using a linear double-stranded DNA gene fragment (Integrated DNA Technologies, Coraville, IA). DNA concentration and purity were measured using a NanoDrop spectrophotometer. The limit of detection was ≥0.25 copy per ng DNA (0.0012 copies per diploid genome) (qPCR) and ≥1 copy per ng RNA (qRT-PCR).
Statistical analyses
Descriptive data are summarized as mean, median, range, and frequencies. Annualized bleed rates (ABRs) are presented as both raw and estimated bleed rates. Raw bleed rates were calculated using the number of bleed events divided by the time under observation. Bleed rate estimations were also performed using a negative binomial model to account for overdispersion of events. Outcomes were modeled using mixed-effects linear regression using restricted maximum likelihood. All tests were 2-tailed with a P value of <.05 used for statistical significance. Analyses were performed using Stata v. 13.0 (StataCorp LLC, TX) and GraphPad Prism v. 9.0.0 for Windows (GraphPad Software, CA).
Results
Eight dogs with severe hemophilia A were treated with AAV-cFVIII (AAV2 = 4, AAV6 = 3, and AAV8 = 1) at a median dose of 1.25 × 1013 vg/kg (6.0 × 1012-2.7 × 1013). A dose escalation protocol (6e12-2.7e13 vg/kg) was used for dogs treated with AAV2 capsid (Table 1). The median age at treatment was 9.5 months (range, 6-15 months), and dogs were followed up for a median of 10.8 years (8.2-12.0 years). Prior to vector infusion, 7 dogs had received previous exposures to canine cryoprecipitate. All animals had negative cFVIII inhibitor screening prior to vector infusions. Vector administration was performed by infusion into the portal vein at laparotomy. Perioperative hemostasis was obtained with pre- and postprocedure canine cryoprecipitate. Vector infusions were well tolerated, although 1 animal (ALX) developed an episode of tachycardia/hypotension and facial swelling. This reaction may relate to the rapidity/temperature of vector infusion (20 seconds).
Study overview and outcomes
| ID sex . | Capsid (dose in vg/kg) . | Treat age (y) . | Follow-up (y) . | Post ABR (pre ABR) . | Pre . | Early WBCT Y0-Y3 CSA FVIII:C Y0-Y4 . | Mid WBCT Y3-Y7 CSA FVIII:C Y4-Y8 . | Late WBCT Y7-Y11 CSA FVIII:C Y8-Y12 . | Final CSA FVIII:C (OSA FVIII:C) . | Liver DNA vg/dg (RNA copies/ng) . | Spleen DNA vg/dg (RNA copies/ng) . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WBCT (min) . | WBCT (min) . | FVIII:C (%) . | WBCT (min) . | FVIII:C (%) . | WBCT (min) . | FVIII:C (%) . | ||||||||
| ELI (F) | 2 6.0e12 | 0.6 | 8.2 | 0.4 (5.2) | 15.9 12.0-22.0 | 5.6 3.6-16.0 | 3.4 2.7-4.0 | 5.7 4.4-7.6 | n/a | 4.8 4.6-5.1 | n/a | n/a (n/a) | 0.044 (6.2) | 0.001 (n/d) |
| VC (M) | 2 1.5e13 | 0.7 | 11.0 | 0.7 (7.2) | 15.4 13.1-17.1 | 5.9 3.0-8.7 | 2.8 2.3-3.3 | n/a | 2.8 1.7-4.0 | 6.4 4.9-7.3 | 3.0 2.3-4.0 | 2.9% (7.3%) | 0.592 (8.3) | n/d (n/d) |
| JUN (M) | 2 2.7e13 | 1.0 | 12.0 | 0.2 (0.0) | 15.9 15.7-16.2 | 5.6 3.4-11.9 | 5.8 4.7-6.9 | 4.2 3.2-4.8 | n/a | 5.6 5.1-6.2 | 6.7 6.1-7.2 | 7.2% (12.7%) | 0.247 (15.9) | 0.005 (n/d) |
| ALX (F) | 6 1.0e13 | 0.7 | 10.5 | 0.0 (2.8) | 14.8 13.0-16.2 | 4.3 2.8-6.3 | 6.6 3.7-9.4 | 4.2 2.1-6.2 | 4.9† | 6.8 6.8-6.9 | 6.7 4.9-8.6 | 8.6% (14.9%) | 0.177 (26.4) | n/d (n/d) |
| MZ (M) | 6 1.0e13 | 1.3 | 10.1 | 0.1 (2.1) | 14.9 10.7-18.7 | 4.7 3.3-5.9 | 4.0 2.4-5.8 | 5.9† | 4.3† | 5.2 5.2-5.2 | 6.2 4.0-7.9 | 7.9% (13.9%) | 0.173 (5.9) | n/d (n/d) |
| FLO (F) | 8 1.0e13 | 1.0 | 10.5 | 0.1 (2.1) | 14.5 13.2-16.3 | 5.3 3.1-7.3 | 2.8 1.8-4.1 | 4.1 1.9-6.3 | 1.6† | 3.2† | 2.5 1.8-3.2 | 1.8% (n/a) | 0.127 (4.4) | 0.033 (n/d) |
| ANG∗ (M) | 2 1.5e13 | 1.0 | 11.5 | 1.5 (5.2) | 15.4 14.0-16.0 | 6.8 4.9-9.9 | n/a | 7.8† | 0.4† | 6.0 4.6-6.9 | 0.5 0.0-0.8 | 0.5% (3.2%) | 0.053 (n/d) | 0.005 (n/d) |
| MG∗ (F) | 6 1.7e13 | 0.5 | 11.5 | 0.9 (10.0) | 16.1 15.9-16.3 | 6.9 4.1-9.7 | n/a | 6.4 5.2-7.7 | 0.8† | 7.7 6.2-13.0 | 0.2 0.0-0.3 | 0.3% (1.7%) | 0.014 (n/d) | n/d (n/d) |
| ID sex . | Capsid (dose in vg/kg) . | Treat age (y) . | Follow-up (y) . | Post ABR (pre ABR) . | Pre . | Early WBCT Y0-Y3 CSA FVIII:C Y0-Y4 . | Mid WBCT Y3-Y7 CSA FVIII:C Y4-Y8 . | Late WBCT Y7-Y11 CSA FVIII:C Y8-Y12 . | Final CSA FVIII:C (OSA FVIII:C) . | Liver DNA vg/dg (RNA copies/ng) . | Spleen DNA vg/dg (RNA copies/ng) . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WBCT (min) . | WBCT (min) . | FVIII:C (%) . | WBCT (min) . | FVIII:C (%) . | WBCT (min) . | FVIII:C (%) . | ||||||||
| ELI (F) | 2 6.0e12 | 0.6 | 8.2 | 0.4 (5.2) | 15.9 12.0-22.0 | 5.6 3.6-16.0 | 3.4 2.7-4.0 | 5.7 4.4-7.6 | n/a | 4.8 4.6-5.1 | n/a | n/a (n/a) | 0.044 (6.2) | 0.001 (n/d) |
| VC (M) | 2 1.5e13 | 0.7 | 11.0 | 0.7 (7.2) | 15.4 13.1-17.1 | 5.9 3.0-8.7 | 2.8 2.3-3.3 | n/a | 2.8 1.7-4.0 | 6.4 4.9-7.3 | 3.0 2.3-4.0 | 2.9% (7.3%) | 0.592 (8.3) | n/d (n/d) |
| JUN (M) | 2 2.7e13 | 1.0 | 12.0 | 0.2 (0.0) | 15.9 15.7-16.2 | 5.6 3.4-11.9 | 5.8 4.7-6.9 | 4.2 3.2-4.8 | n/a | 5.6 5.1-6.2 | 6.7 6.1-7.2 | 7.2% (12.7%) | 0.247 (15.9) | 0.005 (n/d) |
| ALX (F) | 6 1.0e13 | 0.7 | 10.5 | 0.0 (2.8) | 14.8 13.0-16.2 | 4.3 2.8-6.3 | 6.6 3.7-9.4 | 4.2 2.1-6.2 | 4.9† | 6.8 6.8-6.9 | 6.7 4.9-8.6 | 8.6% (14.9%) | 0.177 (26.4) | n/d (n/d) |
| MZ (M) | 6 1.0e13 | 1.3 | 10.1 | 0.1 (2.1) | 14.9 10.7-18.7 | 4.7 3.3-5.9 | 4.0 2.4-5.8 | 5.9† | 4.3† | 5.2 5.2-5.2 | 6.2 4.0-7.9 | 7.9% (13.9%) | 0.173 (5.9) | n/d (n/d) |
| FLO (F) | 8 1.0e13 | 1.0 | 10.5 | 0.1 (2.1) | 14.5 13.2-16.3 | 5.3 3.1-7.3 | 2.8 1.8-4.1 | 4.1 1.9-6.3 | 1.6† | 3.2† | 2.5 1.8-3.2 | 1.8% (n/a) | 0.127 (4.4) | 0.033 (n/d) |
| ANG∗ (M) | 2 1.5e13 | 1.0 | 11.5 | 1.5 (5.2) | 15.4 14.0-16.0 | 6.8 4.9-9.9 | n/a | 7.8† | 0.4† | 6.0 4.6-6.9 | 0.5 0.0-0.8 | 0.5% (3.2%) | 0.053 (n/d) | 0.005 (n/d) |
| MG∗ (F) | 6 1.7e13 | 0.5 | 11.5 | 0.9 (10.0) | 16.1 15.9-16.3 | 6.9 4.1-9.7 | n/a | 6.4 5.2-7.7 | 0.8† | 7.7 6.2-13.0 | 0.2 0.0-0.3 | 0.3% (1.7%) | 0.014 (n/d) | n/d (n/d) |
ABR, annualized bleeding rate; dg, diploid genome; F, female; M, male; n/a, sample not available; nd, not detected.
Nonresponder (CSA < 1%).
Single sample available.
Long-term stable FVIII expression with normalization of the WBCT
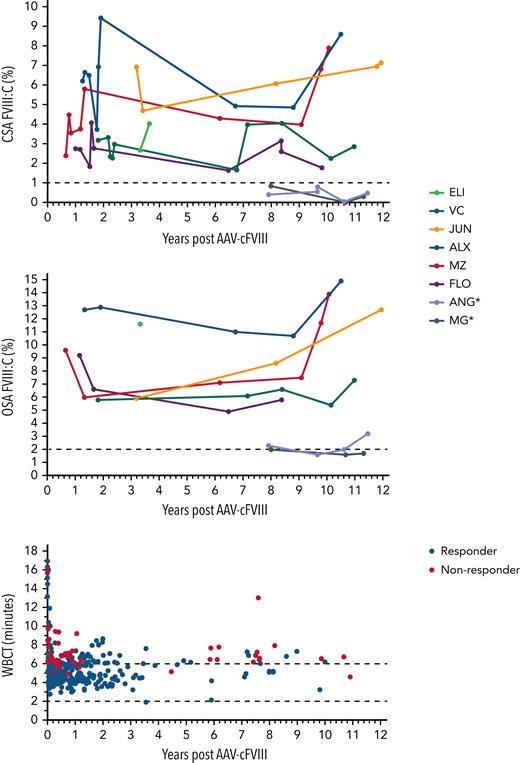
FVIII expression (>1% CSA FVIII:C) was seen in 6 dogs throughout follow-up. The median endpoint CSA and OSA were 7.2% and 12.7%, respectively, in responding dogs after 10.7 years (8.2-12.0 years) of follow-up (Table 1; Figure 2). To evaluate whether changes were seen in FVIII:C over the duration of follow-up, a collection of cryopreserved samples (0.7-12.0 years postdosing) were analyzed using the same FVIII assays per standard, and FVIII:C was modeled using hierarchical linear regression. Within the responding dogs, a trend toward a small increase in FVIII:C per year (coeff = 0.078 per year, P = .12) was seen. In the final sampling points of 2 dogs (MZ and ALX), there appeared to be a small increase in FVIII seen in both CSA and OSA assays (Figure 2). Comparison was then made for FVIII:C values depending on the testing methodology and standard used. Significant correlation (r = 0.76, P < .001) was seen between the results of the OSA and CSA assays when a canine normal pooled plasma standard was used, with higher FVIII:C (median ratio, 1.9; range, 0.9-6.7) for the OSA compared with CSA, in keeping with previous studies.13
Long-term expression of FVIII:C following AAV-FVIII with improvement in global hemostatic profiles. CSAFVIII, chromogenic substrate assay FVIII; OSAFVIII, one-stage assay FVIII; WBCT, normal range: 2-6 minutes. ∗Nonresponder.
Long-term expression of FVIII:C following AAV-FVIII with improvement in global hemostatic profiles. CSAFVIII, chromogenic substrate assay FVIII; OSAFVIII, one-stage assay FVIII; WBCT, normal range: 2-6 minutes. ∗Nonresponder.
Rapid improvements in the WBCT were seen in all dogs within the first weeks following treatment, with a reduction in WBCT observed in all dogs (pre = 15.4 vs post = 5.6 minutes, P < .001). This reduction in WBCT was significant in both responders and nonresponders. There was a significant difference (−1.5 minutes, 95% confidence interval: −2.3 to −0.7; P < .001) in modeled WBCT in the 6 responders (5.1 minutes) compared with the 2 nonresponders (6.6 minutes) (Figure 2). This correlates to a normalization of mean WBCT in the responders (normal range, 2 to 6 minutes). WBCT demonstrated little interindividual variation (±0.2 minutes) and demonstrated no significant change with time (coefficient per year = 0.0025, P = .469).
Long-term improvement in bleeding phenotype following AAV-cFVIII
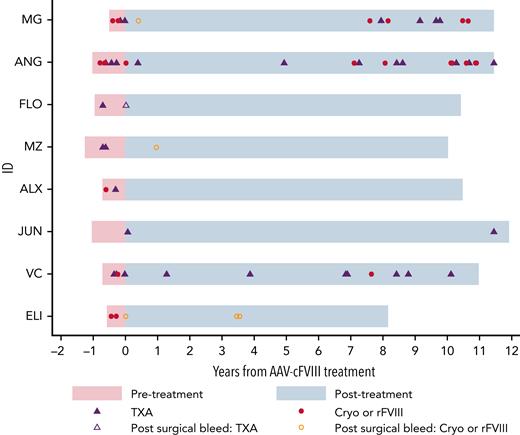
ABRs were compared prior to and following treatment, with a median observation period of 0.8 years (range, 0.5-1.3) and 10.6 years (range, 8.2-12.0), respectively. After performing a sensitivity analysis of events that did not likely represent bleeds (n = 11), there were 84 bleeding events (pre = 30; post = 54), with reduction in mean ABR from 5.4 to 0.6 events per year (P = .014). A subgroup analysis was then performed on clinically significant bleeding events (n = 65), where a hemostatic agent (tranexamic acid, TXA; canine cryoprecipitate or recombinant cFVIII) was administered. There were 24 events prior (TXA = 14 and Cryo = 10) and 41 (TXA = 22 and Cryo/rFVIII = 19) post–gene therapy (Figure 3), with a significant reduction in mean ABR from 4.3 vs 0.5 events per year (P = .009). Most bleeding episodes, post–gene therapy occurred either in the 2 nonresponding animals (63.4%, 26/41) or following surgery in the responders (12.2%, 5/41: post-AAV infusion = 2 and post–liver biopsy = 3). Bleed rates were then modeled to account for overdispersion of events and to directly compare rates in responding and nonresponding animals. In this model, the pretreatment estimated ABR was 3.9, with significantly lower posttreatment estimated bleed rates, in both responding (0.28, P < .001) and nonresponding (1.2, P = .003) dogs. This represents a 13.9-fold (92.8%) and 3.2-fold (69.2%) reduction in bleed rates in responding and nonresponding dogs, respectively.
Reduction in ABR following AAV-cFVIII infusion. Time shown in pink represents pretreatment and blue represents posttreatment. Cryo, canine cryoprecipitate; rFVIII, recombinant canine B-domain deleted FVIII.
Reduction in ABR following AAV-cFVIII infusion. Time shown in pink represents pretreatment and blue represents posttreatment. Cryo, canine cryoprecipitate; rFVIII, recombinant canine B-domain deleted FVIII.
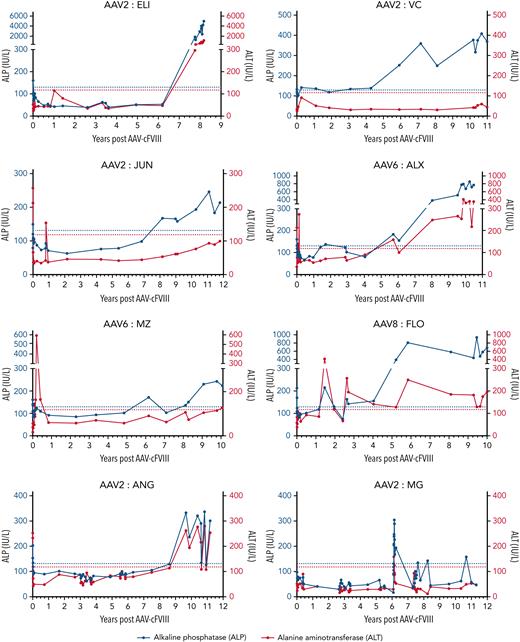
Liver enzyme monitoring following AAV-cFVIII
The predominant change seen on blood testing was asymptomatic elevations in liver enzymes. In the earliest part of the study, mild transient elevation in alkaline phosphatase (ALP) levels was seen postinfusion, most likely relating to surgery and/or corticosteroid premedication. Progressive elevation in ALP levels (≥ upper limit of normal [ULN]) was seen in all animals at a median of 6.9 years following dosing (age = 7.5 years; range, 2.5-10.7 years). At the study endpoint, 7/8 dogs had ALP > ULN (>2 ULN, n = 5, and >ULN and <2 ULN, n = 2). Similar, although less marked changes were seen in the alanine aminotransferase (ALT) levels, with elevation (> ULN) in 5 dogs at a median of 7.5 years (2.4-10.7 years) postdosing (Figure 4B). Similar age-related elevations in liver function tests have been seen in dogs within our colony who have not received gene therapy (data not shown). A summary of all non–bleed-related study events is provided within the supplemental data.
Longitudinal monitoring of liver enzymes following AAV-cFVIII infusion. ALP levels are plotted on the left y-axis (blue). ALT levels are plotted on the right y-axis (red). Horizontal hatched lines represent the ULN for the ALP (blue) and ALT (red) assays.
Longitudinal monitoring of liver enzymes following AAV-cFVIII infusion. ALP levels are plotted on the left y-axis (blue). ALT levels are plotted on the right y-axis (red). Horizontal hatched lines represent the ULN for the ALP (blue) and ALT (red) assays.
Immunogenicity toward the BDD-cFVIII transgene product and AAV capsid
Inhibitor screening was performed in all dogs over the course of the study, with a total of 349 tests performed post-AAV infusion (median, 37.5 per dog; range, 31-41) (supplemental Figure 1). Frequent testing was performed in the first 2 years of follow-up, and a transient early (≤4 weeks’ duration) inhibitor was detected in 1 dog (JUN) 15 days following vector infusion (peak titer 5.6 BU/mL), as previously reported.9 There was spontaneous resolution of this inhibitor, with a negative Bethesda assay at day 43 and improvement in WBCT to <6 minutes by day 54. Further challenge with canine cryoprecipitate in the context of a liver biopsy (3 infusions), performed at day 302, did not result in inhibitor anamnesis.
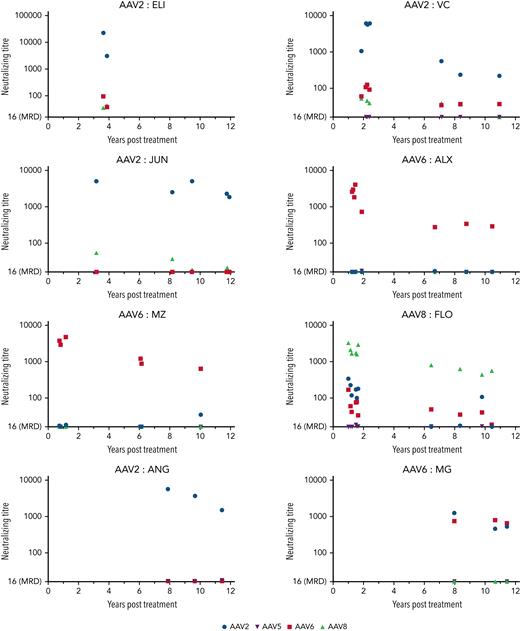
Neutralizing antibodies (nAbs) toward AAV2, 5, 6, and 8 capsids were measured in plasma samples using a sensitive in vitro cell-based luciferase transduction inhibition assay. Samples (n = 44) were available for analysis from all study dogs, with a median of 5.5 time points per animal (range, 2-10). The predominant humoral response was toward the dosed capsid, with a median peak titer of 6485 (range, 761-22 068) at the earliest time point available (3.6 years; range, 1.0-8.0) for analysis. Although some cross-reactivity toward other AAV capsids was seen at earlier time points (<2 years), relatively limited cross-reactivity was seen with longer follow-up (Figure 5). Modeling was performed using a restricted maximum likelihood model, to evaluate the effects of time and capsid toward the dosed neutralizing antibody titer. A significant decrease in titer was seen per year (coefficient −314; P < .001). In addition, lower capsid titers were seen in those dogs treated with AAV6 (coefficient = −2171.1; P < .001) and AAV8 (coefficient = −2171.1; P < .001) compared with those treated with AAV2. Titers on the last available sample showed a median of 2231 (range, 220 - 1864), levels that would very likely prevent redosing toward the originally dosed capsid.
Persistent AAV capsid nAbs are seen following AAV-cFVIII. Neutralizing titer plotted for results above a minimum required dilution of 1/16.
Persistent AAV capsid nAbs are seen following AAV-cFVIII. Neutralizing titer plotted for results above a minimum required dilution of 1/16.
Postmortem examination demonstrates age-related canine liver changes
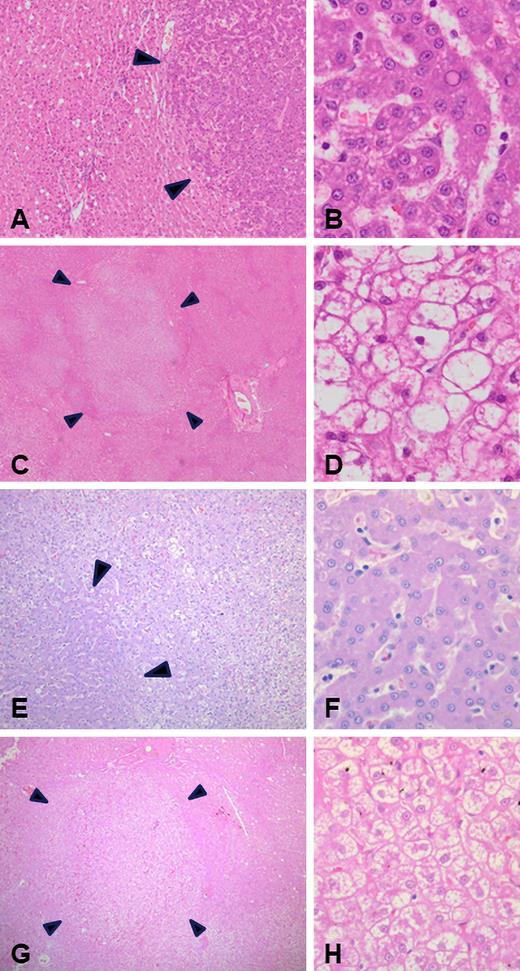
A full postmortem assessment was performed on all dogs, and liver tissue was independently reviewed by 3 blinded veterinary histopathologists, with consensus on hepatic findings (median, 3 sections; range, 2-5; supplemental Table 2). On examination of the liver, there was no evidence of significant parenchymal inflammation, necroinflammatory hepatocyte injury (no hepatitis), or fibrosis. There was no evidence of hepatic adenoma or carcinoma. Focal scarring was seen consistent with previous biopsies. Macroscopically, the predominant finding was that of multifocal parenchymal nodularity, which was seen in all treated dogs. Histopathological assessment demonstrated randomly distributed variable sized areas of hepatocyte proliferative change characterized by preserved reticulin staining with mild focal thickening of liver cell plates, nonencapsulation and retained lobular architecture, and recognizable portal tracts and central veins (Figure 6). These areas of hepatocyte nodular hyperplasia were present in 6 dogs and admixed with foci of liver cell glycogen cytoplasmic vacuolation (periodic acid–Schiff, +ve and periodic acid–Schiff–diastase, −ve), characteristic of canine vacuolar hepatopathy. Additional areas of vacuolar changes were also seen outside of the regions of nodular hyperplasia in several dogs, occasionally in zonal and sometimes multifocal distribution. Additional age-related changes were also seen, including lipogranulomas and variable degrees of pigment accumulation in centrilobular and sometimes mid-zone hepatocytes most likely representing lipofuscin (“wear and tear” pigment). Similar findings of nodular hyperplasia, hepatocyte vacuolation, and periacinar to midzonal pigment accumulation were also seen in an untreated control dog (Figure 6E-H). The etiology for these changes is likely multifactorial, including chronic illness, chronic stress, adrenal hyperplasia (n = 5), corticosteroid therapy (n = 5), and aging. A summary of the additional postmortem findings are shown in supplemental Table 2.
Histopathologic (H&E stain) assessment of canine livers at postmortem in AAV-treated dogs (A-D) and an untreated control (E-H). Foci of hepatocyte nodular hyperplasia (A-B and E-F) characterized by nonencapsulated areas of hepatocyte proliferation (A,E: magnification ×50) with mildly thickened liver cell plates (B,F: magnification ×400). Also observed were ill-defined regions of liver parenchyma pallor due to hepatocyte cytoplasmic clearing (C: magnification ×15; G: magnification ×25), characteristic of canine vacuolar hepatopathy (D,H: magnification ×400).
Histopathologic (H&E stain) assessment of canine livers at postmortem in AAV-treated dogs (A-D) and an untreated control (E-H). Foci of hepatocyte nodular hyperplasia (A-B and E-F) characterized by nonencapsulated areas of hepatocyte proliferation (A,E: magnification ×50) with mildly thickened liver cell plates (B,F: magnification ×400). Also observed were ill-defined regions of liver parenchyma pallor due to hepatocyte cytoplasmic clearing (C: magnification ×15; G: magnification ×25), characteristic of canine vacuolar hepatopathy (D,H: magnification ×400).
Long-term FVIII production following AAV gene therapy is hepatically derived
RT-qPCR was performed on samples of organs collected at postmortem from all 8 study dogs (Table 1; supplemental Table 3). Persistent BDD-cFVIII VGs (DNA) were detected in all postmortem liver samples (median = 30.5 vg/ng; 0.150 vg per diploid genome), including both responding (n = 6) and nonresponding (n = 2) animals. Decline in vg copies was seen relative to biopsies performed earlier in the study (supplemental Table 4).8 BDD-cFVIII VGs were also detected in the spleen in 4 dogs (median = 1.0 vg/ng; 0.005 vg per diploid genome). No AAV BDD-cFVIII VGs were detected within reproductive organs, kidney, lung, lymph node, heart, or skeletal muscle. Similarly, no AAV BDD-cFVIII VGs were detected within a localized soft tissue sarcoma (grade II) seen in 1 dog. To evaluate the source of FVIII expression, messenger RNA (mRNA) was isolated from additional liver and spleen samples and analyzed using qRT-PCR. In keeping with the incorporation of a liver-specific promoter, cFVIII mRNA expression was only detected in the liver (median = 6.1 copies per ng), with no evidence of splenic expression. Location of AAV-cFVIII VGs, cFVIII mRNA, and cFVIII protein was evaluated using in situ hybridization (DNA and RNA in situ hybridization) and IHC, respectively. Most samples demonstrated poor nucleic acid preservation, with the exception for samples from ELI and MG. Detectable hepatocyte FVIII RNA was seen throughout the liver lobe in ELI (responder), but not in MG (nonresponder) (supplemental Figure 4). AAV-cFVIII DNA and cFVIII protein were below the limit of detection using the DNA in situ hybridization and IHC assays (supplemental Figures 2 and 3). In conclusion, the liver is the exclusive source of long-term FVIII production a decade after a single infusion of AAV-cFVIII vector.
cFVIII mRNA is the sole predictor of FVIII expression following AAV gene therapy
Finally, an evaluation of predictors of FVIII activity was performed using univariable linear regression. There was no effect of sex, dose, capsid, or liver VG copies. Within this model, the only predictor of plasma FVIII:C on the final study sample was the level of FVIII mRNA detected by qRT-PCR (supplemental Table 5).
Discussion
In this study, we have demonstrated stable long-term expression for more than a decade following a single portal vein AAV-cFVIII infusion in dogs with hemophilia A. This approach was safe with no significant vector-related toxicities and no evidence of liver carcinoma or chronic liver disease at postmortem. This study represents the longest follow-up (median, 10.8 years; range, 8.2-12.0 years) described to date for successful preclinical gene delivery using an AAV vector.
Stable FVIII:C levels were seen in the 6 responding dogs, with levels in the mild hemophilia range, which resulted in significant clinical benefits with bleed reduction. Similar findings have been seen after a median of 7.3 (2.2-10.1) years follow-up in a cohort of dogs with hemophilia A treated with either a single-chain B-domain deleted cFVIII (n = 4) or separate FVIII heavy and light chain transgenes (n = 5), using AAV8 or AAV9 at the University of North Carolina.14 In that study, 2 dogs demonstrated elevation in FVIII:C 4 years after treatment from steady-state levels of 4% to 10% to 11% at 7 and 10 years, respectively. The proposed mechanism for this rise was from clonal hepatocyte expansion possibly relating to AAV vector integration events, although natural hepatocyte expansion could also explain these findings. Our data show no change with modeling of the FVIII:C over time, although it is interesting to note that some of the later samples demonstrate higher FVIII:C levels (ALX and MZ). These observations are based on a small number of unevenly distributed samples and may reflect an artifact of FVIII:C degradation with time in storage. It is interesting to note that FVIII expression was stable despite an apparent reduction in AAV copies in the endpoint liver sample compared with an earlier biopsy (supplemental data), the mechanism for which is unclear. These data should be interpreted with caution with differences in assay methodology as well as variability seen in VG copies in different regions of the liver.15 Findings of stable expression or rising FVIII expression in these canine hemophilia studies are in contrast to declining FVIII expression seen with time in men with severe hemophilia A treated with an AAV5–hFVIII-SQ construct.16 Although there are a number of differences in the AAV construct design, dosing, and FVIII levels achieved in canine compared with human studies, this may also represent a limitation for the translatability of these canine studies.
Within our study, no response (CSA FVIII:C <1%) was seen in 2 of the dogs, who received treatment with AAV2 (1.5e13 vg/kg) and AAV6 (1.7e13 vg/kg). The mechanisms underlying this lack of therapeutic transgene expression most likely relate from either lower initial vector transduction, vector rearrangement, or inhibition of transgene transcription, given that FVIII mRNA levels were undetectable by qRT-PCR, and only very low levels were detected using a more sensitive droplet digital RT-PCR assay (F.S., unpublished data). It is interesting to note that in these 2 animals, despite undetectable FVIII:C, significant improvements in sensitive global hemostatic testing (WBCT) were seen, and the overall bleed rate was lower. These findings may be of translational relevance and could mirror early data presented in participants with limited response in a phase 3 study using AAV5–hFVIII-SQ.17 Of these 18 participants with FVIII:C <3%, only 4 restarted on prophylaxis and 28% remained bleed free. Further study is required to identify mechanisms resulting in reduction in bleeding in nonresponders and whether global hemostatic assays may provide a sensitive measure to monitor outcomes following gene therapy.
No signs of acute liver toxicity were seen in the early phases (<1 year) of this study. This is in contrast to the experience in human clinical studies where elevation in ALT is the most frequent adverse event reported.17 Asymptomatic elevations in ALP levels were seen in the later phase of the study, which likely reflect the age-related liver findings seen at postmortem. Nodular hyperplasia is a common benign idiopathic finding seen in aging dogs, is well characterized in the literature,18-20 and has been seen at postmortem in untreated dogs within our colony (Figure 6E-F). This process commences at 6 to 8 years of age (incidence 70% to 100% at 14 years), in keeping with the timing of ALP elevations seen in this study.19,20 Nodular hyperplasia is often seen concurrently with age-related hyperplastic changes in other organs,21 a finding that we also saw at postmortem (supplemental Table 2). Given the complexity in diagnosis of nodular hyperplasia, independent review was obtained from 3 blinded veterinary pathologists, with all reports supportive of these findings. Similar findings were not seen in studies reported of AAV-FIX22 or AAV-FVIII14 gene transfer in other longitudinal canine follow-up studies. The reasons for these differences most likely relate to the differences in dog breeds and older age at postmortem. In clinical (human) studies, there have been no reported abnormalities seen on liver imaging during follow-up.23,24 Similarly, liver biopsies performed in 2 patients following treatment using an AAV5-FVIII-SQ construct demonstrated no significant abnormality aside from mild steatosis at 2.7 and 3.9 years.25 In our study, 1 dog developed a localized soft tissue tumor that had no detectable vector copies, suggesting that it was unlikely to be related to the recombinant AAV vector administration.
A strong humoral response was seen toward the dosed AAV capsid, with relatively low cross-reactivity toward nondosed capsids. When considering the translational relevance of these findings, it is important to note that there are differences in the preexisting immunity to AAV in humans and dogs. In our colony, screening for AAV5 capsid nAbs has demonstrated very low frequency of preexisting immunity. Studies in humans have described naturally occurring nAbs toward AAV capsids commonly used for gene therapy, with a higher prevalence toward the AAV2 and AAV6 capsids and a lower prevalence toward AAV8 and AAV5.26-29 Significant preexisting cross-reactivity between capsids is also seen in human studies. In dogs, preexisting nAbs have predominantly been described toward AAV6.30-33 These reports included dogs housed at the University of Missouri and Auburn University, demonstrating the presence of AAV6 nAbs after birth, proposed to be due to passive transfer within the colostrum.30,31 Whether cross-species (human to dog) transfer of AAV may also occur is not clear. The lack of cross-reactivity to other AAV capsids seen in our study may reflect these differences in the natural history of AAV infection in humans and dogs. Long-term immune responses toward the dosed capsids with cross-reactivity have recently been described at up to 15 years in patients treated with intravascular delivery of an AAV2-FIX vector.24 One cause of nonresponse in our study could have been as a result of preexisting capsid nAbs (eg, anti-AAV6) preventing efficient cellular transduction in MG, although no prestudy samples remained to test this hypothesis. Although a reduction in nAb titers was seen with time, if redosing were to be considered, this would have required use of an alternative capsid or experimental intervention, such as plasmapheresis,34 immunoadsorption,35 or immunoglobulin G cleaving endopeptidase (IgG-degrading enzyme of streptococcus pyogenes).36
Conclusion
Stable FVIII expression with improvement in bleeding phenotype without liver toxicity was seen in this long-term follow-up of dogs with hemophilia A following a single portal vein infusion of AAV-cFVIII. Long-lasting immune responses to the AAV capsid suggest the need for additional approaches, including novel capsids or methods for antibody depletion if redosing is required. These studies support the long-term efficacy and safety of this modality of treatment in the current absence of extended follow-up data from human clinical studies.
Acknowledgments
This study was supported by funds from the Canadian Institutes of Health Research Foundation grant FDN 154285 and from Biomarin Pharmaceutical Inc. D.L. is the recipient of a Canada Research Chair in Molecular Hemostasis, and P.B. is the recipient of a SEAMO Clinical Research Fellowship Award.
The visual abstract was created using BioRender.com.
Authorship
Contribution: D.L., P.B., C.H., and S.F. designed the research study; L.H., A.P., and A.W. cared for the animals in the study; A.M.M., C.B., J.I., P.B., B.Y., L.H., and A.P. performed the laboratory assays; P.B., D.H., S.S.I., E.K.R., S.F., and D.L. analyzed the data; P.B., S.F., and D.L. wrote the first draft of the paper. All authors reviewed and critically edited the manuscript.
Conflict-of-interest disclosure: D.L. and P.B. have acted in an advisory role for BioMarin Pharmaceutical Inc. S.F., B.Y., and J.I. are employees and stockholders of BioMarin Pharmaceutical Inc. P.B. has received research support from Biomarin, Grifols, and Octapharma. D.L. has received research support from Bayer, Biomarin, CSL-Behring, Octapharma, and Sanofi. The remaining authors declare no competing financial interests.
Correspondence: David Lillicrap, Department of Pathology and Molecular Medicine, Queen's University, Kingston, Ontario, Canada; e-mail: david.lillicrap@queensu.ca.
References
Author notes
Data concerning any aspect of this report are available upon request fron the corresponding author, David Lillicrap (david.lillicrap@queensu.ca).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal