In this issue of Blood, Batty et al1 report stable lifelong factor VIII (FVIII) expression in the liver of dogs with hemophilia A after a single dose of adeno-associated viral vector (AAV) gene therapy with durable efficacy and reassuring insights on the lack of adverse impact on liver health.
Dogs serve as loyal companions, helpers, even lifesavers, and also work side-by-side with us as protectors and coworkers in the field. I’ve had two dogs in my life: a beagle and an Irish setter, both of whom embraced their roles as loyal companions. However, for a hemophilia investigator, these 2 particular breeds also highlight their coworker role of being “man’s best friend.”
Animal models have been critical to the development of novel hemophilia therapeutics. Whether through naturally occurring or engineered mutations, they inform dosing, efficacy, and safety before human trials. The first hemophilia animal model, established in an Irish setter in 1947 at the University of North Carolina Chapel Hill, replicates the bleeding phenotype of severe hemophilia A and has been used continuously over 7 decades. Another colony of dogs with hemophilia A was established at Queens University in Canada in 1980 from affected miniature schnauzers and spaniels and was then expanded to include beagles.2 Notably, the basis for the FVIII deficiency in both colonies was an intron 22 gene inversion analogous to that found in ∼40% of humans with hemophilia A. The safety and efficacy of replacement therapy with plasma-derived and then recombinant FVIII was first established in dogs. Novel therapies that were not well-tolerated in this model did not progress to human testing.
Animal models have been critical in evaluating strategies to deliver transgenes to tissue targets for expressing missing proteins.3 These approaches often use viral vectors to deliver the complementary DNA to the nucleus of the host cell where, depending on the vector chosen, it integrates into the chromosomal DNA or remains primarily episomal with only relatively rare integrations. Mouse models of hemophilia A engineered via knockout methods have been invaluable because of large litters in the mouse population, short generation time, and well-controlled efficacy measures. However, those models fail to mirror many aspects of human disease, and there are limits to longitudinal studies of long duration. Primates are closer in size for scaling issues and can more faithfully inform relevant human tissue physiology, but no genetic models replicate the hemophilia phenotype. Once again, dogs with hemophilia have proven to be excellent models to assess the short- and long-term safety and efficacy of gene therapy for hemophilia.
The ideal gene therapy for hemophilia A would achieve long-term efficacy after a single treatment with no toxicity from the vector, the transgene, or the FVIII protein. The platform that has advanced the farthest in clinical development is recombinant AAVs (rAAVs) packaged with engineered FVIII transgenes that target the liver to achieve sufficient FVIII expression to correct the bleeding phenotype.4 The best risk:benefit ratio is seen with rAAVs, given their derivation from nonpathogenic replication-defective viruses and the ability to transduce post-mitotic cells in vivo that exhibit high tissue targeting to the liver. Clinical trials have produced clinically relevant expression of FVIII. Expression declines over time,5-7 but it has resulted in reduction of bleeding episodes, elimination of the need for prophylactic FVIII replacement therapy, and improvements in quality of life for at least 5 years after a single infusion event. Safety concerns include asymptomatic liver toxicity manifested by increased transaminases that occur from shortly after the infusion and continue for up to a year or more after dosing. This toxicity is thought to result from immune responses to the vector capsid and has been treated with immunosuppression for resolution and salvage of transgene expression, although other potential mechanisms such as cellular stress from overexpression of the transgene have been hypothesized. Given the high variability of FVIII expression, also seen in all animal models including dogs, high doses (up to 6 × 1013 genome copies per kilogram) have been required to achieve sufficient efficacy. Thus, with quadrillions of vector particles infused, even at a 0.01% rate of integration, millions of integrations can be expected within liver. Accordingly, there is a need for models to evaluate the long-term risks related to insertional mutagenesis and other unintended consequences.
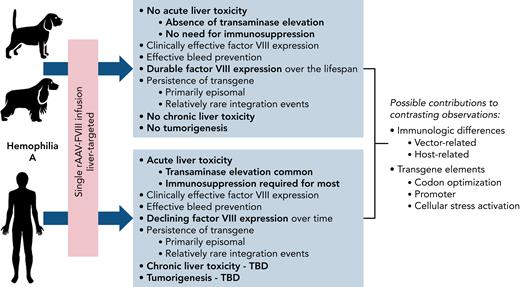
Batty et al now report on 8 dogs with severe hemophilia A treated with a single dose of rAAV containing a canine FVIII; the follow-up was 8 to 12 years, the longest for any animal model to date. Six dogs achieved durable FVIII expression (median FVIII activity one-stage, 12.7%; chromogenic, 7.2%) with accompanying improvement in annualized bleed rates to near zero. Notably, the FVIII transgene was detected postmortem primarily in the liver in all dogs. All FVIII messenger RNA was derived solely from the liver because of the specificity of the transgene promoter elements. Importantly, the liver showed no chronic changes (fibrosis or cirrhosis) or malignancy. The investigators did observe nodular hyperplasia in the liver, which is a common idiopathic finding seen in aging dogs. Nguyen et al8 reported on rAAV-canine FVIII treatment of dogs from the University of North Carolina Chapel Hill that also showed durable FVIII expression. Their integration analysis characterized 1741 integration events and some expanded cell clones, but there was no evidence of altered liver function or tumorigenesis.
No model was able to replicate all of the observations from the clinical trials (see figure). For example, none of the dogs exhibited signs of acute liver toxicity within the first year after rAAV treatment as observed in a high proportion of human patients. No immunologic profiling assessments were conducted in this dog study to evaluate for capsid-directed cellular immune responses, although these will be important features of future prospective experiments. Understanding why the dogs are showing durable expression over a lifespan compared with the waning expression in humans will be critical to moving this field toward a curative gene therapy approach in the future.
Comparison of observations in dogs vs humans after AAV gene therapy in hemophilia A. TBD, to be determined through long-term follow-up studies.
Comparison of observations in dogs vs humans after AAV gene therapy in hemophilia A. TBD, to be determined through long-term follow-up studies.
Overall, these observations should provide reassurance that the rAAV platform is demonstrating a long-term safety profile consistent with the rationale that was posited to move this into human clinical trials. The hemophilia and research community should greatly appreciate the coworker role of these dogs—man’s best friend indeed.
Conflict-of-interest disclosure: S.W.P. has received consulting fees from Apcintex, ASC Therapeutics, Bayer, Biomarin Pharmaceutical, CSL Behring, GeneVentiv, HEMA Biologics, Freeline, LFB, Novo Nordisk, Pfizer, Regeneron/Intellia Therapeutics, Roche/Genentech, Sangamo Therapeutics, Sanofi, Takeda Pharmaceuticals, Spark Therapeutics, and uniQure.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal