In this issue of Blood, Janssen et al1 identified a unique mechanism by which gilteritinib added to the BCL-2 inhibitor venetoclax increased activity in FMS-like tyrosine kinase (FLT3) wild-type (FLT3wt) high-risk acute myeloid leukemia (AML) preclinical models and patients.
AML is a heterogeneous disease of hematopoietic stem cell malignancies characterized by the absence of myeloid differentiation leading to rapid expansion of leukemic progenitors ultimately causing bone marrow failure.2 Outcome of AML is generally dismal owing to relapse or refractoriness of the disease to standard treatment approaches. Disease outcome is particularly poor for elderly patients deemed unfit for intensive treatment strategies.
BCL-2 dependency is a hallmark of most AML cells. The small molecule venetoclax targets BCL-2 selectively and stabilizes proapoptotic proteins.3 Despite promising antileukemic effects in vitro, clinical testing of venetoclax as single agent yielded only modest activity in clinical trials of patients with AML.4 However, the combination of venetoclax with the hypomethylating agent azacitidine showed a significant improvement in response and survival compared with azacitidine alone for elderly unfit patients with newly diagnosed AML leading to approval of venetoclax by the Food and Drug Administration and European Medicines Agency in this setting.5 Despite the significant improvement in outcome, relapsed and resistant disease remains frustratingly frequent. Thus, new approaches are needed, as no standard treatment is available for those patients.
Failure and resistance to venetoclax treatment is mainly mediated by myeloid cell leukemia-1 (MCL-1) overexpression in AML cells.6 Thus, targeting the BCL-2/MCL-1 balance is an attractive drug target in AML. Recently, small molecule MCL-1 inhibitors have been studied in clinical trials, but development has been hampered by dose-limiting toxicities, in particular, cardiac toxicity.7
Thus, novel strategies to target MCL-1 expression are urgently needed to avoid and/or to restore sensitivity of AML cells to venetoclax. Based on this rationale, the findings of Janssen and colleagues to indirectly target MCL-1 in FLT3wt AML cells and patients by combining venetoclax with gilteritinib are of great interest. Both drugs are approved with well-known toxicity profiles for patients with AML.
Gilteritinib is a highly specific inhibitor of FLT3 mutations (FLT3+), including internal tandem mutations, which are the most frequent mutation found in up to 30% of patients with AML, and tyrosine kinase domain mutations. The drug is already approved for the treatment of relapsed or refractory AML in the United States and Europe as a single agent.8 Clinical trials combining gilteritinib and venetoclax for relapsed and refractory mainly FLT3+ patients with AML are underway and have demonstrated feasibility and efficacy.9 Targeting MCL-1 by the combination of gilteritinib and venetoclax has already been reported in preclinical models of FLT3+ AML.10 Efficacy of the gilteritinib-venetoclax combination was also suggested in FLT3wt cells, but the precise mechanisms of action remain unknown.10
The strength of the study of Janssen and colleagues is that they started by identifying the best partner for venetoclax by performing an unsupervised high-throughput ex vivo drug screen with venetoclax and 64 drugs targeting relevant pathways in myeloid malignancies in 31 samples of high-risk patients with AML, including samples from FLT3+ patients. High risk was defined as either treatment-refractory disease or high-risk genetic status according to European Leukemia Net 2017 guidelines. This screen identified gilteritinib as the most potent partner of venetoclax together with the MCL-1 inhibitor MIK665, suggesting that targeting MCL-1 might be a possible mechanism of antileukemic action of gilteritinib in this setting. Surprisingly, gilteritinib combined with venetoclax yielded significantly higher synergy in AML FLT3wt samples, particularly in those with very poor-risk TP53 mutations as compared with FLT3+ patient samples.
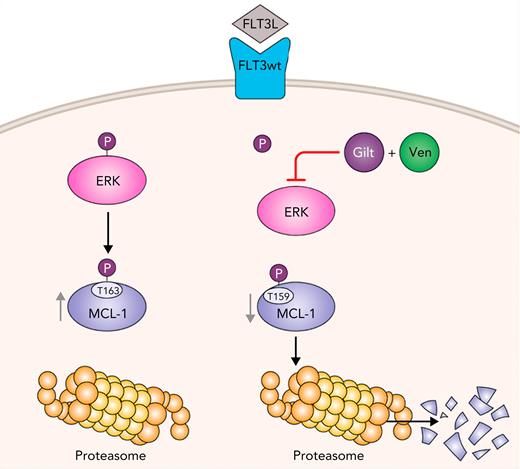
To dissect the precise mechanisms of action of combination treatment with gilteritinib and venetoclax in FLT3wt AML, the authors identified by proteomic studies increased FLT3 pathway signaling (including RAF/MAP, FLT3, and MAPK1/MAPK3 pathways) in patient samples resistant to combined azacitidine and venetoclax treatment compared with those samples sensitive to azacitidine and venetoclax treatment, suggesting that increased FLT3 signaling is responsible for azacitidine-venetoclax resistance. In very elegant experiments, the authors were able to elucidate the mechanism of action of combined gilteritinib and venetoclax treatment (see figure). First, the authors found that only the combination of gilteritinib with venetoclax decreased aberrant MCL-1 expression in AML cells. Furthermore, the investigators demonstrated that the gilteritinib-venetoclax combination inhibited phosphorylation of ERK1/2, which reduced phosphorylation of GSK3A/B and in turn reduced stabilizing phosphorylation of MCL-1 at threonine 163. This resulted in phosphorylation of MCL-1 at serine 159, leading to its proteasomal degradation. Of note, other FLT3+ inhibitors had no significant synergistic effect when combined with venetoclax in FLT3wt AML. The authors demonstrated that inhibition of the receptor tyrosine kinase AXL by giltertinib is essential for efficacy of the gilteritinib-venetoclax combination. AXL is not affected by other FLT3+ inhibitors.
Gilteritinib (Gilt) combined with venetoclax (Ven) leads to proteasomal degradation of MCL-1, which overcomes venetoclax resistance in FLT3wt AML. Gilt and Ven combination dephosphorylates Extracellular signal Regulated Kinase (ERK), which induces phosphorylation of MCL-1 at serine 159 in FLT3wt AML cells. This leads to downregulation of MCL-1 caused by its proteosomal degradation. Professional illustration by Patrick Lane, ScEYEnce Studios.
Gilteritinib (Gilt) combined with venetoclax (Ven) leads to proteasomal degradation of MCL-1, which overcomes venetoclax resistance in FLT3wt AML. Gilt and Ven combination dephosphorylates Extracellular signal Regulated Kinase (ERK), which induces phosphorylation of MCL-1 at serine 159 in FLT3wt AML cells. This leads to downregulation of MCL-1 caused by its proteosomal degradation. Professional illustration by Patrick Lane, ScEYEnce Studios.
To confirm in vitro findings, gilteritinib plus venetoclax was tested in a TP53 mutated FLT3wt patient-derived xenograft AML mouse model in which combination treatment significantly reduced engraftment of AML cells. Importantly, the authors reported the results of off-label treatment with combined gilteritinib and venetoclax after written consent in 4 heavily pretreated patients, including prior azacitidine plus venetoclax exposure. Only modest short-lived responses were observed. Nevertheless, MCL-1 downregulation could be detected in bone marrow blast cells obtained from treated patients, suggesting MCL-1 reduction assessment as biomarker for future clinical trials.
Janssen and colleagues identified increased FLT3 signaling and MCL-1 expression as a hallmark of FLT3wt high-risk AML, which can be targeted efficiently by treatment of gilteritinib and venetoclax. Despite only modest responses in the 4 patients treated with this combination in this study, further clinical investigation in a larger cohort is warranted. Prior testing of FLT3 signaling and MCL-1 expression could help to identify a target AML population who might benefit from this innovative treatment approach. The challenge is to determine the right place for gilteritinib to prevent or overcome venetoclax resistance in different settings, including triple combinations or add-on strategies.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal