In this issue of Blood, Nagahata et al1 provide genetic evidence that phagocytes are the ancestral blood cell type during evolution. They identified CEBPα as the driver of a conserved phagocytosis gene expression program and suggest that its repression by Polycomb eventually permitted the development of alternative blood cell lineages.
What are the evolutionarily most ancient blood cells? Although this question has vexed developmental biologists for a long time, it is now widely agreed that, perhaps counterintuitively, it is not hematopoietic stem cells (HSCs) but macrophage-like cells (“phagocytes”).2 Phagocytes are characterized by their potential to engulf and digest pathogens, dying cells, and particles. They are already observed in sponges while the first lymphoid cells only evolved in lower vertebrates, such as lampreys.3 In mice, nonphagocytic cells of the lymphoid and erythroid lineages have retained the potential to convert into macrophages,4,5 suggesting that the myeloid lineage represents a default pathway.6
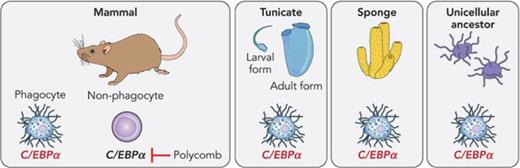
These observations raise several questions: Is there an evolutionarily conserved gene expression program that determines phagocytic capacity? If so, when did it evolve and is it driven by an ancestral transcription factor(s)? Also, how do nonhematopoietic cells arise? To address these questions, Nagahata et al embarked on a genuine tour de force by comparing the gene expression profiles between mouse macrophages with that of sorted phagocytes from the tunicate Ciona intestinalis, which in its larval form resembles chordates, and that of phagocytes from the sponge Amphimedon queenslandica. They also included in this analysis Capsaspora owczarzaki, a unicellular relative of metazoans, which in their ameboidal form resemble mouse macrophages by their phagocytic ability and the presence of filopodia (see figure). They report that tunicate- and sponge-derived phagocytes, as well as Capsaspora cells, show higher transcriptional similarities with macrophages than nonphagocytic cells of mice and also selectively express high levels of the gene encoding the transcription factor CEBPα. Strikingly, Cebpa was also found to be expressed among a small number of genes conserved in Capsaspora, along with the lysosome/phagocytosis-associated gene Pla2g15. It is known that CEBPα plays an important role in myeloid cell specification, that its overexpression in B- and T-cell precursors induces the conversion into functional macrophages,6 and that it is also involved in the specification and function of a number of additional cell types, including adipocytes and hepatocytes.7 Consequently, the authors tested the macrophage-inducing capacity of the CEBPα homologs from Ciona, Amphimedon, and Capsaspora in mouse lymphoid and megakaryocytic precursors. They found that the tunicate and sponge genes are remarkably potent in inducing transdifferentiation into phagocytes while the more distantly related CEBPα homolog from Capsaspora only converted megakaryocyte precursors into myeloid cells.
Diagram summarizing the findings described by Nagahata et al. The authors compared the gene expression programs of phagocytic cells from mice with those from a tunicate (Ciona), sponge (Amphimedon), and the single-celled metazoan precursor Capsaspora, revealing the selective expression of CEBPα homologs. They also found that in nonphagocytic mouse hematopoietic cells, Cebpa is repressed by the Polycomb complex. A representation of a circulating macrophage from mice is shown on the bottom. The image is also used for a generic representation of phagocytes from the more primitive species. Professional illustration by Patrick Lane, ScEYEnce Studios.
Diagram summarizing the findings described by Nagahata et al. The authors compared the gene expression programs of phagocytic cells from mice with those from a tunicate (Ciona), sponge (Amphimedon), and the single-celled metazoan precursor Capsaspora, revealing the selective expression of CEBPα homologs. They also found that in nonphagocytic mouse hematopoietic cells, Cebpa is repressed by the Polycomb complex. A representation of a circulating macrophage from mice is shown on the bottom. The image is also used for a generic representation of phagocytes from the more primitive species. Professional illustration by Patrick Lane, ScEYEnce Studios.
Assuming that cells with phagocytic capacity, filopodia production, and Cebpa expression are the primordial blood cells, how did the other lineages evolve? Here, the data of Nagahata et al suggest that the interplay between CEBPα and Polycomb genes is a critical step in the emergence of nonhematopoietic cells. After observing that chromatinized Cebpa in nonhematopoietic mouse cells is covered with the histone mark H3K27me3, they tested the possibility that the Polycomb complex maintains the gene in a repressed state. Indeed, ablation of the PRC1 genes Ring1a and Ring1b in nonphagocytic mouse hematopoietic cells led to the derepression of Cebpa and the cells’ conversion into phagocytes. Remarkably, similar findings were made in vivo since the hematopoietic system of mice competitively repopulated with bone marrow from floxed Ring1a/b mice consisted almost exclusively of myeloid cells after ablation of the 2 genes. The detection of immunoglobulin and T-cell receptor gene rearrangements in macrophages further demonstrated that the loss of Polycomb complex genes induces the transdifferentiation of B- and T-cell lineage cells into phagocytes. These observations raise the possibility that during evolution the Polycomb complex was co-opted to the Cebpa locus in a subset of phagocytes, giving rise to lymphoid and possibly erythroid/megakaryocytic lineage cells. However, this step cannot be sufficient as nonphagocytic cell lineages are under the control of specific transcription factors, such as Pax5 for B cells and Gata1 for the erythroid/megakaryocytic lineages, which must have likewise evolved. Consistent with this idea, ablation of Pax5 in B-lineage cells leads to the derepression of myeloid genes8 and forced expression of Gata1 induces the transdifferentiation of myeloid precursors into erythroid/megakaryocytic cells.4
Lineage instructive transcription factors are known to work in complexes with other transcription factors, and CEBPα is no exception. Yet Nagahata et al were unable to detect in Capsaspora the expression of PU.1 (Spi1) homolog, an obligate partner of CEBPα in myeloid lineage specification,6 raising the possibility that this gene is less conserved than Cebpa or that another cooperating factor(s) is yet to be found. More fundamentally, the findings described support the idea that primitive phagocytes are evolutionary ancestors of not only myeloid/lymphoid and erythroid/megakaryocytic cells but also HSCs. We are then left with a new, not less fascinating question: How did HSCs evolve?
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal