Key Points
Abnormal uterine bleeding (AUB) is highly incident among women of reproductive age treated with anticoagulants for venous thromboembolism.
Women with AUB, especially new-onset AUB, experience decreased quality of life, which should be a call to action to raise awareness for AUB.
Abstract
Preliminary data and clinical experience have suggested an increased risk of abnormal uterine bleeding (AUB) in women of reproductive age treated with anticoagulants, but solid data are lacking. The TEAM-VTE study was an international multicenter prospective cohort study in women aged 18 to 50 years diagnosed with acute venous thromboembolism (VTE). Menstrual blood loss was measured by pictorial blood loss assessment charts at baseline for the last menstrual cycle before VTE diagnosis and prospectively for each cycle during 3 to 6 months of follow-up. AUB was defined as an increased score on the pictorial blood loss assessment chart (>100 or >150) or self-reported AUB. AUB-related quality of life (QoL) was assessed at baseline and the end of follow-up using the Menstrual Bleeding Questionnaire. The study was terminated early because of slow recruitment attributable to the COVID-19 pandemic. Of the 98 women, 65 (66%) met at least one of the 3 definitions of AUB during follow-up (95% confidence interval [CI], 57%-75%). AUB occurred in 60% of women (36 of 60) without AUB before VTE diagnosis (new-onset AUB; 95% CI, 47%-71%). Overall, QoL decreased over time, with a mean Menstrual Bleeding Questionnaire score increase of 5.1 points (95% CI, 2.2-7.9), but this decrease in QoL was observed only among women with new-onset AUB. To conclude, 2 of every 3 women who start anticoagulation for acute VTE experience AUB, with a considerable negative impact on QoL. These findings should be a call to action to increase awareness and provide evidence-based strategies to prevent and treat AUB in this setting. This was an academic study registered at www.clinicaltrials.gov as #NCT04748393; no funding was received.
Medscape Continuing Medical Education online

In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and the American Society of Hematology. Medscape, LLC is jointly accredited with commendation by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.0 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at https://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 1832.
Disclosures
Laurie Barclay, MD, has the following relevant financial relationships: Formerly owned stocks in: AbbVie Inc.
Learning objectives
Upon completion of this activity, participants will:
Assess incidence of any and new-onset abnormal uterine bleeding in women starting anticoagulant therapy because of acute venous thromboembolism and consequent changes in health-related quality of life, based on the TEAM-VTE study
Evaluate the relevant determinants and predictors of abnormal uterine bleeding in women starting anticoagulant therapy because of acute venous thromboembolism, and the effect of treatment interventions to alleviate abnormal uterine bleeding, based on the TEAM-VTE study
Determine the clinical implications of the incidence, prevalence, and relevance of abnormal uterine bleeding in women starting anticoagulant therapy because of acute venous thromboembolism, based on the TEAM-VTE study
Release date: October 20, 2022; Expiration date: October 20, 2023
Introduction
Abnormal uterine bleeding (AUB) is a highly prevalent condition, affecting 10% to 30% of women of reproductive age, based on objective measurement of menstrual blood loss and self-reported information.1 In general, abnormal menstrual bleeding is associated with negative perceptions and limited social and professional activities as well as poorer quality of life (QoL) compared with patients without abnormal menstrual bleeding: health-related QoL scores in women with AUB have been shown to be below the 25th percentile of scores for the general female population of similar age.1-3 In addition to the burden to the individual, the conservatively estimated annual direct and indirect economic costs of AUB are approximately $1 billion and $12 billion, respectively, underlining its relevance to society at large.1
The prevalence of AUB in women treated with oral anticoagulants is considerably higher than in women who do not take such medications, although exact estimations of the incidence, prevalence, and impact of anticoagulation-induced AUB remain unavailable to date.4 Data from registries and randomized trials have shown that the incidence of major uterine bleeding after initiation of anticoagulant treatment is low.5-7 However, standard major bleeding definitions are insufficient to capture AUB because they fail to account for its chronic and recurrent nature or its major psychological impact. Several studies have suggested that the incidence of AUB is higher in patients treated with oral Xa inhibitors than in patients treated with vitamin K antagonists (VKAs) or oral thrombin inhibitors.8-18 However, randomized trials comparing oral anticoagulant agents are unavailable, and reliable data to guide management decisions in clinical practices are lacking.
To quantify the burden of AUB and identify unmet clinical needs in women given anticoagulant therapy, we set out to acquire high-quality prospective data on the incidence, prevalence, and relevance of AUB in women starting anticoagulant therapy because of acute venous thromboembolism (VTE). More specifically, we aimed to evaluate the incidence of any AUB and new-onset AUB in these patients, consequent changes in health-related QoL, relevant determinants and predictors of AUB, and the effect of treatment interventions aimed to mitigate menstrual bleeding.
Methods
The TEAM-VTE study was an international multicenter observational prospective study conducted at 12 hospitals across 8 countries between August 2018 and September 2021. Its primary aim was to evaluate the incidence of AUB among women of reproductive age who were treated with oral anticoagulants for acute VTE.
The study protocol was approved by the institutional review board of the Leiden University Medical Center (for all participating hospitals in The Netherlands) and by all local institutional review boards of the participating hospitals in Austria, Belgium, France, Germany, Italy, Spain, and Switzerland. All patients provided written informed consent.
Patients
We included consecutive women aged at least 18 years and no older than 50 years of childbearing potential with objectively confirmed symptomatic first or recurrent VTE who fulfilled all of the inclusion criteria and none of the exclusion criteria presenting to one of the participating sites: patients with an active menstrual cycle with or without hormonal regulation of any kind (initiated for contraception or for treatment of abnormal menstrual bleeding) were eligible. Exclusion criteria were premature menopause established before study inclusion, history of hysterectomy or chemically induced menopause, pregnancy or postpartum status (first 3 months), active in vitro fertilization or planned in vitro fertilization treatment during the study period, planned treatment with parenteral anticoagulation without a plan to switch to oral drugs, and inability to comply with study follow-up.
Study procedures
Study enrollment occurred shortly after VTE diagnosis (before the first day of the next menstrual cycle after VTE diagnosis or within 1 month after the VTE diagnosis, whichever came first). At baseline, we collected data on demographic characteristics, VTE, comorbidities, and bleeding history. Participants completed the International Society on Thrombosis and Haemostasis (ISTH) bleeding assessment tool (BAT; cutoff for abnormal bleeding score in women is ≥6 points),19,20 the Menstrual Bleeding Questionnaire (MBQ) to assess menstrual bleeding–specific QoL,21 and the pictorial blood loss assessment chart (PBAC),22 a validated self-reporting tool for assessing menstrual blood loss based on the participant’s experience before VTE. Specifically, the PBAC related to the last menstrual period in retrospect provided an estimation of the amount of blood loss during the last menstrual cycle before VTE diagnosis. In addition, laboratory test results assessed during routine clinical care were collected.
We prospectively followed participants until discontinuation of the anticoagulant treatment or 6 months, whichever occurred first. A PBAC was self-completed prospectively for each menstrual cycle that occurred. Women were contacted every 2 months to evaluate the presence of self-reported AUB and to evaluate the completed PBACs. At the time of anticoagulant agent cessation or at the end of 6-month follow-up, whichever came first, menstrual bleeding–related QoL assessment and laboratory tests were repeated. In case of AUB, a diagnostic workup was suggested to the investigators, consisting of an evaluation of the presence of von Willebrand disease and referral to a gynecologist to rule out coexisting comorbidities. However, this was not dictated by the study protocol. Patients were followed for recurrence of symptomatic VTE, any bleeding event other than AUB, and all-cause mortality, and were managed according to international guidelines, local preference, and standard of care at the participating centers with respect to treatment of VTE as well as management of (abnormal menstrual) bleeding or recurrent VTE. Results of diagnostic workup and details of treatments related to AUB were collected.
A patient panel was involved in the design of the study. They provided advice on the optimal study outcomes and agreed that the burden of the study procedures was acceptable.
Outcomes
The primary outcome was the overall incidence of AUB during the follow-up period and the incidence of new-onset abnormal menstrual bleeding. Because different definitions of AUB are used in the literature, abnormal menstrual bleeding was defined according to 3 definitions in the present study: (1) PBAC score >100 during one menstrual period, (2) PBAC score >150 during one menstrual period, or (3) self-reported increased menstrual volume, regardless of regularity, frequency, or duration according to the International Federation of Gynecology and Obstetrics classification of AUB.22-30 A PBAC score of 100 is indicative of 80 mL of blood loss.22,26 The definitions of PBAC score >100 points and >150 points partly overlap; all women with a PBAC score >150 automatically met the definition of a PBAC score >100. New-onset AUB was defined as AUB (according to any of the 3 definitions) in women with a retrospective PBAC score ≤100 or ≤150, respectively. Details on the assessment of uterine bleeding by the PBAC are provided in supplemental Appendix 1, available on the Blood website.
Statistical analysis
Continuous variables are expressed as means with standard deviation or medians with interquartile range, according to their distribution; categorical variables are reported as frequencies with percentages.
The primary outcome was calculated with corresponding 95% confidence interval (CI) according to the 3 different definitions of AUB. To assess the incidence of new-onset AUB, women without AUB before VTE diagnosis (based on the PBAC completed at baseline for the last menstrual cycle in retrospect) were taken into account. In the primary analysis, we estimated the risk of AUB at any time during the follow-up period with the use of descriptive statistics: median PBAC scores for each menstrual cycle, absolute numbers of women with AUB, and proportion of women with AUB according to the different definitions of AUB. Because menstrual cycles occur irregularly and not all women experienced a cycle every month during the follow-up period, analyses were performed per menstrual cycle.
In the secondary analyses, we evaluated associations of previous AUB based on the retrospective baseline PBAC or previous abnormal bleeding based on the baseline ISTH BAT score with AUB during anticoagulant treatment using crude odds ratios (ORs). We explored median PBAC scores over time and the proportion of women with AUB in 2 subgroup analyses (women with AUB who received treatment vs women who did not receive treatment related to AUB; anticoagulant drug class according to the anticoagulant treatment initiated at VTE diagnosis). To study the impact of (new-onset) AUB on QoL, mean differences in MBQ score between baseline and end of follow-up were calculated with 95% CIs obtained from paired t tests, and mean differences in MBQ score between subgroups were calculated with 95% CIs, provided that the differences were normally distributed. Finally, we described the results of routine diagnostic workup and the proportion of women receiving treatments related to AUB, along with specification of treatment.
An originally considered sample size of 210 was calculated to estimate the primary end point with a 95% CI of a maximum of 6 percentage points above or below the point estimate. As a result of slow recruitment in the COVID-19 pandemic and lack of resources to complete the original study, which was planned to end in August 2022, the study was terminated early. Study enrollment was discontinued after March 2021, followed by completion of the follow-up period on 30 September 2021.
Missing data were not imputed. Analyses were performed in SPSS version 25.0.
Results
From August 2018 to March 2021, 98 women with confirmed symptomatic first or recurrent VTE and active menstrual cycle were included, with a mean age of 34 years (standard deviation, 9.4). The baseline characteristics of the study patients are summarized in Table 1. Pulmonary embolism was diagnosed in 46% of women, deep vein thrombosis in 34%, and both in 20%. Nearly two thirds of women had provoked VTE, with oral contraceptive use or hormone treatment as the most common contributing factor (62%; 40 of 65). The 2-month follow-up visits were completed for all women, including the PBAC scores. One woman did not complete PBAC scores after the 2-month follow-up visit, resulting in 4 missing measurements. The ISTH BAT was completed in 97 of 98 women, and measurements of the MBQ at baseline and the end of follow-up were available in 74 of 98 women.
Baseline characteristics of 98 female patients of reproductive age with VTE
| Patient characteristic . | Value . |
|---|---|
| Mean age ± SD, y | 34 ± 9.4 |
| Mean BMI ± SD, kg/m2∗ | 27 ± 6.8 |
| Obese (BMI ≥30 kg/m2) | 25 (26%) |
| Index VTE diagnosis | |
| DVT | 33 (34%) |
| PE | 45 (46%) |
| Both DVT and PE | 20 (20%) |
| Unprovoked | 33 (34%) |
| Provoked† | 65 (66%) |
| Surgery | 9 |
| Immobilization, including hospitalization | 10 |
| Trauma | 4 |
| Travel (≥6 h), flight | 6 |
| Oral contraceptive use or hormone treatment | 40 |
| Known genetic thrombophilia | 2 |
| Hypercoagulability of other cause (COVID-19, CMV infection, nephrotic syndrome) | 3 |
| Treatment initiated at time of VTE diagnosis | |
| Reperfusion therapy | 2 (2.0%) |
| Anticoagulant therapy | 98 (100%) |
| DOAC | 85 (87%) |
| Apixaban | 26 (27%) |
| Rivaroxaban | 42 (43%) |
| Edoxaban | 10 (10%) |
| Dabigatran | 7 (7.1%) |
| Vitamin K antagonist (LMWH lead-in) | 12 (12%) |
| LMWH | 1 (1.0%) |
| Anticoagulant therapy at 2-mo follow-up | |
| DOAC | 85 (87%) |
| Apixaban | 24 (25%) |
| Rivaroxaban | 41 (42%) |
| Edoxaban | 13 (13%) |
| Dabigatran | 7 (7.1%) |
| Vitamin K antagonist | 11 (11%) |
| LMWH | 1 (1.0%) |
| Fondaparinux | 1 (1.0%) |
| Anticoagulant therapy at end of follow-up | |
| DOAC | 83 (85%) |
| Apixaban | 21 (21%) |
| Rivaroxaban | 42 (43%) |
| Edoxaban | 13 (13%) |
| Dabigatran | 7 (7.1%) |
| Vitamin K antagonist | 11 (11%) |
| LMWH | 3 (3.1%) |
| Fondaparinux | 1 (1.0%) |
| Medical history | |
| Previous VTE | 15 (15%) |
| Active malignancy | 0 |
| Smoking | 24 (25%) |
| Previous gynecological findings‡ | 22 (22%) |
| Abnormal cervical cytology or histology | 2 |
| Endometriosis | 4 |
| Ovarian cyst | 3 |
| Polycystic ovary syndrome | 7 |
| Uterine fibroid(s)/myoma(s), or polyp(s) | 7 |
| Medication use at the moment of VTE diagnosis | |
| Anticoagulation or antiplatelet therapy | 0 |
| Oral contraceptives (estrogenic) | 32 (33%) |
| Other hormonal contraceptives | 9 (9.2%) |
| NuvaRing | 6 |
| Implanon | 1 |
| Evra patch | 2 |
| Intrauterine device | 6 (6.1%) |
| Hormone-containing | 5 |
| Copper | 1 |
| Patient characteristic . | Value . |
|---|---|
| Mean age ± SD, y | 34 ± 9.4 |
| Mean BMI ± SD, kg/m2∗ | 27 ± 6.8 |
| Obese (BMI ≥30 kg/m2) | 25 (26%) |
| Index VTE diagnosis | |
| DVT | 33 (34%) |
| PE | 45 (46%) |
| Both DVT and PE | 20 (20%) |
| Unprovoked | 33 (34%) |
| Provoked† | 65 (66%) |
| Surgery | 9 |
| Immobilization, including hospitalization | 10 |
| Trauma | 4 |
| Travel (≥6 h), flight | 6 |
| Oral contraceptive use or hormone treatment | 40 |
| Known genetic thrombophilia | 2 |
| Hypercoagulability of other cause (COVID-19, CMV infection, nephrotic syndrome) | 3 |
| Treatment initiated at time of VTE diagnosis | |
| Reperfusion therapy | 2 (2.0%) |
| Anticoagulant therapy | 98 (100%) |
| DOAC | 85 (87%) |
| Apixaban | 26 (27%) |
| Rivaroxaban | 42 (43%) |
| Edoxaban | 10 (10%) |
| Dabigatran | 7 (7.1%) |
| Vitamin K antagonist (LMWH lead-in) | 12 (12%) |
| LMWH | 1 (1.0%) |
| Anticoagulant therapy at 2-mo follow-up | |
| DOAC | 85 (87%) |
| Apixaban | 24 (25%) |
| Rivaroxaban | 41 (42%) |
| Edoxaban | 13 (13%) |
| Dabigatran | 7 (7.1%) |
| Vitamin K antagonist | 11 (11%) |
| LMWH | 1 (1.0%) |
| Fondaparinux | 1 (1.0%) |
| Anticoagulant therapy at end of follow-up | |
| DOAC | 83 (85%) |
| Apixaban | 21 (21%) |
| Rivaroxaban | 42 (43%) |
| Edoxaban | 13 (13%) |
| Dabigatran | 7 (7.1%) |
| Vitamin K antagonist | 11 (11%) |
| LMWH | 3 (3.1%) |
| Fondaparinux | 1 (1.0%) |
| Medical history | |
| Previous VTE | 15 (15%) |
| Active malignancy | 0 |
| Smoking | 24 (25%) |
| Previous gynecological findings‡ | 22 (22%) |
| Abnormal cervical cytology or histology | 2 |
| Endometriosis | 4 |
| Ovarian cyst | 3 |
| Polycystic ovary syndrome | 7 |
| Uterine fibroid(s)/myoma(s), or polyp(s) | 7 |
| Medication use at the moment of VTE diagnosis | |
| Anticoagulation or antiplatelet therapy | 0 |
| Oral contraceptives (estrogenic) | 32 (33%) |
| Other hormonal contraceptives | 9 (9.2%) |
| NuvaRing | 6 |
| Implanon | 1 |
| Evra patch | 2 |
| Intrauterine device | 6 (6.1%) |
| Hormone-containing | 5 |
| Copper | 1 |
SD, standard deviation; BMI, body mass index; DVT, deep vein thrombosis; PE, pulmonary embolism; CMV, cytomegalovirus; DOAC, direct oral anticoagulant; LMWH, low molecular weight heparin.
Data available in 95 patients.
Provocative factors were not mutually exclusive. Fifteen of 98 women had more than one provocative factor: 13 women with 2 provocative factors each and 2 with 3 provocative factors each. In 12 women, VTE was provoked by the combination of oral contraceptive use/hormone treatment and another provocative factor.
Previous gynecological findings were not mutually exclusive. One woman had 2 gynecological findings (endometriosis and ovarian cyst).
In 57 of 98 women, anticoagulant treatment was continued during the full 6-month follow-up. Two women underwent anticoagulation dose reduction by the treating physician during the follow-up as a result of AUB temporarily or after the first 3 months of treatment. Three women experienced symptomatic recurrent VTE during the follow-up period: 2 were receiving therapeutic anticoagulant therapy at the time of the recurrence; the third woman did not receive anticoagulant therapy at the time of recurrence because the 3-month treatment for her provoked deep vein thrombosis had already been completed. No major nonmenstrual bleeding events occurred, and no women died.
Incidence of AUB
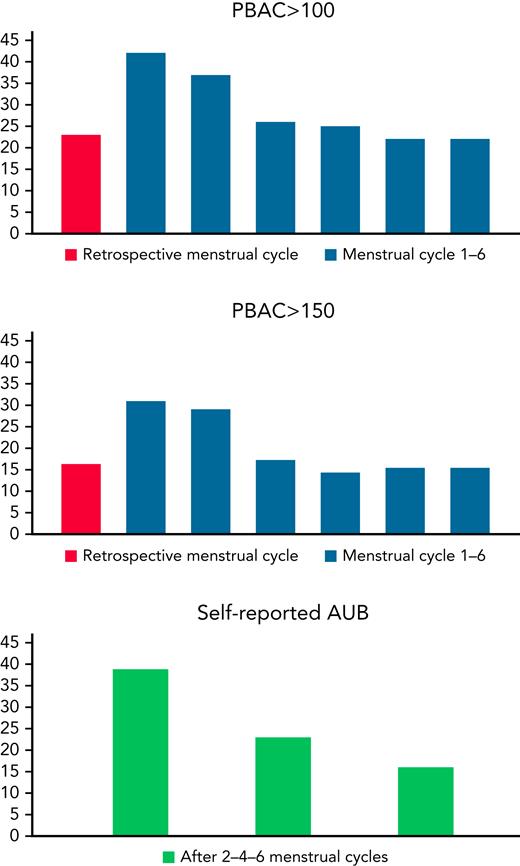
Overall, at least one of the 3 definitions of AUB was met in 66% of the women (65 of 98) at any time during the follow-up period (95% CI, 57%-75%); for the majority of women (90%), this was in the first 2 months following the VTE diagnosis. The incidences of AUB were 57% (56 of 98), 45% (44 of 98), and 48% (47 of 98) according to PBAC score >100, >150, and the self-reported definition, respectively. Of the 65 women with AUB during follow-up, 58% (38 of 65) had an increased PBAC score >100 and self-reported AUB, 14% (9 of 65) met only the self-reported definition of AUB, and 28% (18 of 65) met only the PBAC score >100 definition of AUB. Among women without AUB before VTE based on retrospective PBAC ≤100 and ≤150 thresholds, new-onset AUB according to any of the 3 definitions occurred in 60% (36 of 60; 95% CI, 47%-71%; 37% [36 of 98] of the overall population) and 63% (42 of 67; 95% CI, 51%-73%; 43% [42 of 98] of the overall population), respectively.
Risk according to time, previous bleeding, and treatment type
Median PBAC score increased during the first menstrual cycle compared with the retrospective PBAC assessing blood loss during the last menstrual cycle before VTE diagnosis (median PBAC scores of 35 [interquartile range, 8-114] for the retrospective cycle and 95 [27-248] for the first cycle; median difference, 49; 95% CI, 18-85). After the first menstrual cycle, a gradual decrease in the amount of blood loss was observed, followed by steady median PBAC scores for the second to the sixth menstrual cycle that were still doubled compared to the retrospective PBAC score (Figure 1). A decrease in prevalence of AUB was observed during the follow-up period (Figure 2).
Median PBAC score per menstrual cycle. PBAC scores for the last menstrual cycle before VTE diagnosis (“retrospective menstrual cycle”) were available in 83 of 98 women.
Median PBAC score per menstrual cycle. PBAC scores for the last menstrual cycle before VTE diagnosis (“retrospective menstrual cycle”) were available in 83 of 98 women.
Prevalence of AUB expressed in absolute number of women meeting the definition of AUB based on each of the 3 definitions. PBAC scores for the last menstrual cycle before VTE diagnosis (“retrospective menstrual cycle”) were available in 83 of 98 women.
Prevalence of AUB expressed in absolute number of women meeting the definition of AUB based on each of the 3 definitions. PBAC scores for the last menstrual cycle before VTE diagnosis (“retrospective menstrual cycle”) were available in 83 of 98 women.
Generally, women with self-reported AUB had higher median PBAC scores for all menstrual cycles than women without self-reported AUB, corresponding to more menstrual blood loss (supplemental Table 1). A retrospective PBAC score >100 or >150 (before anticoagulation) was associated with AUB during the follow-up period (OR, 7.0; 95% CI, 1.5-33; and OR, 8.9; 95% CI, 1.1-72, respectively), in contrast to the baseline ISTH BAT, which was not (OR, 0.98; 95% CI, 0.17-5.7).
In contrast to patients treated with oral Xa inhibitors (n = 78) or VKA/low molecular weight heparin (n = 13), the 7 participants treated with dabigatran did not experience an increase in menstrual blood loss during the first menstrual cycle compared with the last menstrual cycle before VTE diagnosis (supplemental Figure 1). Notably, these women had a considerably higher median PBAC score for the last menstrual cycle before VTE diagnosis than those treated with other anticoagulant agents. Comparing the oral Xa inhibitors, the differences in median PBAC score between the first menstrual cycle after VTE diagnosis and the last menstrual cycle before VTE diagnosis were +54 for the apixaban subgroup, +90 for the rivaroxaban subgroup, and +39 for the edoxaban subgroup.
Estrogen-containing contraceptives (oral contraceptives, vaginal ring, contraceptive patch) were discontinued at the time of VTE diagnosis in 15 of 40 women who used estrogenic contraceptives at baseline. The retrospective PBAC score was >100 in 3 of the 15 women and >150 in 2 of them. In a logistic regression analysis, the use and continuation of estrogenic contraceptives at baseline and discontinuation of estrogenic contraceptives at the time of VTE diagnosis or within the first week after VTE diagnosis were not significantly associated with the occurrence of AUB according to any of the 3 definitions at any time during the follow-up period, with an OR of 2.82 (95% CI, 0.72-11.1) for women using estrogenic contraceptives at baseline who stopped at the time of VTE diagnosis or within the first week afterward vs women who did not use estrogenic contraceptives at baseline, and an OR of 2.24 (95% CI, 0.78-6.4) for women who continued estrogenic contraceptives vs women who did not use estrogenic contraceptives at baseline.
Diagnostic workup and management of AUB
Diagnostic workup with transvaginal ultrasound was performed in 51% of the women with AUB and 44% of the women with new-onset AUB, revealing abnormalities in 30% and 25% of the women who underwent transvaginal ultrasound, respectively (supplemental Table 2). None of the women had thrombocytopenia or was diagnosed with von Willebrand disease.
Of the 65 women with AUB according to at least one definition, treatment related to abnormal menstrual bleeding was initiated in 32% (21 of 65). Details about AUB-related treatment are displayed in Table 2. The proportion of women receiving treatments related to AUB was comparable in subgroup analyses for the 3 different definitions separately. Among the women with AUB who had received any treatment related to AUB, a decrease in the PBAC score as well as the prevalence of AUB was observed after the first cycle; this decrease was more pronounced than in women with AUB who did not receive dedicated treatment related to AUB (supplemental Figure 1).
Details of women who received treatment related to abnormal menstrual bleeding within routine clinical care
| Parameter . | Women with AUB (n = 65) . |
|---|---|
| Treatment related to AUB | 21 (32%) |
| Medical treatment | |
| Red blood cell transfusion | 2 |
| Intravenous iron infusion | 4 |
| Oral iron supplements | 7 |
| Reduced dose of anticoagulant | 2 |
| Temporary stop of anticoagulant | 1 |
| Tranexamic acid | 1 |
| Oral contraceptives started or intensified | 8 |
| IUD insertion∗ | 5 |
| Implanon insertion | 1 |
| Surgical treatment | |
| Hysterectomy | 1 |
| Polypectomy† | 1 |
| Parameter . | Women with AUB (n = 65) . |
|---|---|
| Treatment related to AUB | 21 (32%) |
| Medical treatment | |
| Red blood cell transfusion | 2 |
| Intravenous iron infusion | 4 |
| Oral iron supplements | 7 |
| Reduced dose of anticoagulant | 2 |
| Temporary stop of anticoagulant | 1 |
| Tranexamic acid | 1 |
| Oral contraceptives started or intensified | 8 |
| IUD insertion∗ | 5 |
| Implanon insertion | 1 |
| Surgical treatment | |
| Hysterectomy | 1 |
| Polypectomy† | 1 |
Women could have undergone multiple medical and/or surgical treatments. AUB was defined according to at least one of the 3 definitions for AUB at at least one time during follow-up.
IUD, intrauterine device.
Hormone (progesterone)–containing IUD in 3 women; copper IUD in 2 women.
Planned treatment.
Impact of AUB
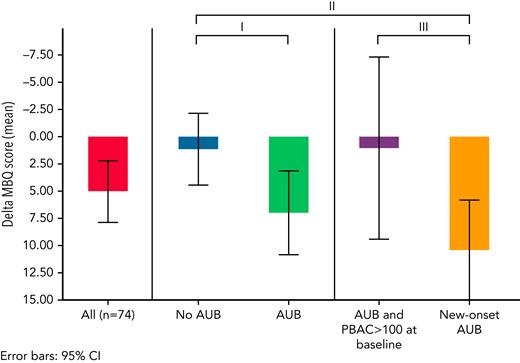
Overall, QoL decreased significantly over time, with a mean increase in MBQ score of 5.1 points (95% CI, 2.2-7.9; Figure 3) between baseline and cessation of anticoagulation or the 6-month follow-up visit. When stratifying by the presence of AUB and new-onset AUB, this decrease in QoL was observed only among women who had new-onset AUB, with a mean difference in MBQ score of +9.2 points for women with new-onset AUB vs women without AUB (95% CI, 3.4-15.0). Moreover, when comparing women with new-onset AUB vs women with preexisting AUB, a decrease in QoL was observed as well (mean difference in MBQ score of +9.3 points; 95% CI, 0.9-17.7).
Change in MBQ score between measurement at the end of follow-up and at baseline. Higher scores indicate worse QoL. Mean difference with corresponding 95% CI: ∗AUB (n = 50) vs no AUB (n = 24): 5.9 (−0.1 to 11.8). ∗∗New-onset AUB (n = 30) vs no AUB (n = 24): 9.2 (3.4-15.0). ∗∗∗New-onset AUB (n = 30) vs AUB with PBAC >100 at baseline (n = 16): 9.3 (0.9-17.7).
Change in MBQ score between measurement at the end of follow-up and at baseline. Higher scores indicate worse QoL. Mean difference with corresponding 95% CI: ∗AUB (n = 50) vs no AUB (n = 24): 5.9 (−0.1 to 11.8). ∗∗New-onset AUB (n = 30) vs no AUB (n = 24): 9.2 (3.4-15.0). ∗∗∗New-onset AUB (n = 30) vs AUB with PBAC >100 at baseline (n = 16): 9.3 (0.9-17.7).
Discussion
In this dedicated prospective multicenter cohort, two thirds of women of reproductive age who received anticoagulant treatment for acute VTE experienced AUB. Importantly, AUB was associated with a considerable negative impact on QoL, which was most pronounced in women with new-onset AUB. Currently, qualifying or quantifying menstrual blood loss in patients with VTE is not part of routine patient care, nor is it recommended by any of the major guidelines. Our findings highlight the critical need to incorporate assessment of AUB in daily practice because of its impact and easily available treatment options.
Our study showed remarkably high rates of AUB compared with rates as high as 30% described in the general population of women of reproductive age,1,2 although it could be expected that the incidence of AUB in women who receive oral anticoagulants is higher than in the general population. Some previous studies estimated the risk of AUB in women receiving oral anticoagulation—however, not all with the use of PBACs—and found rates comparable with our findings.15,32,33 In an observational study, 70% of women reported a change in the bleeding pattern of their menstrual cycle after initiation of VKA therapy, with a mean increase in duration from 5.4 to 6.6 days (P = .0008).32 One study assessed the effect of initiation of VKA on menstrual bleeding in 90 women and found an increase in self-reported menorrhagia from 44% to 71%.33 In a case series of 178 women treated with oral Xa inhibitors, 57 women (32%) had self-reported vaginal bleeding events.10 In these women, 72 self-reported events of vaginal bleeding occurred, including 59 reports of heavy menstrual bleeding, defined as unusually intense or prolonged bleeding related to the menstrual cycle; these were not measured objectively. Of note, in subanalyses of the large phase 3 direct oral anticoagulant trials, the incidence of AUB was estimated to be much lower (0%-8%), but with a likely low sensitivity to detect AUB given the lack of structural assessment of menstrual blood loss.34 Our study supports the proposed association of incident AUB with anticoagulation. In the general population, half of women with AUB have related conditions such as myoma or polyps, whereas this was only 25% to 30% in our cohort.2,35 Moreover, none of the women with AUB in our cohort was diagnosed with von Willebrand disease, whereas the overall prevalence is 13% in the general population of women with AUB.36 The particularly high prevalence of AUB during the first menstrual cycle after the VTE diagnosis in our study could be related to the higher initial dose of anticoagulant agents, in particular rivaroxaban (3 weeks, 15 mg twice daily) and apixaban (1 week, 10 mg twice daily).
The frequent need for medical treatment related to AUB further confirms the relevance of these bleeding events that usually do not meet the ISTH criteria for major bleeding. In the present study, we observed that 32% of women with AUB received dedicated treatment, including invasive procedures such as hysterectomy and polypectomy. Previous studies reported even higher rates of necessary medical treatment or required hospital admission in women with AUB, with one study reporting 43% of women needing medical treatment and another reporting surgical treatment of 14% of women with heavy menstrual bleeding events while receiving oral Xa inhibitors.10,11,17,18 Our study provides important insights into the corresponding impact of AUB on QoL, measured by a validated patient-reported questionnaire developed for women with AUB. Our results show that women with (new-onset) AUB experienced a remarkable decrease in QoL, which is a relevant finding with clinical implications, although a minimal clinically important difference for the MBQ is not available to date. AUB-associated loss of quality-adjusted life years could be one of the explanations for the consistently reported decreased QoL in female vs male pulmonary embolism survivors, another indication that AUB should be taken very seriously and prompt counseling by an expert gynecologist.34,37
In a subgroup analysis, we observed that the few women treated with dabigatran did not experience an increase in menstrual blood loss during the first menstrual cycle after VTE diagnosis, in contrast to women receiving oral Xa inhibitors and VKAs. However, the median retrospective PBAC score was higher in women treated with dabigatran, indicating preexisting AUB in these women. Treatment with dabigatran was found to be associated with a lower incidence of AUB compared with warfarin in a previous study as well, and Xa inhibitors have indeed been suggested to increase the risk of AUB compared with VKAs.8-13,15-18 However, the observational design of our study and the low number of patients exposed to the different drug classes prevent us from drawing any conclusions on differences between direct oral anticoagulant agents. Ongoing randomized trials are expected to shed more light on this important issue.38,39
What are the implications of our study? Despite the early study termination and the resulting lower-than-intended sample size, we provide the most accurate and valid assessment of the risk and impact of AUB in women with VTE to date. Our findings should be a call to action to increase awareness of AUB in patients undergoing anticoagulant therapy. A careful history should be obtained, with special attention to AUB, and VTE caretakers should be trained in the relevant counseling of patients.40 Assessment of the severity of the last menstrual cycle before VTE diagnosis by completing a PBAC at the time of VTE diagnosis predicted the occurrence of AUB after the start of anticoagulant treatment, but not new-onset AUB. The ISTH BAT that assesses the pretest probability of an underlying bleeding disorder, of which none were found in our population, did not prove relevant either. Women diagnosed with AUB should be referred to a gynecologist to rule out alternative causes.41 Treatment options to mitigate the blood loss include tranexamic acid during the menstrual period, use of a (progestogen-containing) intrauterine device, progestin-only therapy, or combined hormonal contraceptives.41 Continuation of hormonal contraceptives is suggested to be safe considering the suppression of the prothrombotic effect of hormonal therapy by therapeutic anticoagulation.40,42 However, combined oral contraceptives should be discontinued some time before anticoagulant cessation.34,40-42 Modifications in anticoagulant treatment including reduced dosing of direct oral anticoagulants or switching of anticoagulant agents could be considered in women with AUB.41,43 A decrease in duration and intensity of menstrual bleeding was found with rivaroxaban 10 mg once daily compared with 20 mg once daily for extended treatment of VTE after completion of 6 to 12 months of anticoagulation.44 The ongoing heavy MEnstrual bleeding in premenopausal women treated with DirEct oral Anticoagulants (MEDEA) randomized clinical trial is currently evaluating management strategies, including switching to dabigatran, in women of reproductive age with AUB while receiving factor Xa inhibitors.39
Strengths of this study include the prospective multicenter observational design and a follow-up period of 6 months (or for the duration of anticoagulant therapy) in which each menstrual cycle that occurred was assessed. The use of PBACs allowed for quantification of the amount of blood loss and comparison of menstrual blood loss before and during anticoagulation. By also using a self-reported definition of AUB, we captured the patients’ perspective in the definition of abnormal menstrual blood loss and were able to show the overlap and discrepancies between prevalence of AUB based on the different definitions. The discrepancy between self-reported and criteria-diagnosed AUB is relevant, as it shows that women may have more than average menstrual blood loss without knowing.
The study has some limitations. First, the originally considered sample size of 210 patients was not reached as a result of slow recruitment in the pandemic and a lack of resources. Consequently, the primary end point contains a wider 95% CI than prespecified in the study protocol. Second, because subgroups were small, no valid comparisons other than descriptive analyses could be performed. Third, the existence of AUB before VTE was determined based on a single retrospective PBAC assessment. However, assessment of multiple PBACs at baseline for several menstrual cycles in retrospect seems infeasible and would increase the risk of recall bias. The clear impact on QoL of new-onset AUB (as defined in our study) shows the relevance of our approach. Fourth, because not all women had a menstrual cycle every month during the follow-up period because of the irregular nature of menstrual cycles, and because the duration of anticoagulant therapy in the study population varied, we could not provide absolute risks per month and realize that the results are therefore less easy to interpret. As a solution for this, we performed analyses per menstrual cycle and calculated the overall prevalence of AUB at any time during the follow-up period. We do consider this to be an adequate approach to analyze the data, reflecting the natural occurrence of menstrual cycles.
In conclusion, this study provides important data on the risk and impact of AUB in women of reproductive age treated with anticoagulants for VTE, showing that AUB is highly incident in this setting and negatively impacts QoL. VTE caretakers should be aware of this, take appropriate action to assess menstrual blood loss in all women with a new VTE diagnosis routinely, and adequately treat incident AUB. Furthermore, our findings underline the need for future studies assessing the optimal strategies for the prevention and management of AUB in this group of patients.
Authorship
Contribution: C.M.M.d.J. and F.A.K performed analyses; C.M.M.d.J., M.B., and F.A.K. drafted the manuscript; and all authors provided important intellectual content and approved the manuscript’s final version.
Conflict-of-interest disclosure: C.A. received personal fees for lectures and participation in advisory boards from Bayer, BMS, Daiichi-Sankyo, Pfizer, and Sanofi. J.B.-W. received honoraria and institutional research support from Bayer AG, Boehringer Ingelheim, Bristol-Myers Squibb/Pfizer, Daiichi Sankyo, DOASENSE, and Alexion. L.B. reports personal fees and nonfinancial support from Aspen, personal fees and nonfinancial support from Bayer, personal fees and nonfinancial support from BMS-Pfizer, personal fees and nonfinancial support from Léo-Pharma; grants, personal fees, and nonfinancial support from MSD; and personal fees and nonfinancial support from Johnson and Johnson, all outside the submitted work. M.P.D. received grants from the Italian Ministry of Health outside the submitted work and paid to his institution. L.J.-P. reports personal fees from Bayer Hispania, Actelion, MSD, Rovi, and Leo-Pharma foundation and grants from Leo-Pharma foundation outside the submitted work. P.R.-A. reports research grants from Rovi outside the submitted work and paid to his institution, consulting fees from Viatris, and payment or honoraria from BMS, Viatris, Leo Pharma, Rovi, and Daiichi. C.T. reports grants from Roche, Bayer, MSD, GSK, and Actelion, all outside the submitted work. T.V. has participated in advisory boards and/or as a speaker for Bayer, Bristol-Myers Squibb/Pfizer, Boehringer-Ingelheim, Leo Pharma, Daiichi Sankyo, and Novartis. F.A.K. reports research grants from Bayer, Bristol-Myers Squibb, Boehringer-Ingelheim, MSD, Leo Pharma, Daiichi-Sankyo, Actelion, The Netherlands Organisation for Health Research and Development, The Dutch Thrombosis Association, The Dutch Heart Foundation, and the Horizon Europe Program, all outside the submitted work and paid to his institution. The remaining authors declare no competing financial interests.
A complete list of the investigators of the TEAM-VTE Study Group appears in “Appendix.”
Correspondence: F. A. Klok, Department of Medicine–Thrombosis and Hemostasis, Leiden University Medical Center, Albinusdreef 2, 2333 ZA, Leiden, The Netherlands; e-mail: f.a.klok@lumc.nl.
Appendix
TEAM-VTE investigators: Cihan Ay, Corinne Bernabe, Laurent Bertoletti, Jan Beyer-Westendorf, Judith Biechele, Marc Blondon, Janet Brantsma, Andrea Buchmuller, Carole Chauvet, Katrien Cludts, Giovanna Colombo, Marco Paolo Donadini, Elke Festerling, Stephan V. Hendriks, Maaike Heuvel, Luis Jara-Palomares, Cloe Jezequel, Cindy M. M. de Jong, Frederikus A. Klok, Stephan Nopp, Miranda Rijnhout, Pedro Ruiz-Artacho, Marianne Spindler, Pauline Stephan, Luise Tittl, Cecile Tromeur, Thomas Vanassche, Kristine Vanheule, Peter E. Westerweel, and Sandra de Zeeuw.
References
Author notes
Complete deidentified participant data collected for this study will be made available after publication to researchers whose proposed use of the data has been approved with a signed data access agreement. Requests for access to the clinical study data can be submitted via e-mail to f.a.klok@lumc.nl. The study protocol will be made available and is part of the supplemental data.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal