Key Points
Patients in remission treated with Oral-AZA had better OS and RFS vs placebo regardless of NPM1 or FLT3 mutational status at AML diagnosis.
Oral-AZA conferred OS and/or RFS benefits in patients with favorable (NPM1mut, no MRD) or adverse (FLT3mut, MRD+) prognostic AML features.
Abstract
The randomized, placebo-controlled, phase 3 QUAZAR AML-001 trial (ClinicalTrials.gov identifier: NCT01757535) evaluated oral azacitidine (Oral-AZA) in patients with acute myeloid leukemia (AML) in first remission after intensive chemotherapy (IC) who were not candidates for hematopoietic stem cell transplantation. Eligible patients were randomized 1:1 to Oral-AZA 300 mg or placebo for 14 days per 28-day cycle. We evaluated relapse-free survival (RFS) and overall survival (OS) in patient subgroups defined by NPM1 and FLT3 mutational status at AML diagnosis and whether survival outcomes in these subgroups were influenced by presence of post-IC measurable residual disease (MRD). Gene mutations at diagnosis were collected from patient case report forms; MRD was determined centrally by multiparameter flow cytometry. Overall, 469 of 472 randomized patients (99.4%) had available mutational data; 137 patients (29.2%) had NPM1 mutations (NPM1mut), 66 patients (14.1%) had FLT3 mutations (FLT3mut; with internal tandem duplications [ITD], tyrosine kinase domain mutations [TKDmut], or both), and 30 patients (6.4%) had NPM1mut and FLT3-ITD at diagnosis. Among patients with NPM1mut, OS and RFS were improved with Oral-AZA by 37% (hazard ratio [HR], 0.63; 95% confidence interval [CI], 0.41-0.98) and 45% (HR, 0.55; 95% CI, 0.35-0.84), respectively, vs placebo. Median OS was improved numerically with Oral-AZA among patients with NPM1mut whether without MRD (48.6 months vs 31.4 months with placebo) or with MRD (46.1 months vs 10.0 months with placebo) post-IC. Among patients with FLT3mut, Oral-AZA improved OS and RFS by 37% (HR, 0.63; 95% CI, 0.35-1.12) and 49% (HR, 0.51; 95% CI, 0.27-0.95), respectively, vs placebo. Median OS with Oral-AZA vs placebo was 28.2 months vs 16.2 months, respectively, for patients with FLT3mut and without MRD and 24.0 months vs 8.0 months for patients with FLT3mut and MRD. In multivariate analyses, Oral-AZA significantly improved survival independent of NPM1 or FLT3 mutational status, cytogenetic risk, or post-IC MRD status.
Introduction
A wide variety of cytogenetic and molecular abnormalities are implicated in the pathogenesis of acute myeloid leukemia (AML).1-3 Among the most common gene mutations in patients with AML are alterations in nucleophosmin 1 (NPM1) and fms-related tyrosine kinase 3 (FLT3) genes, both of which have been shown to be prognostic of therapeutic outcomes and survival.1,4 NPM1 proteins have a variety of cellular functions, including activity related to RNA expression and maturation; DNA replication, transcription, and repair; and preventing protein misfolding and aggregation (molecular chaperoning) for histones and other proteins.5,6NPM1 mutations (NPM1mut) occur in approximately one-third of younger adult patients at AML diagnosis and decrease in frequency with older age.1,7-10NPM1mut AML is recognized as a distinct clinical entity by the World Health Organization.11 Functional FLT3 proteins are expressed by myeloid progenitor cells and play an important role in proliferation, differentiation, and survival of multipotent stem cells.12FLT3 mutations (FLT3mut) are observed in ∼30% of patients at AML diagnosis,1 also decreasing with older age,12 and manifesting as internal tandem duplications (ITD) in ∼15% to 30% of patients, most often located in the juxtamembrane domain of the gene, and as point mutations in the tyrosine kinase domain (TKD) in ∼8% of patients.1,13 FLT3 is overexpressed in AML blasts, and FLT3mut can lead to constitutive phosphorylation of the FLT3 receptor in the absence of FLT3 ligand and activate downstream signaling pathways.14
Current guidelines for AML ascribe disease risk, in part, based on NPM1 and FLT3 mutational status15; these mutations frequently co-occur in patients with AML, implying molecular synergisms that promote AML development.1,6NPM1mut are particularly sensitive to intensive chemotherapy (IC) and typically are associated with favorable prognosis when no co-occurring FLT3-ITD mutation is present or when FLT3-ITD is present at a low allelic ratio (<0.5).4,13,15-17 Conversely, FLT3-ITD alterations generally confer a poor prognosis in the absence of co-occurring NPM1mut, or at a high allelic ratio (≥0.5) when accompanying an NPM1mut.9,13,15 The prognostic implication of FLT3-TKD mutations seems to depend on co-occurring mutational status; although these point mutations generally confer negative outcomes, prognosis mainly is favorable when FLT3-TKD mutations are accompanied by NPM1mut or core-binding factor AML.18-20
Although 40% to 80% of patients with AML can attain complete remission (CR) with IC, most patients eventually relapse because of regrowth of existing (or development of new) leukemic clones.15,21 The presence of measurable residual disease (MRD) after IC is strongly prognostic of poorer overall survival (OS) and relapse-free survival (RFS) for patients with AML in remission.15,21-28 An ongoing need exists for treatments that can suppress regrowth of leukemic cells to maintain remission and prolong OS.29 Oral azacitidine (Oral-AZA [CC-486]) is a hypomethylating agent currently approved in the United States, the European Union, Switzerland, the United Kingdom, and Canada for patients with AML who achieve CR or CR with incomplete blood recovery (CRi) after IC and who are not eligible for curative therapy (eg, hematopoietic stem cell transplantation). Oral administration of azacitidine allows for extended dosing schedules that are not practical with the injectable regimen. In the randomized, phase 3 QUAZAR AML-001 trial (ClinicalTrials.gov identifier: NCT01757535), Oral-AZA significantly improved both OS and RFS from the time of randomization in older patients in first remission after IC compared with placebo.30 Moreover, both OS and RFS were prolonged with Oral-AZA vs placebo regardless of whether patients had post-IC MRD at study entry.31
We performed post hoc analyses of data from the QUAZAR AML-001 trial to understand better the effects of Oral-AZA vs placebo in patients with prognostic mutations at AML diagnosis, specifically NPM1mut and/or FLT3mut, and whether survival outcomes for patients in these gene mutation subgroups were influenced by post-IC MRD status at baseline.
Methods
Patients and assessments
The international, randomized, double-blind, placebo-controlled QUAZAR AML-001 trial was conducted in accordance with the principles outlined in the Declaration of Helsinki. All patients provided written informed consent before study participation. Study design, eligibility criteria, primary and secondary efficacy outcomes, and safety for all enrolled patients have been reported in detail.30 Briefly, eligible patients were 55 years of age or older with newly diagnosed de novo or secondary AML (World Health Organization 2008 classification32) and intermediate- or poor-risk cytogenetic findings at diagnosis (per National Comprehensive Cancer Network 2011 criteria33), had achieved first remission (CR or CRi) after IC (induction chemotherapy with or without subsequent consolidation) and were not candidates for hematopoietic stem cell transplantation. Within 4 months (±7 days) of first CR or CRi, patients were randomized 1:1 to receive Oral-AZA 300 mg or placebo once daily for 14 days of repeated 28-day treatment cycles.
Cytogenetic and molecular gene mutation assessments were performed locally at AML diagnosis (ie, before study screening). Molecular profiling was performed according to institutional practices using standard metaphase cytogenetics and targeted molecular sequencing methods, with no protocol-specified methodology requirements or central confirmation. Mutations in specific genes present at diagnosis were captured on electronic case report forms completed for each patient at study screening. The gene mutation analyses typically were performed using polymerase chain reaction-based or next-generation sequencing methods. FLT3-ITD mutations typically were determined using polymerase chain reaction and fragment analyses. Any variant allele frequency data were reported rarely.
The primary and key secondary trial end points were OS and RFS, respectively, both measured from the time of randomization. OS was assessed until death by any cause, and RFS was the time to relapse (ie, ≥5% blasts in bone marrow) or death, whichever occurred first. To determine the impact of NPM1mut and FLT3mut at AML diagnosis on survival end points for these patients in remission after IC, OS and RFS results were compared within each treatment arm for patients with vs without each mutation, using the placebo arm as the key indicator of prognosis in the absence of active maintenance therapy. The effect of each mutation as a biomarker for survival was evaluated by comparing OS and RFS between the Oral-AZA and placebo arms.34
The influence of post-IC MRD status at screening on survival outcomes also was assessed in patients with NPM1mut or FLT3mut. MRD status was assessed prospectively by multiparameter flow cytometry (Münchner Leukämielabor, Munich, Germany) on samples collected at screening (baseline), on day 1 of cycles 3, 6, 9, 12, 15, 18, 21, 24, 30, and 36, and as clinically indicated using a different-from-normal approach and an MRD positivity cutoff of ≥0.1%, which has been shown to have prognostic relevance.35-38 MRD-related end points included patients with MRD evaluations at baseline and at ≥1 postbaseline visit. The rate of conversion from MRD-positivity at baseline to an MRD− state during treatment (ie, MRD response) was assessed in subgroups defined by NPM1mut status at diagnosis. An MRD response required conversion to MRD− status at 2 or more consecutive MRD assessments. For all patients with NPM1mut, MRD− duration during treatment was computed as the total duration of MRD− status excluding intervals of MRD+ status.
Statistical methods
Baseline characteristics were summarized in patient subgroups defined by NPM1 and FLT3 mutational status using descriptive statistics and compared using χ2 and Fisher exact tests as appropriate. OS and RFS were estimated using Kaplan–Meier methods and were compared within and between treatment arms in mutation-based subgroups with hazard ratios (HR) and 95% confidence intervals (CI) from exploratory Cox proportional hazards regression models and nominal P values from log-rank tests. These analyses were not powered prospectively to detect statistically significant differences in OS or RFS between treatment arms for patient subgroups defined by mutational status. Except when stated otherwise, the FLT3mut subgroup includes all patients with an FLT3-ITD and/or FLT3-TKD mutation.
Multivariate Cox regression analyses were conducted to evaluate treatment effects of Oral-AZA vs placebo on OS and RFS while adjusting for the influence of multiple prognostic variables simultaneously. The covariates in the model were NPM1 mutational status at diagnosis (NPM1mut vs NPM1 wild-type [NPM1wt]), FLT3 mutational status at diagnosis (FLT3mut vs FLT3wt), cytogenetic risk at diagnosis (intermediate vs poor), post-IC MRD status at baseline (MRD+ vs MRD−), and randomized treatment arm (Oral-AZA vs placebo).
Statistical analyses were performed using R statistical software39 version 4.0.3 and the survival package version 3.2-7 (R Foundation for Statistical Computing, Vienna, Austria) and GraphPad Prism software version 8.0 (GraphPad Software, San Diego, CA).
Results
Patients
In all, 472 patients were enrolled in QUAZAR AML-001 and 469 patients (99.4%) had mutational data available at AML diagnosis recorded on electronic case report forms (supplemental Figure 1, available on the Blood website). NPM1mut and FLT3mut were found in 137 patients (29.2%) and 66 patients (14.1%), respectively (Table 1).
NPM1 and FLT3 co-mutational statuses at AML diagnosis
| . | Biomarker cohort∗ (N = 469) . | ||
|---|---|---|---|
| Oral-AZA (n = 236) . | Placebo (n = 233) . | All patients (N = 469) . | |
| Gene mutation, n (%) | |||
| NPM1mut | 66 (28.0) | 71 (30.5) | 137 (29.2) |
| FLT3mut | 30 (12.7) | 36 (15.5) | 66 (14.1) |
| FLT3-ITD+ | 21 (8.9) | 25 (10.7) | 46 (9.8) |
| FLT3-TKDmut | 11 (4.7) | 13 (5.6) | 24 (5.1) |
| FLT3-ITD+ and FLT3-TKDmut | 2 (0.8) | 2 (0.9) | 4 (0.9) |
| NPM1/FLT3-ITD co-mutation status, n (%) | |||
| NPM1mut + FLT3-ITD+ | 12 (5.1) | 18 (7.7) | 30 (6.4) |
| NPM1mut + FLT3-ITD– | 54 (22.9) | 53 (22.7) | 107 (22.8) |
| NPM1wt + FLT3-ITD+ | 9 (3.8) | 7 (3.0) | 16 (3.4) |
| NPM1wt + FLT3-ITD– | 161 (68.2) | 155 (66.5) | 316 (67.4) |
| NPM1/FLT3-TKD co-mutation status, n (%) | |||
| NPM1mut + FLT3-TKDmut | 9 (3.8) | 8 (3.4) | 17 (3.6) |
| NPM1mut + FLT3-TKDwt | 57 (24.2) | 63 (27.0) | 120 (25.6) |
| NPM1wt + FLT3-TKDmut | 2 (0.8) | 5 (2.1) | 7 (1.5) |
| NPM1wt + FLT3-TKDwt | 168 (71.2) | 157 (67.4) | 325 (69.3) |
| . | Biomarker cohort∗ (N = 469) . | ||
|---|---|---|---|
| Oral-AZA (n = 236) . | Placebo (n = 233) . | All patients (N = 469) . | |
| Gene mutation, n (%) | |||
| NPM1mut | 66 (28.0) | 71 (30.5) | 137 (29.2) |
| FLT3mut | 30 (12.7) | 36 (15.5) | 66 (14.1) |
| FLT3-ITD+ | 21 (8.9) | 25 (10.7) | 46 (9.8) |
| FLT3-TKDmut | 11 (4.7) | 13 (5.6) | 24 (5.1) |
| FLT3-ITD+ and FLT3-TKDmut | 2 (0.8) | 2 (0.9) | 4 (0.9) |
| NPM1/FLT3-ITD co-mutation status, n (%) | |||
| NPM1mut + FLT3-ITD+ | 12 (5.1) | 18 (7.7) | 30 (6.4) |
| NPM1mut + FLT3-ITD– | 54 (22.9) | 53 (22.7) | 107 (22.8) |
| NPM1wt + FLT3-ITD+ | 9 (3.8) | 7 (3.0) | 16 (3.4) |
| NPM1wt + FLT3-ITD– | 161 (68.2) | 155 (66.5) | 316 (67.4) |
| NPM1/FLT3-TKD co-mutation status, n (%) | |||
| NPM1mut + FLT3-TKDmut | 9 (3.8) | 8 (3.4) | 17 (3.6) |
| NPM1mut + FLT3-TKDwt | 57 (24.2) | 63 (27.0) | 120 (25.6) |
| NPM1wt + FLT3-TKDmut | 2 (0.8) | 5 (2.1) | 7 (1.5) |
| NPM1wt + FLT3-TKDwt | 168 (71.2) | 157 (67.4) | 325 (69.3) |
Patients with available mutation data at diagnosis on electronic case report forms.
NPM1
Compared with NPM1wt (n = 332), patients with NPM1mut (n = 137) were significantly more likely to be female (P = .033), to have intermediate-risk cytogenetic findings (P < .001) and co-occurring FLT3mut (P < .001) at diagnosis, to have received consolidation chemotherapy after induction (P = .011), and to be without MRD after IC (P = .014) (supplemental Table 1). Baseline characteristics for patients with NPM1mut at AML diagnosis were comparable between the Oral-AZA and placebo arms (supplemental Table 2).
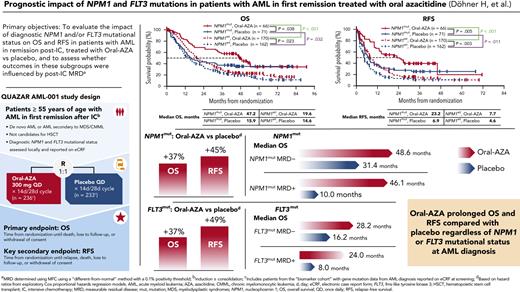
NPM1 mutational status at AML diagnosis was highly prognostic of survival within each treatment arm. In the placebo arm, OS was improved significantly for patients with NPM1mut (n = 71) vs NPM1wt (n = 162; median, 15.9 months vs 14.6 months, respectively) (Figure 1), correlating with a 31% reduced risk of death (OS: HR, 0.69; 95% CI, 0.49-0.97; P = .032) (supplemental Table 3). Within the Oral-AZA arm, patients with NPM1mut (n = 66) had longer median OS than patients with NPM1wt (n = 170; 47.2 months vs 19.6 months, respectively) and a 48% reduced risk of death (OS: HR, 0.52; 95% CI, 0.36-0.75; P < .001). Similarly, patients with NPM1mut at diagnosis had significantly longer median RFS compared with patients with NPM1wt in both the placebo (6.9 months vs 4.6 months) and Oral-AZA (23.2 months vs 7.7 months) arms (Figure 1), reflecting a 35% RFS improvement within the placebo arm (HR, 0.65; 95% CI, 0.47-0.91; P = .011) and a 54% improvement in RFS within the Oral-AZA arm (HR, 0.46; 95% CI, 0.31-0.66; P < .001) (supplemental Table 3).
OS and RFS from randomization by NPM1 mutational status at AML diagnosis and randomized treatment arm. (A) OS. (B) RFS.
OS and RFS from randomization by NPM1 mutational status at AML diagnosis and randomized treatment arm. (A) OS. (B) RFS.
NPM1 mutational status also was predictive of a survival benefit with Oral-AZA compared with placebo. In the NPM1mut subgroup, median OS was substantially longer in the Oral-AZA arm than in the placebo arm: 47.2 months vs 15.9 months, respectively (HR, 0.63; 95% CI, 0.41-0.98; P = .038) (Figure 1; supplemental Table 4). Median RFS in this subgroup was 23.2 months with Oral-AZA vs 6.9 months with placebo (HR, 0.55; 95% CI, 0.35-0.84; P = .005). Among patients with NPM1wt, Oral-AZA also was associated with significant improvements vs placebo in both OS (median, 19.6 months vs 14.6 months, respectively; P = .023) and RFS (median, 7.7 months vs 4.6 months; P = .003) (Figure 1).
FLT3
Of the 66 patients with FLT3mut at diagnosis, 46 patients (9.8% of all patients) had FLT3-ITD, 24 patients (5.1%) had FLT3-TKDmut, and 4 patients (0.9%) had both FLT3-ITD and FLT3-TKDmut (Table 1).
Patients with FLT3mut at diagnosis were significantly younger than those with FLT3wt and significantly more likely to have intermediate-risk cytogenetic findings, a co-occurring NPM1mut, and higher Eastern Cooperative Oncology Group performance status score (supplemental Table 5). FLT3 mutational status was not correlated with post-IC MRD status at baseline. Baseline characteristics for patients with FLT3mut at diagnosis generally were balanced between the Oral-AZA and placebo arms (supplemental Table 6).
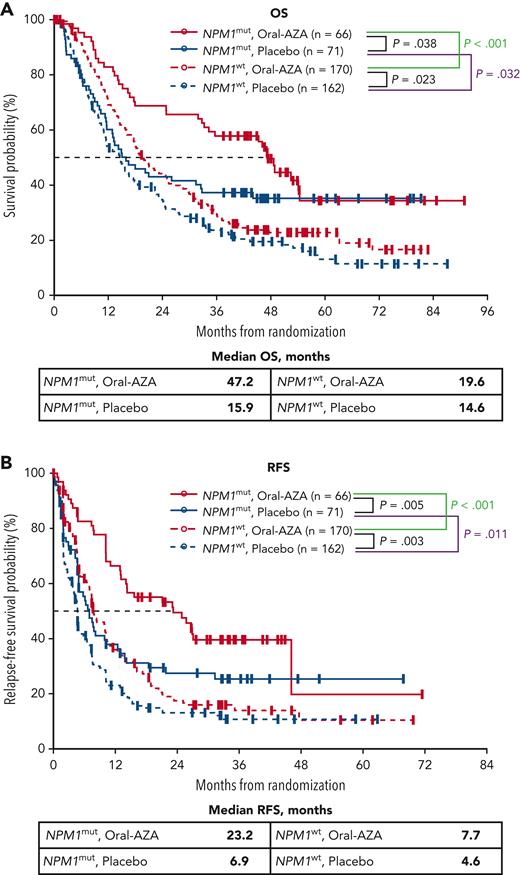
Presence of FLT3mut seemed to confer a negative, but nonsignificant, prognostic effect for patients who received placebo: median OS for patients with FLT3mut (n = 36) and FLT3wt (n = 197) in this arm was 9.7 months vs 15.2 months, respectively (Figure 2), and risk of death was increased by 25% for patients with FLT3mut vs FLT3wt status (HR, 1.25; 95% CI, 0.83-1.89; P = .280) (supplemental Table 3). In contrast to the placebo arm, median OS within the Oral-AZA arm was similar in the FLT3mut subgroup (n = 30) and the FLT3wt subgroup (n = 206), with median OS of 28.2 and 24.7 months, respectively (HR, 0.96; 95% CI, 0.60-1.54; P = .871).
OS and RFS from randomization by FLT3 mutational status at AML diagnosis and randomized treatment arm. (A) OS. (B) RFS. FLT3mut includes both FLT3-ITD and FLT3-TKD mutations.
OS and RFS from randomization by FLT3 mutational status at AML diagnosis and randomized treatment arm. (A) OS. (B) RFS. FLT3mut includes both FLT3-ITD and FLT3-TKD mutations.
Maintenance therapy with Oral-AZA was associated with nominally prolonged median OS vs placebo in patients with FLT3mut at diagnosis (28.2 months vs 9.7 months, respectively; P = .114) and significantly improved median OS for patients with FLT3wt (24.7 months vs 15.2 months; P = .013) (Figure 2; supplemental Table 4). RFS also was improved substantially with Oral-AZA vs placebo in the FLT3mut subgroup, with median RFS of 23.1 months vs 4.6 months in the Oral-AZA and placebo arms, respectively (HR, 0.51; 95% CI, 0.27-0.95; P = .032) (supplemental Table 4). Median RFS with Oral-AZA vs placebo in the FLT3wt subgroup was 10.2 months vs 4.9 months, respectively (P = .001) (Figure 2).
NPM1 and FLT3-ITD co-mutations
At diagnosis, 30 patients (6.4%) had co-occurring NPM1mut and FLT3-ITD, 107 patients (22.8%) had NPM1mut but no FLT3-ITD, and 16 patients (3.4%) had NPM1wt and FLT3-ITD (Table 1); the small number of patients in the latter subgroup precluded meaningful comparison of survival outcomes between the Oral-AZA (n = 9) and placebo (n = 7) arms.
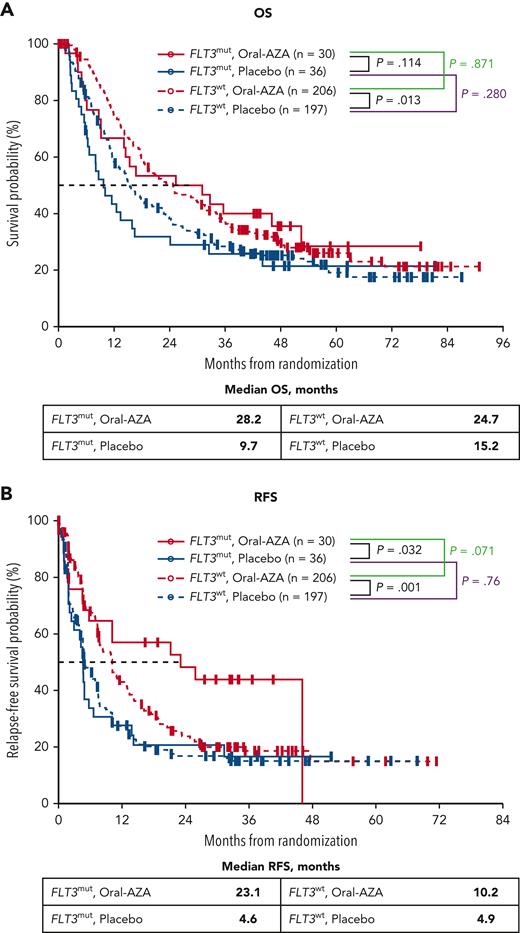
Co-occurrence of NPM1mut and FLT3-ITD at diagnosis showed a generally negative prognostic trend, as indicated by differences in median OS within the placebo arm: 18.0 months for patients with NPM1mut and no FLT3-ITD vs 11.5 months in patients with both mutations (Figure 3). In contrast, within the Oral-AZA arm, the presence or absence of co-occurring FLT3-ITD in patients with NPM1mut did not impact survival meaningfully: median OS was 46.1 months for patients with co-occurring FLT3-ITD and 48.6 months for those with no FLT3-ITD. Oral-AZA nominally prolonged OS vs placebo in patients with NPM1mut with or without co-occurring FLT3-ITD (Figure 3).
OS from randomization in patients with NPM1mut, with or without co-occurring FLT3-ITD, at AML diagnosis. (A) NPM1mut without co-occurring FLT3-ITD. (B) NPM1mut with co-occurring FLT3-ITD. “Other” includes study patients without the specific genetic status at diagnosis.
OS from randomization in patients with NPM1mut, with or without co-occurring FLT3-ITD, at AML diagnosis. (A) NPM1mut without co-occurring FLT3-ITD. (B) NPM1mut with co-occurring FLT3-ITD. “Other” includes study patients without the specific genetic status at diagnosis.
NPM1/FLT3 mutations and post-IC MRD status
Overall, the MRD-evaluable cohort included 463 patients (98.1%; Oral-AZA, n = 236; placebo, n = 227). The rate of post-IC MRD negativity at screening was 61.7% (82/133 patients) for patients with NPM1mut at diagnosis, vs 48.9% (160/327 patients) for patients with NPM1wt (P = .014).
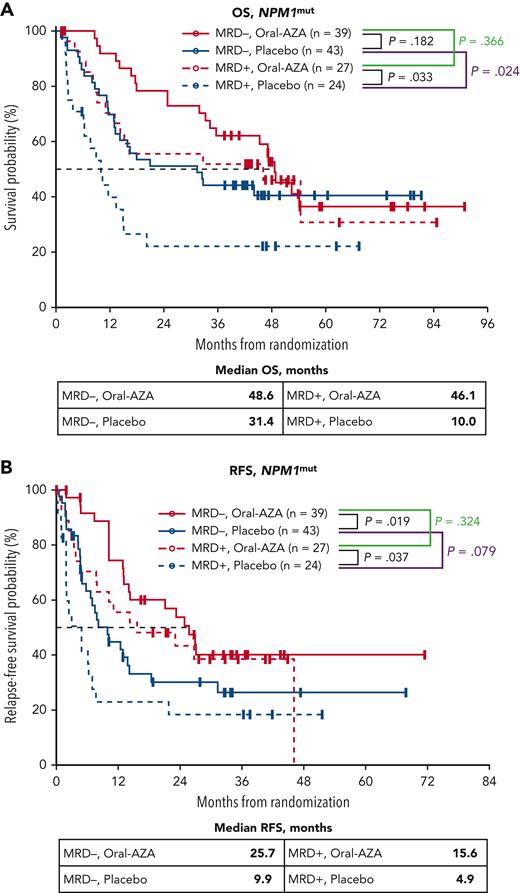
In patients with NPM1mut, MRD status after IC was prognostic of survival in the placebo arm, with median OS of 31.4 months for patients without MRD at screening vs 10.0 months for those with MRD (P = .024). In contrast, median OS was not influenced meaningfully by MRD status at screening in patients with NPM1mut receiving Oral-AZA (median, 48.6 months vs 46.1 months for patients without MRD and with MRD, respectively; P = .366) (Figure 4). Median RFS for patients with NPM1mut at diagnosis who were MRD− or MRD+ at screening within the placebo arm was 9.9 and 4.9 months, respectively (P = .079), and within the Oral-AZA arm was 25.7 and 15.6 months, respectively (P = .324) (Figure 4).
OS and RFS from randomization for patients with NPM1mutat AML diagnosis by MRD status at baseline (after chemotherapy) and randomized treatment arm. (A) OS. (B) RFS. MRD was determined centrally at study entry by multiparameter flow cytometry using a different-from-normal method with a 0.1% positivity threshold.
OS and RFS from randomization for patients with NPM1mutat AML diagnosis by MRD status at baseline (after chemotherapy) and randomized treatment arm. (A) OS. (B) RFS. MRD was determined centrally at study entry by multiparameter flow cytometry using a different-from-normal method with a 0.1% positivity threshold.
Similar to findings in the overall study population,31 median OS was improved numerically with Oral-AZA vs placebo in patients with NPM1mut AML, regardless of MRD status at study entry. Median OS for patients without MRD but with NPM1mut in the Oral-AZA and placebo arms was 48.6 months vs 31.4 months, respectively (P = .182), and was 46.1 months vs 10.0 months (P = .033) for patients with MRD at study entry (Figure 4). Thus, patients with MRD at baseline who received Oral-AZA had nominally longer median OS than patients without MRD who received placebo. Oral-AZA also prolonged RFS relative to placebo in the NPM1mut subgroup regardless of MRD status at baseline (Figure 4).
Among patients with NPM1mut at diagnosis, maintenance therapy with Oral-AZA after remission was associated with a higher rate of MRD response (conversion from MRD at baseline to no MRD on study) compared with placebo (63% [17/27] vs 33% [8/24], respectively; P = .051). For all patients with NPM1mut at diagnosis, duration of no MRD was significantly longer in the Oral-AZA arm vs the placebo arm (15.6 months vs 7.1 months; P = .006).
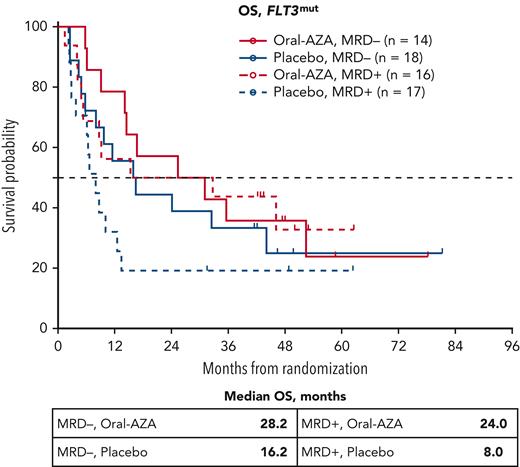
Median OS was similar within the Oral-AZA arm in patients with FLT3mut at diagnosis regardless of whether patients were without MRD after IC at baseline (n = 14; 28.2 months) or with MRD after IC at baseline (n = 16; 24.0 months); whereas within the placebo arm, median OS for patients with FLT3mut who were without MRD at baseline (n = 18; 16.2 months) was twice that of patients who were with MRD at baseline (n = 17; 8.0 months) (Figure 5). The proportion of patients with FLT3mut who converted from having MRD at baseline to being without MRD during treatment (MRD responders) was 50% (8/16 patients) in the Oral-AZA arm, compared with 18% (3/17 patients) in the placebo arm.
OS from randomization for patients with FLT3mutat AML diagnosis by MRD status at baseline (after chemotherapy) and randomized treatment arm. MRD was determined at study entry by multiparameter flow cytometry using a different-from-normal method with a 0.1% positivity threshold. FLT3mut includes both FLT3-ITD and FLT3-TKD mutations.
OS from randomization for patients with FLT3mutat AML diagnosis by MRD status at baseline (after chemotherapy) and randomized treatment arm. MRD was determined at study entry by multiparameter flow cytometry using a different-from-normal method with a 0.1% positivity threshold. FLT3mut includes both FLT3-ITD and FLT3-TKD mutations.
In treatment comparisons, median OS in patients with FLT3mut at diagnosis and without MRD at baseline was 28.2 months vs 16.2 months (P = .495) in the Oral-AZA and placebo arms, respectively, and in patients with FLT3mut and MRD at baseline was 24.0 months vs 8.0 months, respectively (P = .158) (Figure 5).
Multivariate analysis
In multivariate analyses, treatment with Oral-AZA (vs placebo) was an independent prognostic factor of improved OS (P = .004) and RFS (P < .001) after controlling for NPM1 mutational status, FLT3 mutational status, and cytogenetic risk at AML diagnosis and for post-IC MRD status at baseline (Table 2). Each other covariate in the model also was independently predictive of OS: NPM1 (P = .001), FLT3 (P = .036), cytogenetic risk (P < .001), and MRD status (P < .001). All covariates in the model, except FLT3 mutational status at diagnosis (P = .851), were independently predictive of RFS.
Multivariate analysis of effects of select prognostic variables and randomized treatment on overall survival and relapse-free survival on study
| Survival . | HR . | 95% CI . | P value . |
|---|---|---|---|
| Overall | |||
| Randomized treatment: Oral-AZA vs placebo | 0.732 | 0.5898-0.9074 | .004 |
| NPM1 mutation status at diagnosis: NPM1mut vs NPM1wt | 0.624 | 0.469-0.829 | .001 |
| FLT3 mutation status at diagnosis: FLT3mut (ITD/TKD) vs FLT3wt | 1.444 | 1.023-2.039 | .036 |
| Cytogenetic risk at diagnosis: poor vs intermediate | 1.853 | 1.376-2.495 | <.001 |
| MRD status at screening: MRD+ vs MRD– | 1.716 | 1.380-2.133 | <.001 |
| Relapse-free | |||
| Randomized treatment: Oral-AZA vs placebo | 0.631 | 0.509-0.782 | <.001 |
| NPM1 mutation status at diagnosis: NPM1mut vs NPM1wt | 0.614 | 0.467-0.808 | <.001 |
| FLT3 mutation status at diagnosis: FLT3mut (ITD/TKD) vs FLT3wt | 1.034 | 0.727-1.472 | .851 |
| Cytogenetic risk at diagnosis: poor vs intermediate | 1.753 | 1.294-2.375 | <.001 |
| MRD status at screening: MRD+ vs MRD– | 1.984 | 1.596-2.466 | <.001 |
| Survival . | HR . | 95% CI . | P value . |
|---|---|---|---|
| Overall | |||
| Randomized treatment: Oral-AZA vs placebo | 0.732 | 0.5898-0.9074 | .004 |
| NPM1 mutation status at diagnosis: NPM1mut vs NPM1wt | 0.624 | 0.469-0.829 | .001 |
| FLT3 mutation status at diagnosis: FLT3mut (ITD/TKD) vs FLT3wt | 1.444 | 1.023-2.039 | .036 |
| Cytogenetic risk at diagnosis: poor vs intermediate | 1.853 | 1.376-2.495 | <.001 |
| MRD status at screening: MRD+ vs MRD– | 1.716 | 1.380-2.133 | <.001 |
| Relapse-free | |||
| Randomized treatment: Oral-AZA vs placebo | 0.631 | 0.509-0.782 | <.001 |
| NPM1 mutation status at diagnosis: NPM1mut vs NPM1wt | 0.614 | 0.467-0.808 | <.001 |
| FLT3 mutation status at diagnosis: FLT3mut (ITD/TKD) vs FLT3wt | 1.034 | 0.727-1.472 | .851 |
| Cytogenetic risk at diagnosis: poor vs intermediate | 1.753 | 1.294-2.375 | <.001 |
| MRD status at screening: MRD+ vs MRD– | 1.984 | 1.596-2.466 | <.001 |
Discussion
Of the several aberrant genes detected in patients with AML, NPM1mut and FLT3mut are among the most frequent and among the few molecular genetic abnormalities with established (albeit context-dependent) prognostic implications in AML.1,15,40,41 The proportion of patients with NPM1 mutations at diagnosis in this study (29.2%) was approximately equal to historical rates in other AML populations,1,3 which would be expected, because these patients generally tend to respond well to IC.1,42 Consistent with findings in other populations of older patients with newly diagnosed AML,1,42,43 the presence of NPM1mut at diagnosis in this cohort of patients was associated with longer remission duration and prolonged survival compared with those with NPM1wt within each treatment arm. Notably, however, treatment with Oral-AZA maintenance therapy was associated with further improvements in both OS and RFS vs placebo in patients with NPM1mut, and even patients treated with Oral-AZA with NPM1wt at diagnosis showed longer median OS and median RFS than patients with NPM1mut who received placebo.
Up to 90% of all patients with AML in remission with detectable MRD will experience morphologic relapse in the absence of post-remission therapy, typically within 6 to 12 months.4 In the subgroup of patients with NPM1mut who had MRD post-IC at baseline in this study, approximately twice as many patients in the Oral-AZA arm achieved an MRD response (converted to MRD− state) compared with the placebo arm, and duration of MRD negativity overall in the Oral-AZA arm was more than twice as long. Among patients with NPM1mut at diagnosis who had no MRD at screening, Oral-AZA prolonged median RFS by approximately 15 months compared with placebo, suggesting that even patients with particularly favorable prognostic disease features can experience extended remission with Oral-AZA maintenance therapy. Multivariate analysis confirmed the significant independent prognostic benefit of Oral-AZA maintenance therapy vs placebo on OS and RFS, regardless of NPM1 mutational status at diagnosis and post-IC MRD status.
Fourteen percent of patients in this study carried FLT3mut at diagnosis, proportionately less than what has been reported historically,1,3 which may reflect the observed lower incidence of these mutations in older patients or low rates of CR or CRi achieved with IC in patients with these mutations.44 Consistent with other studies,1,23,41 in this study, the presence of a FLT3mut at diagnosis in the placebo arm was associated with a negative prognosis, whereas median OS for patients with FLT3mut within the Oral-AZA arm was comparable with that of patients with FLT3wt. Similar to the findings in the NPM1 analyses, patients with FLT3mut at diagnosis treated with Oral-AZA showed better OS and RFS than patients with FLT3wt in the placebo arm, and multivariate analysis demonstrated the significant prognostic benefit of Oral-AZA treatment vs placebo regardless of FLT3 mutational status.
OS and RFS outcomes for patients with FLT3mut at diagnosis in these analyses may be confounded because this subgroup included patients with FLT3-ITD and/or FLT3-TKD mutations, which have differing prognostic implications.13,18,19,45 The number of patients with FLT3-TKD mutations in these analyses was too small to make meaningful comparisons within or between treatment arms. For patients with NPM1mut, the median OS in the Oral-AZA arm was approximately 4 years, regardless of whether patients had a co-occurring FLT3-ITD mutation, and more than 2 years longer than the median OS in patients without co-occurring FLT3-ITD in the placebo arm. However, these subgroups were small and further investigation of Oral-AZA effects in patients with co-occurring NPM1mut and FLT3-ITD is required.
The mechanisms by which Oral-AZA therapy augments clinical benefits in patients with NPM1mut and may attenuate the negative prognostic effect of FLT3mut are not clear. In animal models and leukemic cells from patients with AML, FLT3-ITD mutations can collaborate with mutations in epigenetic regulating genes (eg, TET2, IDH1/2), leading to DNA hypermethylation, altered gene expression, and impaired cellular differentiation.46 It is possible that DNMT1 inhibition and hypomethylating activity with Oral-AZA47,48 ameliorates DNA hypermethylation and restores gene expression and downstream gene signaling pathways in leukemic cells of patients with AML with NPM1mut or FLT3-ITD, but this remains to be determined.
These analyses have some limitations, most notably that mutational assessments were conducted locally at the time of patient diagnosis, before AML treatment with IC and before entry into the QUAZAR AML-001 trial. Although mutational data were collected for almost all patients on the screening electronic case report form for this study, individual mutation calls may have been influenced by a variety of testing approaches with differing gene panels, assay sensitivities, and methodologies performed by institutions worldwide. Further, over the duration of patient enrollment in this study, methodologic advancements in genetic testing and understanding of the genetic landscape of AML were evolving rapidly. Before initiation of this study, the World Health Organization AML diagnostic recommendations included testing for NPM1mut and FLT3mut (at least in patients with normal cytogenetic findings), so it is likely that the mutational subgroups evaluated here included most patients with these mutations. Diagnostic mutational data were binary; that is, the mutation was present or not, and the variant allele frequencies of individual mutations were unknown, limiting interpretation of outcomes in the FLT3-ITD subgroup (or 2017 European LeukemiaNet risk segments) because of the prognostic importance of the FLT3-ITD allelic ratio. Moreover, FLT3-ITD mutations may have been cleared by the time of study entry, after IC. Thus, we cannot be certain about whether or to what extent the positive prognostic effect of co-occurring NPM1mut may have influenced survival outcomes for patients with FLT3-ITD. Finally, patients in this study obtained remission from treatment with IC; it is unknown how Oral-AZA maintenance therapy might influence outcomes of patients who obtain remission by other means, such as treatment with CPX-351 or a venetoclax-based regimen. Despite these limitations, the double-blind, randomized design of the QUAZAR AML-001 trial allows for cross-treatment comparisons of Oral-AZA vs placebo because these shortcomings should apply broadly to both treatment arms.
Patients’ post-IC mutational status at study screening and during treatment in QUAZAR AML-001 are of high interest, and these data currently are under investigation. Because patients entered the trial in morphologic remission with <5% bone marrow blasts, quantitating post-IC mutations with widely different detection thresholds in a small number of leukemic blasts at baseline has been a challenge. Deep sequencing methods are being refined to track gene mutations during the course of Oral-AZA treatment, particularly the types of mutations occurring at relapse.
These data suggest that Oral-AZA maintenance therapy can benefit substantially patients with AML in remission who are not candidates for hematopoietic stem cell transplantation and who have poor prognostic disease features (FLT3mut at diagnosis, MRD after IC) and further can improve survival outcomes for patients with more favorable prognostic disease characteristics (NPM1mut at diagnosis, no MRD after IC) compared with placebo.
Acknowledgments
The authors thank all the patients who participated in the study.
This study was sponsored by Celgene, a Bristol-Myers Squibb Company. Editorial support of an early draft was provided by Sheila Truten of Medical Communication Company, Inc., and by Brian Kaiser and Miriam de Boeck of Excerpta Medica, funded by Bristol Myers Squibb.
The authors are fully responsible for all content and editorial decisions.
Authorship
Contribution: The sponsors collected and analyzed data in conjunction with all authors. H. Döhner and D.L.d.M. wrote the first draft of the manuscript; the data were reviewed and verified by H. Döhner, B.S., W.L.S., M.U., A.R., E.T.C., A.T., C.L.B., and D.L.d.M.; and all authors revised the manuscript and reviewed and approved the final version.
Conflict-of-interest disclosure: H. Döhner received honoraria from and served as a consultant to AbbVie, Agios, Amgen, Astellas, AstraZeneca, Berlin-Chemie, BMS, Celgene, GEMoaB, Gilead, Janssen, Jazz, Novartis, and Syndax; received research funding from AbbVie, Agios, Amgen, Astellas, BMS, Celgene, Jazz Pharmaceuticals, Kronos Bio, and Novartis; and received travel expenses from Servier. A.H.W. served on the speaker’s bureau and advisory board of and received travel expenses from BMS. G.J.R. served as a consultant to or on the advisory board or data and safety monitoring committee of AbbVie, Agios, Amgen, Astellas, AstraZeneca, BMS, Blueprint Medicines, bluebird bio, Celgene, GSK, Janssen, Jasper Therapeutics, Jazz, MEI Pharma (IDMC Chair), Mesoblast, Novartis, Pfizer, Syndax, and Takeda (IRC Chair); and received research support from Janssen. P.M. served as a consultant to, received research funding from, and served on the speaker’s bureau of BMS. F.R.T. served on the advisory board of AbbVie, Astellas, BMS/Celgene, Jazz, Novartis, and Pfizer; and received research support from BMS/Celgene and Novartis. F.R. received research funding from and served on the advisory board of BMS/Celgene. H. Dombret received honoraria from Incyte. K.P. received research funding from BMS/Celgene, Incyte, and Novartis. I.S. served as a consultant to and on the advisory board of Amgen, BMS/Celgene, Janssen, Kite/Gilead, Pfizer, Sanofi, and Takeda; and has stock ownership in illumiSonics Inc. B.S. is employed by BMS. W.L.S. is a contractor for and has a patent filed with BMS. M.U. is employed by and has a patent filed with BMS. A.R., A.T., and D.L.d.M. are employed by, have stock ownership in, and have a patent filed with BMS. E.T.C. and C.L.B. are employed by and have stock ownership in BMS.
Correspondence: Hartmut Döhner, Professor of Medicine, Medical Director, Department of Internal Medicine III, Ulm University Hospital, Albert-Einstein-Allee 23, 89081 Ulm, Germany; e-mail: hartmut.doehner@uniklinik-ulm.de.
References
Author notes
BMS policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal