Abstract
Epstein-Barr virus (EBV) is a ubiquitous human tumor virus, which contributes to the development of lymphoproliferative disease, most notably in patients with impaired immunity. EBV-associated lymphoproliferation is characterized by expression of latent EBV proteins and ranges in severity from a relatively benign proliferative response to aggressive malignant lymphomas. The presence of EBV can also serve as a unique target for directed therapies for the treatment of EBV lymphoproliferative diseases, including T cell–based immune therapies. In this review, we describe the EBV-associated lymphoproliferative diseases and particularly focus on the therapies that target EBV.
Introduction
Epstein-Barr virus (EBV) is the most common human tumor virus, infecting >90% of adults throughout their lifetime.1,2 EBV was first isolated from a Burkitt lymphoma cell line in 1964, and its relationship to cancers has been extensively researched since.3 The primary infection of EBV frequently occurs in childhood, with mild to no symptoms, or in adolescence, with symptoms of infectious mononucleosis. After infection, the virus persists for the lifetime of the host.2,4,5 EBV infects epithelial cells of the oropharynx, followed by replication and spread to B cells, but EBV can also infect various types of human cells (especially in pathogenic settings), including T cells, natural killer (NK) cells, and epithelial cells.6,7 In most immunocompetent individuals, the virus remains latent in memory B cells, controlled by a robust cytotoxic T lymphocyte response.6,8-10 Lifelong persistence of the virus is typically asymptomatic, but especially in those with impaired cell-mediated immunity, the virus can proliferate in an unregulated fashion, with malignant potential.6,8,11,12 The most common EBV-associated malignancy is gastric carcinoma, followed by nasopharyngeal carcinoma and lymphoma.13 We review EBV-associated lymphoproliferative diseases and treatments leveraging EBV as a target.
Lymphoproliferative diseases
EBV infections associated with lymphoid proliferation range from lymphoproliferation with no malignant potential to aggressive lymphoma. Those with no or minimal malignant potential include infectious mononucleosis and EBV+ hemophagocytic lymphohistiocytosis (HLH), with increasing malignant potential apparent in some settings, including chronic active EBV disease (CAEBV).7,14 EBV-associated lymphomas are usually of B-cell origin (eg, Hodgkin lymphoma [HL] or B-cell non-HL [NHL]) but in rarer circumstances can be of NK/T-cell origin. EBV-associated lymphoproliferative diseases develop in those with either congenital or acquired immunodeficiencies, including iatrogenic or posttransplantation lymphoproliferative disease (PTLD), HIV-related lymphoproliferative disorders, and immunodeficiencies associated with primary immune disorders13 (Table 1).
EBV-associated lymphoproliferative diagnoses
| Classification . | Diagnosis . |
|---|---|
| Reactive lymphoid proliferation | HLH CAEBV, B cell, and T/NK cell Hydroa vacciniforme–like lymphoproliferative disorder EBV+ mucocutaneous ulcer Severe mosquito bite allergy |
| B-cell malignancies | Hodgkin Lymphoma Diffuse large B-cell lymphoma Burkitt lymphoma Plasma cell neoplasms Lymphomatoid granulomatosis Plasmablastic lymphoma |
| NK- and T-cell malignancies | Systemic EBV+ T-cell lymphoma of childhood Aggressive NK-cell leukemia Angioimmunoblastic T-cell lymphoma* Follicular T-cell lymphoma* Peripheral T-cell lymphomas Extranodal NK/T-cell lymphoma, nasal type EBV+ nodal T- and NK-cell lymphoma Peripheral T-cell lymphoma, NOS |
| Immunodeficiency related | Posttransplantation lymphoproliferative disorder HIV related Lymphoproliferative disease associated with primary immune deficiencies |
| Classification . | Diagnosis . |
|---|---|
| Reactive lymphoid proliferation | HLH CAEBV, B cell, and T/NK cell Hydroa vacciniforme–like lymphoproliferative disorder EBV+ mucocutaneous ulcer Severe mosquito bite allergy |
| B-cell malignancies | Hodgkin Lymphoma Diffuse large B-cell lymphoma Burkitt lymphoma Plasma cell neoplasms Lymphomatoid granulomatosis Plasmablastic lymphoma |
| NK- and T-cell malignancies | Systemic EBV+ T-cell lymphoma of childhood Aggressive NK-cell leukemia Angioimmunoblastic T-cell lymphoma* Follicular T-cell lymphoma* Peripheral T-cell lymphomas Extranodal NK/T-cell lymphoma, nasal type EBV+ nodal T- and NK-cell lymphoma Peripheral T-cell lymphoma, NOS |
| Immunodeficiency related | Posttransplantation lymphoproliferative disorder HIV related Lymphoproliferative disease associated with primary immune deficiencies |
Shown are diagnoses with proliferative or malignant cells that can demonstrate EBV positivity.
NOS, not otherwise specified.
Angioimmunoblastic T-cell lymphoma and follicular T-cell lymphoma have a follicular T-cell phenotype, but EBV+ B-cell blasts may be present.
EBV protein expression and EBV latency types
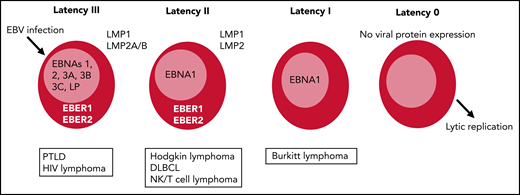
EBV contains multiple gene products that express proteins and small untranslated RNAs, some of which are involved in the lytic or productive cycle of the virus, whereas others are expressed in the latent cycle, allowing the virus to persist long term.15 There are many functions of the different latency EBV proteins, including proliferation, mechanisms to avoid immune surveillance, and resistance to cell death, that contribute to development of lymphoproliferative diseases.12,15 Each EBV latency type is defined by the expression of viral-encoded antigens (Figure 1). These include 6 nuclear antigens (EBNA1, EBNA2, EBNA3A, EBNA3B, EBNA3C, and EBNA-LP), 3 latent membrane proteins (LMP1, LMP2A, and LMP2B), and 2 short noncoding RNAs (EBER1 and EBER2), in addition to >40 microRNAs.4,14,16 In latency type III, there is expression of all EBV antigens (EBNAs, EBERs, and LMPs). The expression of the immunodominant EBNA3 antigens renders type III latency tumors highly immunogenic, and this tumor develops in patients with impaired cell-mediated immunity, including PTLD and other immunocompromised states.6,17 Latency type II tumors express EBNA1, EBERs, and the 3 LMPs. Type II latency is associated with EBV-associated HL and NHL of B- and NK/T-cell origin. LMP1 is the main oncogenic protein described, but expression has been variable in NK/T-cell lymphomas, because these lymphomas have likely also developed virus-independent methods of persistence.7,18,19 EBV+ DLBCL is associated with both latency type II and type III, with some tumors expressing EBNA2 and/or EBNA3.6,20,21 Latency type I expresses EBNA1, which maintains the EBV episomal genome, among other functions, as well as the 2 EBERs. Latency type I is poorly immunogenic and associated with Burkitt lymphoma, with evidence that EBNA1 activates C-MYC expression and mediates some of the antiapoptotic effects.15,22
Latency types after EBV infection range from latency III to latency I/0. Latency III expresses all EBV-encoded proteins and is the most immunogenic, with expression of the immunodominant EBNA3 antigens. Latency II is less immunogenic, expressing only EBNA1, with 2 EBERs and LMP1 and LMP2. Latency III tumors are most amenable to adoptive cellular therapy, although LMP-specific T cells have also shown efficacy for latency II tumors. DLBCL, diffuse large B-cell lymphoma.
Latency types after EBV infection range from latency III to latency I/0. Latency III expresses all EBV-encoded proteins and is the most immunogenic, with expression of the immunodominant EBNA3 antigens. Latency II is less immunogenic, expressing only EBNA1, with 2 EBERs and LMP1 and LMP2. Latency III tumors are most amenable to adoptive cellular therapy, although LMP-specific T cells have also shown efficacy for latency II tumors. DLBCL, diffuse large B-cell lymphoma.
Although the proteins expressed in the latent stages of EBV can serve as targets for EBV-targeted therapy, more recent research suggests lytic EBV antigen expression can also contribute to oncogenesis. Multiple studies have demonstrated presence of lytic gene expression in a variety of lymphomas, with lytic cycle proteins contributing to tumorigenesis via antiapoptotic and immunomodulatory effects.23,24 For this reason, the expression of lytic antigens may also serve as a potential target for novel therapies.1,24,25
Classification
Reactive proliferation
As described, EBV-associated lymphoproliferation ranges from reactive proliferation to malignant lymphoma. CAEBV and HLH are reactive disorders that can lead to significant morbidity and mortality, including fulminant lymphoma. CAEBV not only has the potential to progress to high-grade NHL but can also precipitate secondary HLH, coronary artery aneurysms, and liver failure.8 CAEBV is a chronic disease in immunocompetent hosts defined by sustained EBV DNA load in the peripheral blood. It is typically associated with B-cell proliferation, but CAEBV can also be associated with NK/T-cell proliferation, especially in East Asian countries and patients of Asian descent. There is evidence that many patients with B-cell CAEBV have an underlying immune deficiency disorder.8,26,27 Recently, it was demonstrated that patients with CAEBV have evidence of myeloid-derived suppressor cells that inhibit T-cell responses in vitro, which may contribute to the disease process in patients.28 CAEBV is characterized by fever, hepatosplenomegaly, hepatitis, and lymphadenopathy after the initial infectious mononucleosis. Cutaneous forms of CAEBV include hydroa vacciniforme–like lymphoproliferative disorder and severe mosquito bite allergy.8,26,27
EBV is the most common virus associated with HLH. HLH can be secondary to the virus itself or primary HLH as an underlying genetic defect triggered by EBV.7,29 HLH should be considered in the setting of EBV infection when typical features of HLH are present, including fever, splenomegaly, cytopenias, coagulopathy, and central nervous system disturbances.29 Symptoms of HLH may be similar to those of acute EBV infection but typically are more severe in a patient who appears ill. Dexamethasone and etoposide are the mainstays of treatment of HLH, but with presence of EBV, rituximab may be added for treatment unless the EBV disease is NK and/or T cell mediated.29
Lymphomas
EBV is associated with lymphoid neoplasms, according to the 2016 World Health Organization classification, including mature B-cell neoplasms, mature T- and NK-cell neoplasms, HL, and posttransplantation lymphoproliferative disorders.30 Of the mature B-cell lymphomas, DLBCL is the most common lymphoma associated with EBV, and although endemic Burkitt lymphoma is associated with EBV in up to 95% of patients, in the United States it is only associated with ∼20%.31 EBV+ DLBCL not otherwise specified is a disease entity classified separately, but other B-cell lymphomas that can be given a more specific diagnosis can also have evidence of EBV.30,32 HL is also frequently EBV+, and up to 30% of HLs in North America are EBV associated.33
NK- and T-cell lymphomas demonstrated to be associated with EBV include systemic EBV+ T-cell lymphoma of childhood, aggressive NK-cell leukemia, and peripheral T-cell lymphomas, which are further classified as either extranodal or nodal. Systemic EBV+ T-cell lymphoma of childhood is a rapidly progressive and fatal disease comprising a monoclonal expansion of EBV+ T cells. It occurs mainly in East Asia, with some cases in Latin America, but it is much rarer in the Western population.7 Aggressive NK-cell leukemia is also a rare fatal disease characterized by proliferation of NK cells. Extranodal NK/T-cell lymphoma, nasal type, is an aggressive lymphoma that leads to vascular damage and ischemic necrosis. EBV+ nodal T- and NK-cell lymphoma is also a rare peripheral T-cell lymphoma without other extranodal site involvement. Angioimmunoblastic T-cell lymphoma and follicular T-cell lymphomas have a T follicular helper phenotype but may also contain B-cell blasts, which are often EBV+.30
PTLD
PTLD encompasses lymphoid disorders occurring after either solid organ transplantation (SOT) or hematopoietic stem cell transplantation (HSCT) and the iatrogenic immunosuppression required. It was first described in patients undergoing renal transplantation by Doak et al34 in 1968, and the term PTLD was introduced by Starzl et al35 in 1984.36 Both T-cell quality and quantity are affected by the required immunosuppression after transplantation, leading to potential reactivation of EBV and unregulated proliferation of the latently EBV-infected B cells.10,37
PTLD following SOT differs from that in patients undergoing HSCT, because PTLD in those undergoing SOT arises typically from host lymphocytes, whereas in HSCT, the cell origin is more often donor derived.38,39 After SOT, EBV-associated PTLD tends to occur within the first year but can occur later, because immunosuppression is continued long term.37,40 EBV-associated PTLD cases after HSCT typically occur within the first 6 months before reconstitution of EBV-specific T lymphocyte activity, and PTLD diagnosed later is more likely EBV−.40,41 The incidence of PTLD post-HSCT is <2%, whereas in those undergoing SOT, the estimated incidence is up to 20%.13,37,42
Several factors increase the risk of PTLD in patients, including recipient age, high-dose immunosuppression, infection (cytomegalovirus in addition to EBV), type of allograft, and graft rejection.36,43 A recipient who is EBV seronegative receiving an EBV seropositive graft or organ has a higher association with development of PTLD, and children are more likely to be seronegative and develop PTLD after conversion.38 After HSCT, factors that impair T-cell function or lead to more significant T-cell depletion increase EBV-associated PTLD risk.44 Intestinal transplantations have a higher risk of PTLD than other solid organ transplantations, with renal transplantations posing the least amount of risk.37 Although serial monitoring of EBV viral DNA is important to identify patients at risk of PTLD with detection of rising EBV load, the best methods and limits for treatment have not been defined.13,31
World Health Organization classification divides PTLD into 4 types: early lesions (plasmacytic hyperplasia, infectious mononucleosis-like, and florid follicular hyperplasia), polymorphic PTLD, monomorphic PTLD, and classic HL–like.30 Most PTLDs are of B-cell origin, with a minority of NK/T-cell origin. Polymorphic PTLD and monomorphic B-cell PTLD (DLBCL-like) are the 2 most common forms of PTLD seen in childhood, and a vast majority of these are EBV+.
PTLD can present with lymphadenopathy; however, the significant lymphadenopathy associated with other lymphomas is not as frequent, and B symptoms may or may not occur.10,38 Nonspecific and extranodal involvement is common, including of the gastrointestinal tract, lungs, skin, bone marrow, and central nervous system.42 EBV disease should be suspected with lymphadenopathy, hepatosplenomegaly, or lymphomatous growth in the transplanted organ, as applicable in the setting of high EBV DNA load, but ideally, EBV disease is proven through detection of EBV-encoded RNA by in situ hybridization in a tissue specimen.
Treatment
The treatment of EBV-associated lymphoproliferative diseases is unique compared with other lymphoma treatments, because the virus itself can serve as a target for treatment as well as the signaling pathways in which the virus is involved. EBV-associated lymphomas have evidence of immune evasion, including avoidance of apoptosis, persistent proliferation, and evasion of growth suppressors; therefore, as with other lymphomas, inhibitors of these processes can potentially be used for treatment.12 Additional treatments demonstrate induction of lytic viral activation, including chemotherapeutic agents, radiation, steroids, and histone deacetylase (HDAC) inhibitors.33 Although multiple treatment strategies are potentially available, very few large prospective trials exist that have comprehensively evaluated treatment of EBV-associated lymphoproliferative diseases, especially PTLD.37,45 The reasons for this are multifactorial but are associated with the relative rarity of the disease, variety of histologic subtypes, and other complicating factors in these patients, such as pretransplantation diagnoses, risk of allograft rejection, and varying immunosuppressive regimens.
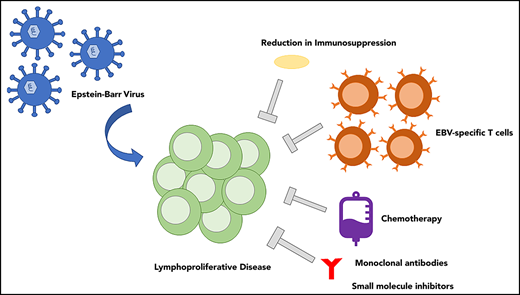
Treatment of PTLD has unique challenges, because treatment must be balanced with risk of graft rejection, graft-versus-host disease (GVHD), delays in immune reconstitution, and opportunistic infections.37 Restoring the immune defects present posttransplantation provides the best framework for disease eradication.43 For PTLD and other immune deficiency–related lymphoproliferations, the goal of treatment therefore is to remove the infected B cells and recover the EBV-specific T cell (EST)-mediated immune response.10Figure 2 outlines an algorithm for treatment of EBV-associated PTLD. Below, we further explore these therapeutic strategies that use EBV as a target.
Algorithm for the treatment of PTLD. Early clinical trial enrollment is pertinent to expand accessibility of treatment options. Reduction in immunosuppression (RIS; as tolerated to limit risk of GVHD or graft rejection) is an initial method of controlling EBV-associated PTLD. Rituximab should be initiated in both polymorphic and monomorphic PTLD, with evidence that complete response (CR) to rituximab alone should be treated with an extended course of rituximab. Without response to rituximab, chemotherapy should be added to the treatment regimen as well as the administration of ESTs (as available in clinical trials). ISH, in situ hybridization.
Algorithm for the treatment of PTLD. Early clinical trial enrollment is pertinent to expand accessibility of treatment options. Reduction in immunosuppression (RIS; as tolerated to limit risk of GVHD or graft rejection) is an initial method of controlling EBV-associated PTLD. Rituximab should be initiated in both polymorphic and monomorphic PTLD, with evidence that complete response (CR) to rituximab alone should be treated with an extended course of rituximab. Without response to rituximab, chemotherapy should be added to the treatment regimen as well as the administration of ESTs (as available in clinical trials). ISH, in situ hybridization.
Reduction and modification of immunosuppression
In the setting of immunosuppression and EBV-associated lymphoproliferative disorders, improving the immune defect can serve as frontline treatment.33 For patients with HIV, continuing antiretroviral therapy is not sufficient but is of significant importance to improve immune function. In patients receiving immunosuppression posttransplantation, identifying high-risk patients and diagnosing early lymphoproliferation can allow for earlier reduction of immunosuppression to prevent disease progression.41 However, reduction of immunosuppression is frequently not a viable option in patients undergoing HSCT because of the risk of GVHD and time until immune reconstitution, and in many solid organ transplant recipients, the risk of rejection may outweigh the potential benefit. Reduction of immunosuppression alone has been documented in retrospective reviews, with responses ranging from 43% to 63% of patients diagnosed with PTLD. However, patients who received reduction of immunosuppression alone are very limited in number, and for most patients, reduction of immunosuppression alone is insufficient, and additional therapies are required.46-49 If reduction in immunosuppression cannot be tolerated, modifying immunosuppression such as with mTOR inhibitors instead of calcineurin inhibitors could potentially be beneficial, although clear evidence is lacking.33
Monoclonal antibodies
Rituximab, a chimeric anti-CD20 monoclonal antibody, has become a standard addition to treatment regimens for multiple B-cell malignancies since its approval in 1997.50 Rituximab targets malignant CD20+ B cells and eliminates the EBV+ latently infected CD20+ memory B-cell population. However, this therapy also targets healthy uninfected CD20+ B cells, which can further immune suppress this vulnerable population.
Cases detailing the treatment of PTLD with rituximab as a single agent to elicit a CR were reported as early as 1999.51 Since then, rituximab has become a first-line treatment of PTLD and has shown efficacy as a monotherapy for PTLD.44 Multiple prospective trials have evaluated rituximab once per week for 3 or 4 weeks as a monotherapy for PTLD after SOT, with overall response rates ranging from 44% to 69% (CR, 25%-53%).45,52-57 Use of rituximab as monotherapy for PTLD in those undergoing allogeneic HSCT is extrapolated from retrospective reviews, with response rates 63% to 70% and documented CRs in up to 84% (43 of 51 patients) when combined with reduction of immunosuppression.44,58,59 Treatment with ofatumumab, a fully human monoclonal anti-CD20 antibody, has been retrospectively reported for patients who could not tolerate rituximab, but this agent has not been evaluated in prospective trials.60
Brentuximab is an antibody-drug conjugate of an antimitotic agent linked to a chimeric anti-CD30 monoclonal antibody that has been shown to have therapeutic benefit for some lymphoma subtypes, such as HL.36,61 Currently, there is limited evidence of therapeutic potential of brentuximab for CD30+ EBV-associated lymphoproliferative disorders. However, successful management of refractory EBV-associated PTLD has been reported with a combination of brentuximab with ESTs.62 In a small series of primary immunodeficiency patients with lymphoproliferative disease, treatment with brentuximab resulted in CR in 6 of 8 patients.63
Checkpoint inhibitors
EBV has been associated with upregulation of PD-1 and its ligands (PD-L1/PD-L2) in a variety of lymphomas, including PTLD.64-66 The blockade of PD-1 signaling has proven successful for treatment of HL (including EBV+ HL), and the US Food and Drug Administration has approved nivolumab for patients with classic HL that has relapsed or progressed after autologous stem cell transplantation and posttransplantation brentuximab vedotin.67 A variety of trials have continued to demonstrate success of PD-1/PD-L1 blockade for relapsed HL, and recently, an overall response rate of 38% was reported for relapsed/refractory NK/T-cell lymphomas.68 However, when considering treatment of EBV-associated PTLD, the risk of graft rejection and GVHD must be taken into account, and there is risk that these checkpoint inhibitors could precipitate rejection. Case reports and series have documented that PD-1 blockade can be effective, although a retrospective series recorded GVHD in up to 55% of patients who received a PD-1 inhibitor post-HSCT for treatment of relapsed lymphoma.69 Therefore, special considerations must be taken to minimize risk of GVHD if checkpoint inhibitors are used to treat EBV-associated lymphoproliferative disorders post-HSCT. The National Cancer Institute is currently leading a phase 2 trial evaluating nivolumab for the treatment of EBV lymphoproliferative disorders (lymphomatoid granulomatosis, CAEBV, EBV-associated PTLD, and DLBCL) in patients age ≥12 years, excluding patients with PTLD after SOT (registered at www.clinicaltrials.gov as #NCT03258567).
Multiple case reports and retrospective reviews have demonstrated rates of rejection of solid organ transplants up to 40% in patients who received checkpoint inhibitors posttransplantation for treatment of malignancy, a majority of which were melanomas.70,71 Therefore, although there is potential efficacy of checkpoint inhibitors for EBV-associated PTLD, there is currently insufficient evidence to suggest benefits would outweigh risks for patients with PTLD post-SOT. Results of ongoing and future trials may help elucidate the role and safety of PD-1 inhibitors in treatment of EBV-associated lymphoproliferative disorders.
Conventional chemotherapy and radiation
EBV+ lymphomas that are classified the same as EBV− lymphomas of the same histology are generally treated with standard lymphoma regimens.33 Early identification of high-risk patients facilitates enrollment in clinical trials and access to novel therapies.72 Chemotherapy for PTLD is appropriate as first-line therapy in monomorphic PTLD and classical HL–like histologic subtypes, as is salvage therapy if initial reduction of immunosuppression and rituximab do not result in CR in early lesions or polymorphic PTLD.
There have been 2 groups with large prospective clinical trials evaluating chemotherapy after rituximab for PTLD, a Children’s Oncology Group (COG) trial in pediatric patients and the PTLD-1 study in Europe in adult patients undergoing SOT. The COG ANHL0221 study evaluated a regimen combining rituximab with prednisone and low-dose cyclophosphamide for EBV+ CD20+ PTLD. In this 55-patient study, 69% achieved complete remission, with a 2-year overall survival (OS) rate of 83% and event-free survival (EFS) rate of 71%.73 This study was subsequent to a prior study that demonstrated the success of prednisone and low-dose cyclophosphamide after failure of immunosuppression in pediatric patients with PTLD, with an overall response rate of 83%, 2-year OS rate of 73%, and progression-free survival rate of 69% in 36 evaluable patients.74 PTLD-1 evaluated sequential treatment with 4 cycles of rituximab followed by 4 cycles of the CHOP regimen (cyclophosphamide, doxorubicin, vincristine sulfate [Oncovin], and prednisone), with an amended trial to include an extended course of rituximab after initial induction for those with CR to rituximab monotherapy.75 Of the 148 patients, 25% had a CR after initial rituximab and continued rituximab monotherapy. Overall response rate of the risk-stratified sequential treatment with chemotherapy was 88% (CR, 70%), with a 3-year OS estimate of 70%.45 Therefore, use of this risk-stratified approach suggests that responding patients do not need chemotherapy, which could potentially reduce infection risks and mortality secondary to chemotherapy in this population.45
As with conventional chemotherapy, combined treatment with radiation is included in treatment plans for EBV-associated lymphomas if considered standard treatment for their respective histologic subtype. Radiation may also prove to have some benefit in localized disease for PTLD, when used in combination with other treatments.76,77
ESTs
ESTs are an adoptive T-cell therapy that can be generated from allogeneic, autologous, or third-party donors. In immunosuppressed patients, restoring EBV-specific T lymphocyte activity may prevent or treat EBV-associated PTLD.41 Allogeneic ESTs can be generated from HSCT donors, and autologous ESTs can be generated ex vivo for EBV-associated lymphomas before transplantation as well as for SOT recipients.
ESTs for PTLD
The earliest use of ESTs was reported in the mid 1990s for the prevention and treatment of PTLD. Their use for more than 2 decades has shown both safety and efficacy in phase 1/2 trials.33 Early experiences used donor-derived T cells to prevent and treat EBV-associated lymphomas after HSCT. Although donor lymphocyte infusions demonstrated effective treatment of lymphoproliferative disorders after transplantation, strategies for manufacturing ESTs have been developed to reduce the risk of GVHD seen with donor lymphocyte infusions.78
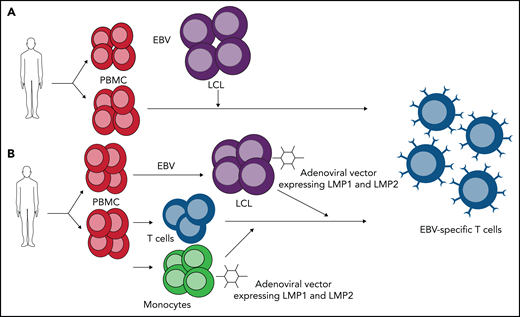
ESTs are designed to target EBV antigens that are presented on the cell surface by major histocompatibility complex molecules. When EBV infects B cells in vitro, the cells are activated to proliferate and grow, leading to lymphoblastoid cell lines (LCLs).15 LCLs express all 10 EBV latency antigens and can be used as antigen-presenting cells to selectively expand ESTs from HSCT donors targeting the immunodominant antigens to treat latency type III tumors in patients undergoing HSCT.10 Rooney et al79 first reported HSCT donor-derived ESTs to prevent or control EBV-associated PTLD, and since then, several groups have reported the successful use of donor-derived ESTs. Heslop et al80 describes a series of 114 patients who received ESTs either as treatment or prophylaxis around transplantation for PTLD, and of the 13 patients with active disease, 11 achieved CR, and those without disease remained PTLD free. Importantly, no patients developed de novo GVHD after EST infusion.10,80 Doubrovina et al81 from Memorial Sloan Kettering Cancer Center (MSKCC) also demonstrated success of donor-derived ESTs for PTLD, with CR in 10 of 14 patients.
Because solid organ transplant recipients frequently do not have a living donor available or are not HLA matched with the donor, autologous ESTs have been developed with similar methods using LCLs to expand ESTs targeting type III latency tumors. Savoldo et al82 demonstrated that autologous ESTs can be generated from those undergoing SOT, and in their original series of 12 treated patients (2 with active disease), none had significant toxicity or graft rejection after infusion. Other small series have also demonstrated prevention of or durable responses in PTLD with autologous ESTs after SOT,38,83,84 but ongoing immunosuppression, time to generate ESTs, and possibility of seronegativity render this approach challenging for broader application.38
LMP-specific T cells targeting type II and III latency tumors
ESTs have also demonstrated therapeutic benefit in HLs and NHLs (including NK/T-cell lymphomas) with latency type II patterns, which are less immunogenic, using methods to enhance specificity, including adenoviral vectors to promote LMP1/2 specificity.85,86 Good Manufacturing Practices–compliant methods of the most common approaches have been detailed: peripheral blood mononuclear cell–derived monocytes transduced with adenovirus vector expressing inactive LMP1 and LMP2 matured into dendritic cells are used as initial antigen presenting cells, with LCLs transduced with LMP1 and LMP2 adenoviral vector as antigen presenting cells for future stimulations of the expanding LMP-specific T-cell population (Figure 3).86 When generated from autologous donors and infused into 50 patients (21 with relapsed/resistant EBV+ HL or NHL and 29 in remission from high-risk or multiple-relapse disease), those with resistant/recurrent disease had a 2-year EFS rate of ∼50%, and those in remission had an 82% EFS rate; 11 of the 21 patients treated exclusively with LMP-specific ESTs (7 of 13 with B-cell lymphoma and 3 of 8 with NK/T-cell lymphoma) had sustained CR.87 For patients with EBV+ B- and T-cell lymphomas undergoing allogeneic HSCT, the administration of donor-derived LMP-specific ESTs seemed to improve outcomes over historical expected survival rates. Specifically, 7 patients with relapsed disease at the time of infusion had an OS rate of 43% at 2 years (with an otherwise expected EFS rate of ∼20%), and those in remission had a 57% EFS rate and 78% OS rate at 2 years.85 Thus, these data suggest that autologous and donor-derived allogeneic LMP-specific ESTs may play a role in maintaining remission or treating relapsed disease.
ESTs generated targeting latency type III vs latency type II EBV-associated lymphoproliferative disorders. Mononuclear cells are isolated from peripheral blood from autologous or allogeneic donor. (A) Laboratory-strain EBV infects peripheral blood mononuclear cells (PBMCs) to generate LCLs. LCLs express all EBV-encoded proteins and are used as antigen-presenting cells in coculture with PBMCs to expand ESTs, which target immunodominant EBNA3 and other EBV latent antigens. (B) Laboratory-strain EBV infects PBMCs to generate LCLs, which are transduced with adenoviral vector expressing LMP1 and LMP2. Monocytes are also transduced with adenoviral vector expressing LMP1 and LMP2 and cocultured with isolated T cells. Transduced LCLs are used in second stimulation to further expand LMP-specific T cells.
ESTs generated targeting latency type III vs latency type II EBV-associated lymphoproliferative disorders. Mononuclear cells are isolated from peripheral blood from autologous or allogeneic donor. (A) Laboratory-strain EBV infects peripheral blood mononuclear cells (PBMCs) to generate LCLs. LCLs express all EBV-encoded proteins and are used as antigen-presenting cells in coculture with PBMCs to expand ESTs, which target immunodominant EBNA3 and other EBV latent antigens. (B) Laboratory-strain EBV infects PBMCs to generate LCLs, which are transduced with adenoviral vector expressing LMP1 and LMP2. Monocytes are also transduced with adenoviral vector expressing LMP1 and LMP2 and cocultured with isolated T cells. Transduced LCLs are used in second stimulation to further expand LMP-specific T cells.
Third-party off-the-shelf ESTs for type III latency tumors
The time needed to manufacture ESTs for patients with PTLD or relapsed EBV+ lymphoma and the availability of ESTs only through clinical trials prohibit a majority of patients from benefiting from these therapies. The feasibility and efficacy of third-party banks of ESTs are actively being studied to maximize accessibility and minimize time to treatment.88-92 The third-party approach was first tested in clinic by Haque et al,93 with selection of product based on best HLA match. Multiple studies have shown the safety of third-party ESTs, with few adverse events (Table 2 lists third-party trials, including multivirus-specific T cells).81,90,92,94-97 The third-party approach allows for more rapid allotment of ESTs for patients with active disease, and products are chosen based on HLA match as well as HLA restriction of viral activity (Figure 4). Withers et al92 detail the establishment of a bank to effectively treat patients, and Prockop et al90 from MSKCC recently described a large third-party allogeneic EST bank that includes 330 EST therapy products. In this MSKCC study, 33 patients undergoing HSCT and 13 undergoing SOT were treated posttransplantation, with 68% of HSCT and 54% of SOT patients demonstrating CR or sustained partial response, further indicating that the third-party bank is safe and feasible. The COG ANHL1522 trial is evaluating rituximab and third-party LMP-specific cytotoxic T lymphocytes in pediatric patients with EBV+ CD20+ PTLD undergoing SOT. Commercialization of ESTs is actively being pursued by both Atara and AlloVir, with active phase 2 and 3 trials evaluating Atara’s tabelecleucel; this would allow for increased availability, because currently, patients must rely on clinical trials for access to treatment.98 Additional genetic modifications to ESTs are also actively being studied, including chimeric antigen receptors targeting lytic proteins (eg, GP350) and surface markers, resistance to immunosuppressive medications, and resistance to immune modulating effects of the tumor environment.99,100Table 3 lists active clinical trials for ESTs.
Published third-party EST trials
| Study . | Year . | Target . | N . | Serious adverse events . | Clinical results . |
|---|---|---|---|---|---|
| 94 | 2007 | EBV | 33 | None | 52% CR/PR |
| 81,95 | 2010, 2012 | EBV | 5 | None | 4/5 CR |
| 97 | 2013 | CMV, EBV, Adv | 50 | 8 cases GVHD (2 de novo) | 74% CR/PR |
| 96 | 2017 | CMV, EBV, Adv, BK, HHV6 | 38 | 2 cases of de novo GVHD (grade 1) | 92% CR/PR |
| 92 | 2018 | CMV, EBV, Adv | 30 | 2 cases of de novo GVHD | 93% CR/PR |
| 90 | 2020 | EBV | 46 | None | 68% CR/PR (BMT) 54% CR/PR (SOT) |
| Study . | Year . | Target . | N . | Serious adverse events . | Clinical results . |
|---|---|---|---|---|---|
| 94 | 2007 | EBV | 33 | None | 52% CR/PR |
| 81,95 | 2010, 2012 | EBV | 5 | None | 4/5 CR |
| 97 | 2013 | CMV, EBV, Adv | 50 | 8 cases GVHD (2 de novo) | 74% CR/PR |
| 96 | 2017 | CMV, EBV, Adv, BK, HHV6 | 38 | 2 cases of de novo GVHD (grade 1) | 92% CR/PR |
| 92 | 2018 | CMV, EBV, Adv | 30 | 2 cases of de novo GVHD | 93% CR/PR |
| 90 | 2020 | EBV | 46 | None | 68% CR/PR (BMT) 54% CR/PR (SOT) |
Shown are published results of prior clinical trials demonstrating safety of third-party ESTs.
Adv, adenovirus; BMT, bone marrow transplantation; CMV, cytomegalovirus; PR, partial response.
Product selection of third-party ESTs. Recipient HLA is compared with 2 separate third-party products along with antiviral activity. Product 1 is a 5/8 HLA match with the patient, whereas product 2 is a 6/8 HLA match. On prior evaluation of products to determine through which alleles the product has antiviral activity, product 1 demonstrated antiviral activity in 3 of these shared alleles (HLA restriction), whereas product 2 had antiviral activity in only 1 shared allele. Because of this antiviral activity in more shared alleles, product 1 is chosen as the initial suitable product.
Product selection of third-party ESTs. Recipient HLA is compared with 2 separate third-party products along with antiviral activity. Product 1 is a 5/8 HLA match with the patient, whereas product 2 is a 6/8 HLA match. On prior evaluation of products to determine through which alleles the product has antiviral activity, product 1 demonstrated antiviral activity in 3 of these shared alleles (HLA restriction), whereas product 2 had antiviral activity in only 1 shared allele. Because of this antiviral activity in more shared alleles, product 1 is chosen as the initial suitable product.
Active EST trials
| Intervention . | Clinical trial . | Location . | Phase . |
|---|---|---|---|
| Tabelecleucel | NCT04554914 | Emory University Washington University | 2 |
| Tabelecleucel | NCT03394365, NCT03392142 | Multiple locations (sponsor Atara Biotherapeutics) | 3 |
| CD30 CAR EBVSTs (allogeneic, autologous) | NCT04288726, NCT01192464 | Houston Methodist Hospital Texas Children’s Hospital | 1 |
| EBVST cells with nivolumab | NCT02973113 | Houston Methodist Hospital Texas Children’s Hospital | 1 |
| Allogeneic LMP1/LMP2-specific cytotoxic T cells and rituximab | NCT02900976 | Multiple locations (sponsor COG) | 2 |
| Third-party LMP-, BARF1-, and EBNA1-specific CTLs | NCT02287311 | Houston Methodist Hospital Texas Children’s Hospital | 1 |
| LMP1/2 CTLs | NCT01956084 | Children’s National Medical Center | 1 |
| ESTs | NCT01555892 | Houston Methodist Hospital Texas Children’s Hospital | 1 |
| Biologically/genetically modified T cells (19-28z CAR EBV CTLs) | NCT01430390 | MSKCC | 1 |
| CD19 ESTs | NCT00709033 | Houston Methodist Hospital Texas Children’s Hospital | 1 |
| TGF-β–resistant LMP-specific CTLs | NCT00368082 | Houston Methodist Hospital Texas Children’s Hospital | 1 |
| Intervention . | Clinical trial . | Location . | Phase . |
|---|---|---|---|
| Tabelecleucel | NCT04554914 | Emory University Washington University | 2 |
| Tabelecleucel | NCT03394365, NCT03392142 | Multiple locations (sponsor Atara Biotherapeutics) | 3 |
| CD30 CAR EBVSTs (allogeneic, autologous) | NCT04288726, NCT01192464 | Houston Methodist Hospital Texas Children’s Hospital | 1 |
| EBVST cells with nivolumab | NCT02973113 | Houston Methodist Hospital Texas Children’s Hospital | 1 |
| Allogeneic LMP1/LMP2-specific cytotoxic T cells and rituximab | NCT02900976 | Multiple locations (sponsor COG) | 2 |
| Third-party LMP-, BARF1-, and EBNA1-specific CTLs | NCT02287311 | Houston Methodist Hospital Texas Children’s Hospital | 1 |
| LMP1/2 CTLs | NCT01956084 | Children’s National Medical Center | 1 |
| ESTs | NCT01555892 | Houston Methodist Hospital Texas Children’s Hospital | 1 |
| Biologically/genetically modified T cells (19-28z CAR EBV CTLs) | NCT01430390 | MSKCC | 1 |
| CD19 ESTs | NCT00709033 | Houston Methodist Hospital Texas Children’s Hospital | 1 |
| TGF-β–resistant LMP-specific CTLs | NCT00368082 | Houston Methodist Hospital Texas Children’s Hospital | 1 |
Shown are current active EST trials registered on clinicaltrials.gov at time of submission.
CAR, chimeric antigen receptor; CTL, cytotoxic T lymphocyte; EBVST, EBV-specific T cells; TGF, transforming growth factor.
Antivirals
Antivirals have currently not been proven to have a significant impact on treatment of EBV-associated lymphoproliferative disorders. The role of the lytic cycle in EBV-associated lymphoproliferation has not been thoroughly demonstrated, and antivirals that inhibit viral replication have not been shown to have a significant effect on tumors.5,10 The viral enzyme target of nucleoside-type antiviral agents (ganciclovir and acyclovir), thymidine kinase, is expressed only in the lytic phase of the virus, rendering them unsuccessful as treatment of EBV-associated lymphoproliferative disorders.101 Therefore, reactivation of these lytic genes may render these tumors more susceptible to antiviral therapy. Combinations of small-molecule inhibitors inducing lytic replication, such as HDAC inhibitor arginine butyrate, with antiviral agents have demonstrated in small case series to have some antitumor effects, but none have yet been further developed clinically.101
Small-molecule inhibitors
Currently, a variety of small-molecule inhibitors are being evaluated for the treatment of EBV-associated lymphoproliferative diseases, with some in clinical trials. EBV uses host pathways to induce lymphomagenesis, and targeting these using small-molecule inhibitors may aid in treatment of EBV-associated malignancies.12 Current inhibitors being studied include EBNA1 inhibitors, HDAC inhibitors, proteasome inhibitors (bortezomib and ixazomib), cyclin-dependent kinase inhibitors, PI3K inhibitors, AKT inhibitors, and mTOR inhibitors.12,102,103 Hydroxyurea, a ribonucleotide reductase inhibitor, has been shown to eliminate EBV episomes from Burkitt lymphoma cells and EBV-immortalized lymphoblastoid cell lines in vitro and has induced long-lasting responses in small series of patients with EBV-associated primary central nervous system lymphoma associated with AIDS.104 Other small series have shown benefits with a variety of small-molecule inhibitors in combination with other treatments for refractory lymphoproliferative diseases.102 Combining small-molecule inhibitors may increase the efficacy of either treatment alone, and using small-molecule inhibitors with ESTs may enhance immunotherapeutic effects of the T-cell product in vivo.
Vaccines
There is currently no vaccine available for the treatment of EBV.105 However, conceptually, preventing the latent infection while mounting an effective immune response could potentially prevent lymphoproliferative disorders.15 Vaccine trials to evaluate the reduction in rate of EBV+ cancers is challenging because of the long latency periods between initial infection and cancer development. Vaccines targeting lytic proteins have been explored for the treatment of lymphoproliferative disorders.105 In addition, a vaccine targeting GP350 (a glycoprotein present on both the surface of the virus and virus-infected cells that is important for viral attachment to B cells)105 has been evaluated in pediatric patients before SOT, with suboptimal response, and currently, no data exist to support EBV-directed cancer vaccines.106
Summary
EBV is a ubiquitous human tumor virus that can lead to development of lymphoproliferative diseases, including CAEBV, HLH, and various lymphomas, in patients with acquired or congenital immunodeficiencies. Further research into the mechanisms of lymphomagenesis caused by EBV may lead to improvements in prevention strategies for patients at high risk, although currently, data are conflicting regarding prophylactic measures. Although conventional chemotherapy plays an appreciable role in the treatment of many of these lymphoproliferative disorders, more targeted therapies are actively being studied to improve treatment of patients. ESTs have shown significant therapeutic success, especially in PTLD. Ongoing clinical trials and commercialization of ESTs may allow more expedient access to this treatment.
Acknowledgments
This work was supported, in part by award T32 HL110841 from the National Heart, Lung and Blood Institute, National Institutes of Health (NIH), and a fellowship from the Mark Foundation for Cancer Research. This work was also supported by grants from the National Cancer Institute (2P01 CA148600 and P01 CA225618) (C.M.B.), NIH, and the Board of Visitors of the Children’s National Health System.
The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the National Heart, Lung and Blood Institute or the NIH.
Authorship
Contribution: K.T. and C.M.B. designed the review, analyzed data and literature review, and wrote the paper.
Conflict-of-interest disclosure: C.M.B. is on the scientific advisory boards for Catamaran Bio and Mana Therapeutics with stock options and/or ownership, is on the Board of Directors for Caballeta Bio with stock options, and has stock in Neximmune and Torque Therapeutics. K.T. declares no competing financial interests.
Correspondence: Catherine M. Bollard, Center for Cancer and Immunology Research, Children’s National Health System, 111 Michigan Ave NW, Washington, DC 20010; e-mail: cbollard@childrensnational.org.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal