TO THE EDITOR:
Primary central nervous system lymphoma (PCNSL) is a rare diffuse large B-cell lymphoma (DLBCL) with poor prognosis. With a median overall survival (OS) of 6.8 months,1 patients with relapsed/refractory (R/R) disease represent an unmet medical need, in particular when they are not eligible for autologous stem cell transplantation (ASCT) or in relapse after ASCT. Anti-CD19 chimeric antigen receptor (CAR) T-cells demonstrated high efficacy in systemic DLBCL. Axicabtagene ciloleucel (axi-cel)2,3 and tisagenlecleucel (tisa-cel)4 have been approved and are commercialized in Europe for R/R DLBCL after 2 or more previous lines of therapy. Because of safety concerns related to immune effector cell‐associated neurotoxicity syndrome (ICANS) after CAR T-cell therapy,5 patients with PCNSL were excluded from the initial pivotal trials. Nonetheless, recent evidence has shown an acceptable safety profile and some activity of anti-CD19 CAR T-cell treatment of secondary CNS lymphoma (sCNSL).6-8 Here we report a cohort of patients with R/R PCNSL treated with CAR T-cells within the French national expert network for oculo-cerebral lymphomas (LOC).1
Since January 2020, anti-CD19 CAR T cells were discussed as a therapeutic option within the LOC network for immunocompetent patients with R/R PCNSL after at least 2 previous conventional lines of therapy. We retrospectively identified patients treated in this fashion from our national database to evaluate the safety and efficacy of CAR T-cells in this setting. Patients received a single intravenous infusion of axi-cel or tisa-cel following the recommended lymphodepletion regimen with cyclophosphamide and fludarabine. Response was centrally reviewed (CH, SC), according to the International Primary CNS Lymphoma Collaborative Group (IPCG) criteria.9 Cytokine release syndrome (CRS) and ICANS were graded according to the ASTCT 2019 guidelines.10 Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 was used to report other adverse events. Progression-free survival (PFS) was defined as the time between CAR T-cell infusion and progression or death of any cause. OS was defined as the time from CAR T-cell infusion until death of any cause. Duration of response (DoR) was defined as the time from first complete or partial response to disease progression/relapse. This retrospective analysis was approved by the LOC Institutional Review Board. All patients gave written informed consent.
Between May 2020 and March 2021, 9 patients with R/R PCNSL were treated with anti-CD19 CAR T-cells: 7 received tisa-cel and 2 received axi-cel (Table 1). At time of infusion, the median age was 67 years old (range, 48-75 years) and median Eastern Cooperative Oncology Group (ECOG) scale of Performance Status was 1 (range, 0-4). Eight patients had brain parenchymal involvement, including 1 with intraocular localization, and 1 patient had an isolated cerebrospinal fluid (CSF) relapse. The median number of prior therapies before leukapheresis was 3 (range, 2-5), including ASCT in 7 patients. Bridging therapy was necessary in all but 1 patient because of the rapid evolution of R/R PCNSL and the time to manufacture CAR T-cells. At time of CAR T-cell infusion, following bridging treatment, 4 patients had progressive disease (PD) and 5 patients were in partial response (PR). One patient had ongoing corticosteroid treatment. The median follow-up after CAR T-cell infusion was 8.5 months. Seven patients experienced CRS (any grade), including 1 grade 3 after tisa-cel. ICANS of any grade occurred in 5 patients, including 1 grade 3 after tisa-cel and 1 grade 4 after axi-cel. Symptoms of ICANS included confusion, impaired handwriting, seizures, and status epilepticus. The median times from infusion to the onset of CRS and ICANS were 5 (range, 1-6) and 8 days (range, 5-21), respectively. The median times to resolution of CRS and ICANS were 3 (range, 2-9) and 4 days (range, 1-70), respectively. Four patients had grade 3 or higher cytopenia lasting more than 28 days. At 1 month (M1), OR was observed in 6 of 9 patients, including complete response (CR) in 3 of 9 patients. At M3, OR and CR were observed in 6 of 9 and 5 of 9 patients, respectively. Best response to CAR T-cells was PR in 1 of 9 (tisa-cel) and CR in 5 of 9 patients (2 axi-cel, 3 tisa-cel). Two patients died, 1 from PD and 1 from COVID-19 while still in CR. Median PFS was 122 days, increasing to 210 days for responders. Median DoR was not reached. Six-month OS, PFS, and DoR were 89%, 44%, and 67%, respectively (Figure 1A; supplemental Figure 1).
Summary of the main patient characteristics, response, and toxicity of CAR Tcells
| ID . | Sex . | Age (y) at time of CAR T-cell infusion . | ECOG . | No. and types of prior lines of therapy . | Bridging therapy . | Disease localization at time of CAR T-cell infusion . | Disease status at time of CAR T-cell infusion . | Infused CAR T-cell product . | Maximum CRS/ICANS . | Required treatment of CRS/ICANS . | Cytopenia not resolved by day 28, grade ≥ 3 (type) . | Response at M1 . | Response at M3 . | Subsequent treatment, most recent response . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 69 | 1 | 2 R-MPVA, R-ICE + ASCT (thiotepa-BCNU) | Ibrutinib | Several small nodular left parietal lesions | PR | Tisa-cel | Grade 2 CRS, grade 2 ICANS | Tocilizumab and corticosteroids | No | PR | PR (concurrent ibrutinib) | Progression at M2 ≥ Ibrutinib ≥ CRu finally followed by PD at day 178 Still alive at day 295 |
| 2 | M | 51 | 4 | 3 R-MTX-cytarabine + ASCT (TBC), R-ICE, ibrutinib | MTX-cytarabine- lenalidomide | Necrotic hemorrhagic left temporoparietal lesion of 98 × 74 mm | PD | Tisa-cel | Grade 2 CRS, no ICANS | Tocilizumab and corticosteroids | Yes (thrombocytopenia, neutropenia) | SD | NE | Deceased at day 37 |
| 3 | F | 71 | 3 | 4 R-MTX-cytarabine, R-ICE + ASCT (TBC), temozolomide, ibrutinib | None | Eye + several lesions of the right hemisphere, including a 28 × 23 mm frontal lesion | PD | Axi-cel | Grade 2 CRS, grade 4 ICANS | Corticosteroids | No | PR | CR by brain MRI Eye NE | Persistent CR by brain MRI and clinical improvement at day 350 Eye examination not feasible High level of IL-10 in the anterior chamber (1200 pg/mL) at day 176 |
| 4 | F | 68 | 3 | 4 R-MBVP, R-ICE + ACST (TBC), intrathecal MTX and cytarabine, ibrutinib | R-ibrutinib-lenalidomide | CSF only | PD | Tisa-cel | No CRS, grade 3 ICANS | No | No | CR | CR | Relapse at day 122 Still alive at day 458 after multiple treatments (lenalidomide, ibrutinib, intrathecal rituximab, pomalidomide) |
| 5 | F | 67 | 1 | 3 R-MPVA, R-ICE, ocular RT | R-MTX-procarbazine | Necrotic hemorrhagic bifrontal lesion (right: 28 × 14 mm. Left: 18 × 7 mm) | PR | Tisa-cel | No CRS, no ICANS | NA | No | PD | PD | Still alive at day 261 after multiple treatments (lenalidomide, ibrutinib, pomalidomide, temozolomide) |
| 6 | M | 49 | 1 | 5 R-MTX-cytarabine- temozolomide + ASCT (TB), R-ICE, R-ibrutinib-lenalidomide, ibrutinib-pomalidomide, pomalidomide-nivolumab | MTX-cytarabine | Several small nodular left frontal lesions | PD | Tisa-cel | Grade 2 CRS, grade 1 ICANS | No | No | PD (suspected flare effect) | PD (suspected flare effect) | PD at day 256: near disappearance of all enhanced lesions but increase of an occipital lesion No additional treatment |
| 7 | F | 65 | 1 | 3 R-MPVA, R-ICE, R-ibrutinib-lenalidomide + ASCT (TBC) | MTX | Ventriculitis (right temporal ventricular horn) + 5 mm diameter right frontal lesion | PR | Tisa-cel | Grade 3 CRS, no ICANS | Tocilizumab and corticosteroids | Yes (thrombocytopenia, neutropenia) | PR | CR | Persistent CR at day 209 |
| 8 | F | 48 | 0 | 2 R-MBVP-cytarabine + ASCT (TB), R-lenalidomide | Ibrutinib | 3-mm diameter hypothalamic lesion | PR | Tisa-cel | Grade 1 CRS, no ICANS | Tocilizumab | Yes (pancytopenia) | CR | CR | Persistent CR at day 145 |
| 9 | M | 75 | 0 | 2 R-MPVA, R-MTX | R-lenalidomide | Left temporal lobe (20.5 × 17 × 8.5mm) + perilesional edema | PR | Axi-cel | Grade 1 CRS, grade 1 ICANS | Tocilizumab and corticosteroids | Yes (thrombocytopenia, neutropenia) | CR | CR | Persistent CR at day 210 Deceased from COVID-19 |
| ID . | Sex . | Age (y) at time of CAR T-cell infusion . | ECOG . | No. and types of prior lines of therapy . | Bridging therapy . | Disease localization at time of CAR T-cell infusion . | Disease status at time of CAR T-cell infusion . | Infused CAR T-cell product . | Maximum CRS/ICANS . | Required treatment of CRS/ICANS . | Cytopenia not resolved by day 28, grade ≥ 3 (type) . | Response at M1 . | Response at M3 . | Subsequent treatment, most recent response . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 69 | 1 | 2 R-MPVA, R-ICE + ASCT (thiotepa-BCNU) | Ibrutinib | Several small nodular left parietal lesions | PR | Tisa-cel | Grade 2 CRS, grade 2 ICANS | Tocilizumab and corticosteroids | No | PR | PR (concurrent ibrutinib) | Progression at M2 ≥ Ibrutinib ≥ CRu finally followed by PD at day 178 Still alive at day 295 |
| 2 | M | 51 | 4 | 3 R-MTX-cytarabine + ASCT (TBC), R-ICE, ibrutinib | MTX-cytarabine- lenalidomide | Necrotic hemorrhagic left temporoparietal lesion of 98 × 74 mm | PD | Tisa-cel | Grade 2 CRS, no ICANS | Tocilizumab and corticosteroids | Yes (thrombocytopenia, neutropenia) | SD | NE | Deceased at day 37 |
| 3 | F | 71 | 3 | 4 R-MTX-cytarabine, R-ICE + ASCT (TBC), temozolomide, ibrutinib | None | Eye + several lesions of the right hemisphere, including a 28 × 23 mm frontal lesion | PD | Axi-cel | Grade 2 CRS, grade 4 ICANS | Corticosteroids | No | PR | CR by brain MRI Eye NE | Persistent CR by brain MRI and clinical improvement at day 350 Eye examination not feasible High level of IL-10 in the anterior chamber (1200 pg/mL) at day 176 |
| 4 | F | 68 | 3 | 4 R-MBVP, R-ICE + ACST (TBC), intrathecal MTX and cytarabine, ibrutinib | R-ibrutinib-lenalidomide | CSF only | PD | Tisa-cel | No CRS, grade 3 ICANS | No | No | CR | CR | Relapse at day 122 Still alive at day 458 after multiple treatments (lenalidomide, ibrutinib, intrathecal rituximab, pomalidomide) |
| 5 | F | 67 | 1 | 3 R-MPVA, R-ICE, ocular RT | R-MTX-procarbazine | Necrotic hemorrhagic bifrontal lesion (right: 28 × 14 mm. Left: 18 × 7 mm) | PR | Tisa-cel | No CRS, no ICANS | NA | No | PD | PD | Still alive at day 261 after multiple treatments (lenalidomide, ibrutinib, pomalidomide, temozolomide) |
| 6 | M | 49 | 1 | 5 R-MTX-cytarabine- temozolomide + ASCT (TB), R-ICE, R-ibrutinib-lenalidomide, ibrutinib-pomalidomide, pomalidomide-nivolumab | MTX-cytarabine | Several small nodular left frontal lesions | PD | Tisa-cel | Grade 2 CRS, grade 1 ICANS | No | No | PD (suspected flare effect) | PD (suspected flare effect) | PD at day 256: near disappearance of all enhanced lesions but increase of an occipital lesion No additional treatment |
| 7 | F | 65 | 1 | 3 R-MPVA, R-ICE, R-ibrutinib-lenalidomide + ASCT (TBC) | MTX | Ventriculitis (right temporal ventricular horn) + 5 mm diameter right frontal lesion | PR | Tisa-cel | Grade 3 CRS, no ICANS | Tocilizumab and corticosteroids | Yes (thrombocytopenia, neutropenia) | PR | CR | Persistent CR at day 209 |
| 8 | F | 48 | 0 | 2 R-MBVP-cytarabine + ASCT (TB), R-lenalidomide | Ibrutinib | 3-mm diameter hypothalamic lesion | PR | Tisa-cel | Grade 1 CRS, no ICANS | Tocilizumab | Yes (pancytopenia) | CR | CR | Persistent CR at day 145 |
| 9 | M | 75 | 0 | 2 R-MPVA, R-MTX | R-lenalidomide | Left temporal lobe (20.5 × 17 × 8.5mm) + perilesional edema | PR | Axi-cel | Grade 1 CRS, grade 1 ICANS | Tocilizumab and corticosteroids | Yes (thrombocytopenia, neutropenia) | CR | CR | Persistent CR at day 210 Deceased from COVID-19 |
Response was centrally reviewed by 2 experts and determined according to the IPCG criteria9; grading of CRS and ICANS was determined by the ASTCT scale10; grading of cytopenia was performed with the use of the CTCAE version 5.0.
F, female; M, male; MTX, methotrexate; NE, not evaluable; R-ICE, rituximab, ifosfamide, carboplatine, etoposide; R-MBVP, rituximab, methotrexate, BCNU, etoposide, prednisone; R-MPVA, rituximab, methotrexate, procarbazine, vincristine, aracytine; RT, radiotherapy; TBC, thiotepa, busulfan, cyclophosphamide.
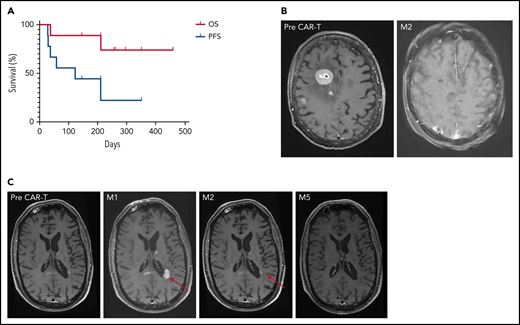
Survival and imaging of patients with R/R PCNSL treated with commercial anti-CD19 CAR T-cells. (A) OS and PFS. Median OS was not reached. Median PFS was 122 days for the whole cohort, increasing to 210 days for responders, vs 29 days for non-responders. (B) Patient 3: right frontal lesion at baseline and CR 2 months after CAR T-cell infusion. (C) Illustration of the flare effect suspected in patient 6: appearance of a left periventricular lesion 1 month after CAR T-cell infusion that dramatically decreased 2 and 5 months later. Despite high suspicion of a flare effect, response was reported as progressive disease according to the IPCG criteria, thus potentially underestimating PFS.
Survival and imaging of patients with R/R PCNSL treated with commercial anti-CD19 CAR T-cells. (A) OS and PFS. Median OS was not reached. Median PFS was 122 days for the whole cohort, increasing to 210 days for responders, vs 29 days for non-responders. (B) Patient 3: right frontal lesion at baseline and CR 2 months after CAR T-cell infusion. (C) Illustration of the flare effect suspected in patient 6: appearance of a left periventricular lesion 1 month after CAR T-cell infusion that dramatically decreased 2 and 5 months later. Despite high suspicion of a flare effect, response was reported as progressive disease according to the IPCG criteria, thus potentially underestimating PFS.
Despite a remarkable efficacy in systemic DLBCL, resistance to CD19 CAR T-cells can occur.11 Our series illustrates such an issue for PCNSL, and representative cases are highlighted here.
Patient 4 presented with a relapse at day122 after CAR T-cell infusion. She was treated with lenalidomide, followed by ibrutinib, because of the potential capacity of both drugs to enhance CAR T-cell functions.12-15 Unfortunately, a subsequent CD19-negative CSF relapse was documented (supplemental Figure 2), antigen loss or downregulation being a well-described mechanism of relapse after CAR T-cell therapy.11
Patient 3 presented with high tumor burden and PD at time of CAR T-cell infusion, associated with an ocular infiltration. A remarkable response to CAR T-cells was observed, with a persistent CR on brain magnetic resonance imaging (MRI; Figure 1B). Because of a grade 4 ICANS with status epilepticus, an eye examination was not feasible. The level of interleukin-10 was measured at 1200 pg/mL in the anterior chamber at M6.16 A larger series is needed to evaluate CAR T-cell homing in the ocular compartment.
Patient 6 had an early brain MRI 9 days after CAR T-cell infusion because of clinical deterioration. New multiple contrast-enhanced nodular lesions of several millimeters were observed. On MRIs performed 7 and 19 days later, without any additional treatment, these lesions significantly decreased, but 2 new lesions appeared in the corpus callosum and left periventricular region (Figure 1C). These lesions then markedly decreased on subsequent MRIs at M2, M3, and M6. In parallel, clinical status dramatically improved, raising the potential of pseudoprogression linked to CAR T-cell therapy and the difficulty to assess the response.
Few data on CAR T-cells for CNS lymphoma have been published to date.17 Frigault et al7 reported a retrospective analysis on 8 patients with sCNSL who received tisa-cel. The treatment was well tolerated, and responses were observed in 4 patients (2 CR, 2 PR) at M1. Preliminary results with short follow-up from an ongoing anti-CD19 CAR T-cell trial described 1 CR and 3 PR among 7 patients (3 PCNSL, 4 sCNSL), with no grade 3 or higher CRS or ICANS.18 Li et al19 published results with a longer follow-up for 5 patients (1 PCNSL, 4 sCNSL) included in a clinical trial of anti-CD19 plus anti-CD22 CAR T-cells. All patients achieved an objective response, but 4 patients relapsed within 3 to 8 months. The immunosuppressive brain microenvironment could have contributed to lymphoma recurrence.20
To our knowledge, our study represents the first and largest cohort of patients with R/R PCNSL treated with commercial anti-CD19 CAR T-cells. We observed a manageable toxicity, despite the recent identification of CD19-expressing mural cells surrounding the brain endothelium as potential off-tumor targets for CAR T-cells.21 Our series showed activity of CAR T-cells in the brain and CSF compartments of heavily pretreated patients. No conclusion can be drawn regarding CAR T-cell intraocular activity, as only 1 patient presented with an ocular involvement.
Our results support the development of CAR T -cells in R/R PCNSL. Prospective clinical trials are needed to confirm these encouraging results. Additionally, synergic associations with immunomodulatory drugs should be tested.
Acknowledgments
The authors acknowledge the LOC network for patient recruitment and treatment recommendations. The authors also thank Joshua Waterfall, Institut Curie, Paris, France, for insightful comments on the manuscript.
Authorship
Contribution: M.A., C.H., C.S., and S.C. analyzed and interpreted data, wrote the manuscript, and created the table and figures; M.A., M.-T.R., and S.C. collected data; C.H., M.B., L.W., A.W.R., and C.S. addressed the patients to the CAR T reference centers; M.-T.R., L.S., D.R.-W., V.M., M.U., C.M., M.D.-C., S.N., N.S., D.P., N.W., N.J., S.S., N.G., M.L.C., F.N., and S.C. provided patient care; M.L.G.-T., K.M., and C.B. performed CSF analyses; and all authors agreed to the final version of the manuscript.
Conflict-of-interest disclosure: M.A. performed scientific and medical consulting for Novartis and Janssen. A.W.R. performed medical consulting for Janssen and AbbVie. S.C. served on the scientific advisory board for Gilead, Novartis, Roche France, Abbvie, Sandoz, Sanofi, Janssen, Celgene-BMS, and Takeda. All remaining authors declare no competing financial interests.
Correspondence: Marion Alcantara, Center for Cancer Immunotherapy, Institut Curie, 26 rue d’Ulm, 75005 Paris, France; e-mail: marion.alcantara@curie.fr.
The online version of this article contains a data supplement.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal