TO THE EDITOR:
Myelodysplastic syndromes (MDS) result in abnormal blood cell development, cytopenias, and risk of progression to acute myeloid leukemia (AML).1 Most patients with lower-risk MDS (LR-MDS) have anemia, but patients can also have neutropenia and/or thrombocytopenia with significant clinical implications.2-4 Treatments for anemia include red blood cell (RBC) transfusions, erythropoiesis-stimulating agents (ESAs), hypomethylating agents (HMAs),5 or lenalidomide.5 However, RBC transfusions can result in iron overload6,7; patients can become resistant to ESAs,4,8 and HMAs and lenalidomide have been associated with grade 3 or 4 neutropenia and thrombocytopenia.9,10 Luspatercept is a first-in-class erythroid maturation agent that binds several transforming growth factor-β (TGF-β) superfamily ligands to diminish Smad2/3 signaling and enhance late-stage erythropoiesis.11 Its efficacy and safety were demonstrated in the phase 3, placebo-controlled MEDALIST trial in RBC transfusion-dependent patients with LR-MDS with ring sideroblasts (RS).12 In this study, significantly more luspatercept-treated patients achieved RBC transfusion independence for ≥8 weeks during weeks 1 to 24 (37.9% vs 13.2%; P < .001).12 Significantly more patients in the luspatercept arm achieved hematologic improvement-erythroid (HI-E), as per 2006 International Working Group (IWG) criteria,13 during weeks 1 to 24 (52.9% vs 11.8%; P < .001) and weeks 1 to 48 (58.8% vs 17.1%; P < .001).12 Here, we report the effect of luspatercept on lineages outside the erythroid compartment, including platelets and neutrophils, and the HI for these lineages in MEDALIST patients cytopenic at baseline.
Full details of the MEDALIST trial (NCT02631070) have been published.12 Briefly, 229 adults with LR-MDS (defined as very low-, low-, or intermediate-risk MDS per the Revised International Prognostic Scoring System [IPSS-R]14) with RS (either ≥15% or ≥5% if SF3B1 mutation was present), who were refractory to, intolerant of, or unlikely to respond to ESAs (serum erythropoietin >200 U/L) and required RBC transfusions, were randomized 2:1 to receive luspatercept (n = 153) or placebo (n = 76) subcutaneously every 3 weeks for 24 weeks.
Data cutoff for the current analysis was July 1, 2019. The secondary endpoints reported are mean neutrophil and platelet counts; mean neutrophil and platelet changes from baseline; proportions of patients achieving absolute increases in neutrophil and platelet counts of ≥0.5 × 109/L and ≥30 × 109/L, respectively; proportions of patients achieving HI-neutrophil (HI-N) and HI-platelet (HI-P) during weeks 1 to 24 and 1 to 48; and hematological toxicities (neutropenia and thrombocytopenia). HI-N is defined as neutrophil increase of >0.5 × 109/L and ≥100% among patients with pretreatment levels <1 × 109/L.13 HI-P is defined as platelet increase, without platelet transfusion, of ≥30 × 109/L (>20 × 109/L at baseline), or of >20 × 109/L and ≥100% increase (<20 × 109/L at baseline) among patients with pretreatment levels <100 × 109/L.13
Median age of patients in the MEDALIST trial was 71 years; 62.9% were male.12 Most patients (95.6%) had refractory cytopenia with multilineage dysplasia and RS (RCMD-RS), and 91.0% of those with available data had SF3B1 mutations (Table 1).12
Baseline patient and treatment characteristics
| Characteristic . | ITT population . | Patients with neutropenia* . | Patients with thrombocytopenia† . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Luspatercept (n = 153) . | Placebo (n = 76) . | Total (n = 229) . | Luspatercept (n = 15) . | Placebo (n = 10) . | Total (n = 25) . | Luspatercept (n = 8) . | Placebo (n = 6) . | Total (n = 14) . | |
| Age, median, y (range) | 71 (40-95) | 72 (26-91) | 71 (26-95) | 72 (60-86) | 69.5 (43-79) | 72 (43-86) | 72.5 (58-79) | 73.5 (65-80) | 73.5 (58-80) |
| Male, n (%) | 94 (61.4) | 50 (65.8) | 144 (62.9) | 9 (60.0) | 6 (60.0) | 15 (60.0) | 5 (62.5) | 5 (83.3) | 10 (71.4) |
| MDS WHO 2008 classification, n (%) | |||||||||
| RS and multilineage dysplasia | 1 (0.7) | 0 | 1 (0.4) | NA | NA | NA | NA | NA | NA |
| RCMD-RS | 145 (94.8) | 74 (97.4) | 219 (95.6) | 15 (100.0) | 9 (90.0) | 24 (96.0) | 8 (100.0) | 6 (100.0) | 14 (100.0) |
| RARS | 7 (4.6) | 2 (2.6) | 9 (3.9) | NA | 1 (10.0) | 1 (4.0) | NA | NA | NA |
| Mutated SF3B1,‡ n/N with data (%) | 138/148 (93.2) | 64/74 (86.5) | 202/222 (91.0) | 12 (80.0) | 10 (100.0) | 22 (88.0) | 5 (62.5) | 4 (66.7) | 9 (64.3) |
| ANC, mean, ×109/L (SD) | 2.8 (2.1) | 2.7 (2.0) | 2.8 (2.0) | 0.8 (0.19) | 0.8 (0.11) | 0.8 (0.16) | 3.6 (5.00) | 1.8 (0.96) | 2.8 (3.83) |
| ANC category, n (%) | |||||||||
| <0.5 × 109/L | 1 (0.7) | 0 | 1 (0.4) | 1 (6.7) | 0 | 1 (4.0) | NA | NA | NA |
| 0.5 to <1.0 × 109/L | 14 (9.2) | 10 (13.2) | 24 (10.5) | 14 (93.3) | 10 (100.0) | 24 (96.0) | 2 (25.0) | 2 (33.3) | 4 (28.6) |
| ≥1.0 × 109/L | 138 (90.2) | 66 (86.8) | 204 (89.1) | NA | NA | NA | 6 (75.0) | 4 (66.7) | 10 (71.4) |
| Platelet count, mean, ×109/L (SD) | 259 (123) | 252 (124) | 257 (123) | 160.5 (58.13) | 179.1 (80.97) | 167.9 (67.20) | 78.1 (14.16) | 84.7 (12.74) | 80.9 (13.48) |
| Platelet count category, n (%) | |||||||||
| <100 × 109/L | 8 (5.2) | 6 (7.9) | 14 (6.1) | 2 (13.3) | 2 (20.0) | 4 (16.0) | 8 (100.0) | 6 (100.0) | 14 (100.0) |
| 100-400 × 109/L | 128 (83.7) | 61 (80.3) | 189 (82.5) | 13 (86.7) | 8 (80.0) | 21 (84.0) | NA | NA | NA |
| >400 × 109/L | 17 (11.1) | 9 (11.8) | 26 (11.4) | NA | NA | NA | NA | NA | NA |
| ICT use, n (%) | 71 (46.4) | 40 (52.6) | 111 (48.5) | 10 (66.7) | 8 (80.0) | 18 (72.0) | 3 (37.5) | 5 (83.3) | 8 (57.1) |
| Characteristic . | ITT population . | Patients with neutropenia* . | Patients with thrombocytopenia† . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Luspatercept (n = 153) . | Placebo (n = 76) . | Total (n = 229) . | Luspatercept (n = 15) . | Placebo (n = 10) . | Total (n = 25) . | Luspatercept (n = 8) . | Placebo (n = 6) . | Total (n = 14) . | |
| Age, median, y (range) | 71 (40-95) | 72 (26-91) | 71 (26-95) | 72 (60-86) | 69.5 (43-79) | 72 (43-86) | 72.5 (58-79) | 73.5 (65-80) | 73.5 (58-80) |
| Male, n (%) | 94 (61.4) | 50 (65.8) | 144 (62.9) | 9 (60.0) | 6 (60.0) | 15 (60.0) | 5 (62.5) | 5 (83.3) | 10 (71.4) |
| MDS WHO 2008 classification, n (%) | |||||||||
| RS and multilineage dysplasia | 1 (0.7) | 0 | 1 (0.4) | NA | NA | NA | NA | NA | NA |
| RCMD-RS | 145 (94.8) | 74 (97.4) | 219 (95.6) | 15 (100.0) | 9 (90.0) | 24 (96.0) | 8 (100.0) | 6 (100.0) | 14 (100.0) |
| RARS | 7 (4.6) | 2 (2.6) | 9 (3.9) | NA | 1 (10.0) | 1 (4.0) | NA | NA | NA |
| Mutated SF3B1,‡ n/N with data (%) | 138/148 (93.2) | 64/74 (86.5) | 202/222 (91.0) | 12 (80.0) | 10 (100.0) | 22 (88.0) | 5 (62.5) | 4 (66.7) | 9 (64.3) |
| ANC, mean, ×109/L (SD) | 2.8 (2.1) | 2.7 (2.0) | 2.8 (2.0) | 0.8 (0.19) | 0.8 (0.11) | 0.8 (0.16) | 3.6 (5.00) | 1.8 (0.96) | 2.8 (3.83) |
| ANC category, n (%) | |||||||||
| <0.5 × 109/L | 1 (0.7) | 0 | 1 (0.4) | 1 (6.7) | 0 | 1 (4.0) | NA | NA | NA |
| 0.5 to <1.0 × 109/L | 14 (9.2) | 10 (13.2) | 24 (10.5) | 14 (93.3) | 10 (100.0) | 24 (96.0) | 2 (25.0) | 2 (33.3) | 4 (28.6) |
| ≥1.0 × 109/L | 138 (90.2) | 66 (86.8) | 204 (89.1) | NA | NA | NA | 6 (75.0) | 4 (66.7) | 10 (71.4) |
| Platelet count, mean, ×109/L (SD) | 259 (123) | 252 (124) | 257 (123) | 160.5 (58.13) | 179.1 (80.97) | 167.9 (67.20) | 78.1 (14.16) | 84.7 (12.74) | 80.9 (13.48) |
| Platelet count category, n (%) | |||||||||
| <100 × 109/L | 8 (5.2) | 6 (7.9) | 14 (6.1) | 2 (13.3) | 2 (20.0) | 4 (16.0) | 8 (100.0) | 6 (100.0) | 14 (100.0) |
| 100-400 × 109/L | 128 (83.7) | 61 (80.3) | 189 (82.5) | 13 (86.7) | 8 (80.0) | 21 (84.0) | NA | NA | NA |
| >400 × 109/L | 17 (11.1) | 9 (11.8) | 26 (11.4) | NA | NA | NA | NA | NA | NA |
| ICT use, n (%) | 71 (46.4) | 40 (52.6) | 111 (48.5) | 10 (66.7) | 8 (80.0) | 18 (72.0) | 3 (37.5) | 5 (83.3) | 8 (57.1) |
ANC, absolute neutrophil count; ICT, iron chelation therapy; ITT, intention to treat; NA, not applicable; RARS, refractory anemia with RS; RCMD-RS, refractory cytopenia with multilineage dysplasia and RS; SD, standard deviation; WHO, World Health Organization.
Patients from the ITT population with neutropenia defined per IWG 2006 criteria as neutrophil level <1 × 109/L.
Patients from the ITT population with thrombocytopenia defined per IWG 2006 criteria as platelet level <100 × 109/L.
No patients with SF3B1 mutation had RS <15%.
Mean baseline absolute neutrophil count (ANC) was 2.8 × 109/L, and 25 patients (10.9%) had neutropenia (neutrophils <1 × 109/L per IWG 2006 criteria13): 15 (9.8%) of the luspatercept arm and 10 (13.2%) of the placebo arm (Table 1). Mean baseline platelet count was 257 × 109/L, and 14 (6.1%) patients had thrombocytopenia (platelets <100 × 109/L per IWG 2006 criteria13): 8 (5.2%) of the luspatercept arm and 6 (7.9%) of the placebo arm (Table 1). Table 1 also lists characteristics of patients with baseline neutropenia or thrombocytopenia.
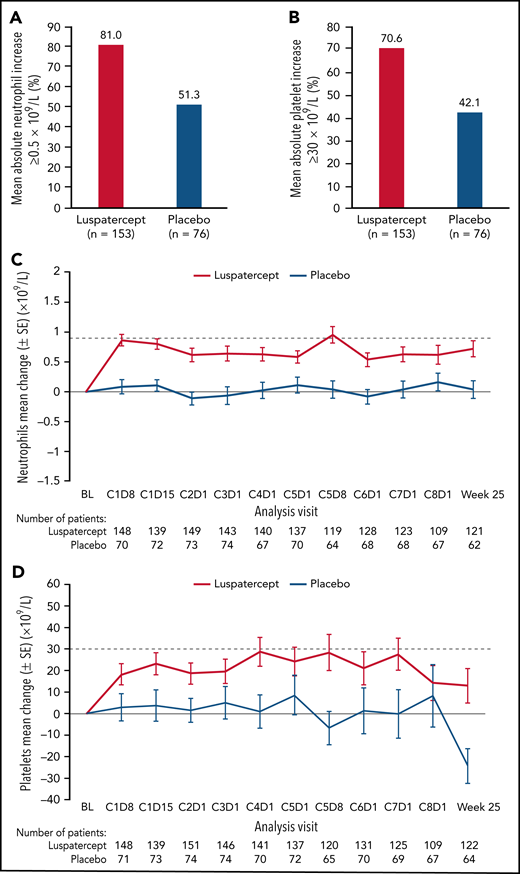
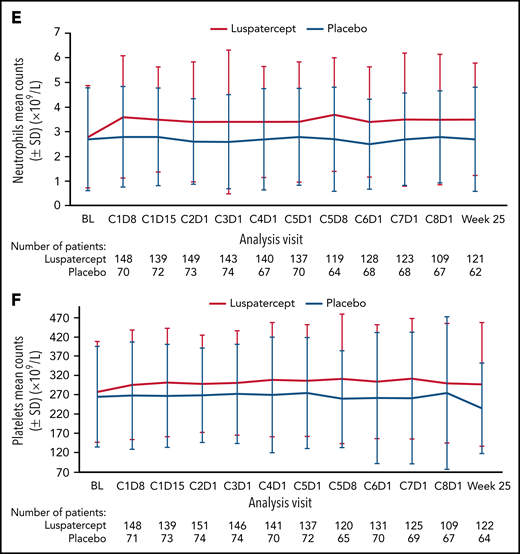
Among all randomized patients, 124 (81.0%) vs 39 (51.3%) patients in the luspatercept and placebo arms, respectively, achieved mean absolute increase in neutrophils of ≥0.5 × 109/L for 56 consecutive days compared with baseline (Figure 1A). Similarly, 108 (70.6%) vs 32 (42.1%) patients in the luspatercept vs placebo arms achieved mean absolute increase in platelets of ≥30 × 109/L (Figure 1B), maintained through week 25. By cycle 5, day 8, mean change from baseline in neutrophils was 0.95 × 109/L vs 0.04 × 109/L in the luspatercept and placebo arms, respectively (Figure 1C). By cycle 4, day 1, mean change from baseline in platelets was 28.7 × 109/L in the luspatercept arm and 0.9 × 109/L in the placebo arms (Figure 1D). Mean neutrophil and platelet counts are presented in Figure 1E-F. Although the increased levels of both neutrophils and platelets were maintained throughout luspatercept treatment (weeks 1-24), they did not exceed the upper-limits-of-normal values for adults to be considered a safety concern. The observed mean absolute increases in neutrophils and platelets were not dose-dependent.
Neutrophil and platelet improvements. Achievement of mean (A) absolute neutrophil increase ≥0.5 × 109/L and (B) absolute platelet increase ≥30 × 109/L. Mean change from baseline in (C) neutrophils and (D) platelets over time. Mean counts of (E) neutrophils and (F) platelets. Dashed lines indicate (C) a mean change from baseline of 0.9 × 109/L and (D) a mean change from baseline of 30 × 109/L. BL, baseline; C, cycle; D, day; SD, standard deviation; SE, standard error.
Neutrophil and platelet improvements. Achievement of mean (A) absolute neutrophil increase ≥0.5 × 109/L and (B) absolute platelet increase ≥30 × 109/L. Mean change from baseline in (C) neutrophils and (D) platelets over time. Mean counts of (E) neutrophils and (F) platelets. Dashed lines indicate (C) a mean change from baseline of 0.9 × 109/L and (D) a mean change from baseline of 30 × 109/L. BL, baseline; C, cycle; D, day; SD, standard deviation; SE, standard error.
Of the 25 patients evaluable for HI-N, more of those randomized to luspatercept vs placebo achieved HI-N during weeks 1 to 24 (13.3% vs 0.0%) and weeks 1 to 48 (20.0% vs 10.0%). Similarly, of the 14 patients evaluable for HI-P, more luspatercept- vs placebo-treated patients achieved HI-P during weeks 1 to 24 (50.0% vs 33.3%) and weeks 1 to 48 (62.5% vs 33.3%). These findings potentially support the use of luspatercept to treat patients with LR-MDS with RS who are often neutropenic and/or thrombocytopenic and anemic. However, the HI-N and HI-P responses in the placebo arm might highlight the normal oscillations seen in blood counts of patients with LR-MDS. Coupled with the low numbers of patients evaluable for HI-N and HI-P, these results should be interpreted with caution.
Treatment-emergent grade 3 or 4 neutropenia was infrequently reported, with lower incidence in the luspatercept vs the placebo group (7/153 [4.6%] vs 6/76 [7.9%]) and may have represented normal fluctuations in patients’ blood counts. No grade 3 or 4 treatment-emergent thrombocytopenia was reported in either treatment arm. These rates of grade 3 or 4 cytopenias are much lower than those observed with other therapies for MDS, including decitabine,15 azacytidine,16 and lenalidomide, which in a phase 3, randomized, placebo-controlled trial in patients with lower-risk non-del(5q) MDS showed high rates of grade 3 or 4 neutropenia (61.9% vs 12.7%) and thrombocytopenia (35.6% vs 3.8%).10
Despite the increase in neutrophil counts, there was a slight increase in infection rate with luspatercept compared with placebo. Infection was reported in 4 of 9 (44.4%) and 3 of 7 (42.9%) luspatercept- and placebo-treated patients, respectively, who experienced neutropenia (any grade) during the study. Overall infection rates for luspatercept and placebo patients were 53.6% and 40.8%, respectively. The infections were not opportunistic and were mostly grade 1 to 2 in severity. The differences in infection rates were not assessed, as this study was not designed or powered for this purpose. Bleeding was not reported in any luspatercept- or placebo-treated patients who experienced thrombocytopenia (any grade) on study. Among patients who achieved HI-N or HI-P, 1 patient in the luspatercept arm progressed to higher-risk MDS, but none progressed to AML.
Although only a minority of patients were evaluable for HI-P/HI-N, luspatercept treatment resulted in a mean increase from baseline in platelet and neutrophil counts in most patients overall vs placebo. Mean neutrophil and platelet count increases were observed early on luspatercept treatment and persisted to week 25. This could be associated with the positive effect of luspatercept on hematopoietic stem and progenitor cell expansion by modulating the structure of extracellular matrix17 or by direct inhibition of transforming growth factor-β signaling.18 In the 25 patients with baseline neutropenia and 14 patients with baseline thrombocytopenia, higher proportions of patients in the luspatercept vs placebo arms achieved HI-N and HI-P during weeks 1 to 24 and weeks 1 to 48. As meaningful statistical analyses were not possible because of small sample sizes, these results should be treated with caution.
Acknowledgments
The authors thank all the patients who participated in the study.
This study was sponsored by Celgene, a Bristol Myers Squibb Company, Princeton, New Jersey in collaboration with Acceleron Pharma. The authors received writing support in the preparation of this report from Karolina Lech of Excerpta Medica, supported by Bristol Myers Squibb.
The authors are fully responsible for all content and editorial decisions. All the patients in the MEDALIST study provided written informed consent.
Authorship
Contribution: G.G.-M., V.S., U.P., and R.S.K. designed the study; G.G.-M., G.J.M., P.F., R.B., V.S., M.D-C., C.F., O.I., M.A.S., A.M.Z., U.P., and R.S.K. collected data; G.G-M., G.J.M., P.F., R.B., V.S., M.D-C., C.F., O.I., M.A.S., A.M.Z., U.P., R.S.K., R.I., J.Z., A.R., D.S., and J.T.B. analyzed and interpreted the data; R.I. and A.R. supervised the clinical study; and J.Z. performed statistical analysis.
Conflict-of-interest disclosure: G.G-M. served in a consulting or advisory role for Acceleron Pharma, Astex Pharmaceuticals, Bristol Myers Squibb, Helsinn Therapeutics, and Jazz Pharmaceuticals; received honoraria from AbbVie, Acceleron Pharma, Astex Pharmaceuticals, Bristol Myers Squibb, and Helsinn Therapeutics; and received research funding from AbbVie, Amphivena Therapeutics, Astex Pharmaceuticals, Bristol Myers Squibb, H3 Biomedicine, Helsinn Therapeutics, Merck, Novartis, and Onconova Therapeutics. G.J.M. served in a consulting or advisory role for AbbVie and Novartis; and received research funding from Bristol Myers Squibb and Novartis. P.F. served in a consulting or advisory role for and received honoraria from AbbVie, Bristol Myers Squibb, Janssen, and Jazz Pharmaceuticals; and received accommodations, expenses, travel from Jazz Pharmaceuticals. R.B. served in a consulting or advisory role for and received honoraria and research funding from Bristol Myers Squibb and TAIHO; and received research funding from Takeda. V.S. received honoraria from Bristol Myers Squibb and Novartis; and served in a consulting or advisory role for Astex, Bristol Myers Squibb, Geron, Gilead, Menarini, and Novartis. M.D.-C. served in a consulting or advisory role for, received honoraria and research funding from, and had membership on an entity's board of directors or advisory committee of Bristol Myers Squibb and Novartis. C.F. served in a consulting or advisory role for and on the speakers bureau of Bristol Myers Squibb, Janssen, Novartis, and Takeda; and received research funding from Bristol Myers Squibb. M.A.S. served in a consulting or advisory role for Bristol Myers Squibb, Millennium, and Syros Pharmaceuticals; and received research funding from Pfizer and Takeda. A.M.Z. served in a consulting or advisory role for and received honoraria from AbbVie, Acceleron Pharma, Agios, Astellas, Beyond Spring, Boehringer-Ingelheim, Bristol Myers Squibb, Cardiff Oncology, Cardinal Health, Daiichi Sankyo, Epizyme, Incyte, Ionis, Jazz Pharmaceuticals, Novartis, Pfizer, Otsuka, Seattle Genetics, Taiho, Takeda, and Trovagene; and received other from AbbVie, ADC Therapeutics, Aprea, Astex, Boehringer-Ingelheim, Bristol Myers Squibb, Incyte, MedImmune/AstraZeneca, Novartis, Pfizer, Takeda, and Trovagene; Cardiff Oncology, CCITLA, and Leukemia and Lymphoma Society. R.I. ended employment in the past 24 months with and received stock and other ownership interests from Bristol Myers Squibb; and is employed by and received stock and other ownership interests from Eli Lilly and Company. J.Z. is employed by and received stock and other ownership interests in Bristol Myers Squibb. D.S. ended employment in the past 24 months with Bristol Myers Squibb; and is employed by CRISPR Therapeutics. A.R. is employed by and received travel, accommodations, expenses, stock, and other ownership interests from Bristol Myers Squibb. J.T.B. is employed by and received stock and other ownership interests from Acceleron Pharma; and received stock and other ownership interests from Bristol Myers Squibb. U.P. served in a consulting role for and received honoraria from AbbVie, Bristol Myers Squibb, and Novartis. R.S.K. served in a consulting or advisory role for Agios, Bristol Myers Squibb, Daiichi Sankyo, Incyte, Janssen, Novartis, and Pfizer; served on the speakers bureau for Alexion Pharmaceuticals, Jazz Pharmaceuticals, and Novartis; and received stock and other ownership interests from AbbVie. O.I. declares no competing financial interests.
Correspondence: Guillermo Garcia-Manero, Department of Leukemia, The University of Texas MD Anderson Cancer Center, 515 Holcombe Blvd, Houston, TX 77030, USA; e-mail: ggarciam@mdanderson.org.
REFERENCES
Author notes
U.P. and R.S.K. are joint senior authors.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal