In this issue of Blood, McCurdy et al1 report findings from the first study to directly compare immune restoration after haploidentical and matched donor hematopoietic cell transplantation (HCT) using high-dose posttransplant cyclophosphamide (PTCy)–based graft-versus-host disease (GVHD) prophylaxis.
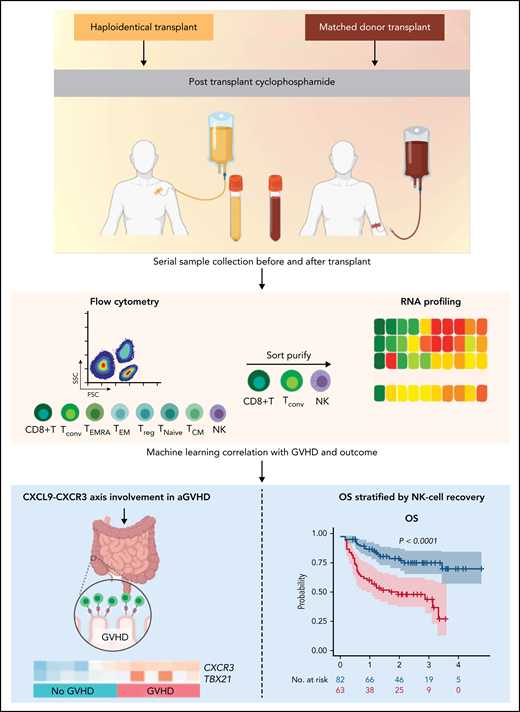
The investigators collected data on surface cell markers and cytokines, and they performed RNA sequencing (RNA-seq) of purified cellular subsets from a large cohort of patients who underwent myeloablative bone marrow transplantation followed by the leveraging of machine-learning algorithms to make sense of the potpourri (see figure). Interestingly, only minor differences in immune reconstitution were seen between donor types, with a transient early delay in CD4+ and CD8+ T-cell recovery in the haploidentical setting, likely attributable to additional early immunosuppression with tacrolimus and mycophenolate. Digging deeper into their large reservoir of analytes, they identified a potential novel GVHD biomarker and therapeutic target, CXCL9-CXCR3. They also correlated early quantitative natural killer (NK)–cell deficiency with reductions in progression-free survival (PFS) and overall survival (OS).
Haploidentical and matched donor HCT patients treated with PTCy underwent serial sample collection before and after receiving a transplant. Established and exploratory analytes along with key immune cell subsets were measured. A subset of mechanistically relevant immune cells were sort purified and interrogated via RNA sequencing, and machine learning was used to correlate with key transplantation-related outcomes including GVHD and survival. The CXCR3-CXCL9 axis was implicated as being important for the development of aGVHD, and quantitative NK-cell deficits during recovery were associated with inferior OS. The figure was created (in part) with BioRender.com.
Haploidentical and matched donor HCT patients treated with PTCy underwent serial sample collection before and after receiving a transplant. Established and exploratory analytes along with key immune cell subsets were measured. A subset of mechanistically relevant immune cells were sort purified and interrogated via RNA sequencing, and machine learning was used to correlate with key transplantation-related outcomes including GVHD and survival. The CXCR3-CXCL9 axis was implicated as being important for the development of aGVHD, and quantitative NK-cell deficits during recovery were associated with inferior OS. The figure was created (in part) with BioRender.com.
The use of PTCy on days 3 and 4 after HCT, first pioneered in humans at Johns Hopkins University more than 2 decades ago, represents a simple, inexpensive, and highly effective platform for preventing acute GVHD (aGVHD) and chronic GVHD (cGVHD) while seemingly preserving graft-versus-tumor activity, which led to increasing adoption by the worldwide community.2 After demonstrating safety and efficacy in a multicenter phase 2 trial of haploidentical HCT,3 extending it to be used in matched and mismatched unrelated donors was the next logical step. Emerging data suggest that PTCy is potentially superior to conventional calcineurin inhibitor–based GVHD prophylaxis.4
The long-held belief regarding the putative mechanism of PTCy-mediated protection against GVHD revolved around the proclivity for alloreactive adoptively transferred T cells to undergo high-dose cyclophosphamide–mediated deletion. However, recent work has refined this narrative and suggests that rather than deletion, it is functional impairment of alloreactive effectors and rapid preferential recovery of FoxP3+ regulatory T cells (Tregs) that afford protection.5 Indeed, careful modeling demonstrated that expression of aldehyde dehydrogenase by Tregs is protective against cyclophosphamide-mediated destruction. Furthermore, murine models have shown that Tregs are absolutely requisite for PTCy-mediated GVHD protection.6,7
Given the established importance of Tregs after PTCy, McCurdy et al carefully scrutinized levels of Tregs, ratio of Tregs to conventional T cells (Tconv), and the RNA expression profile of purified Tregs at early and late time points after transplantation, and they stratified their analysis by patients who developed aGVHD. Surprisingly, neither qualitative nor quantitative Treg deficits were sufficient to account for the development of aGVHD. Instead, they found highly proficient Tregs that expressed canonical markers, including interferon-γ (IFN-γ) and granzyme A (expression profiles that persisted after successful treatment of GVHD), which suggest that Tregs cooperate with pharmacologic therapy to resolve GVHD.
By using machine learning to make sense of the panoply of cytokines, surface markers, and clinical variables, the authors identified higher levels of CXCL9, which when combined with high levels of Tconv at day 28, predicted subsequent development of aGVHD. The role of CXCL9 as an IFN-γ–mediated regulator of leukocyte tissue trafficking provides a putative mechanism for its role in the development of GVHD. Next, by assessing RNA expression profiles of purified Tconv, the investigators found skewing toward an inflammatory Th1 phenotype with upregulation of IFN-γ–related genes previously related to GVHD, including CXCR3 and TBX21.8 This finding was rewarding from a biological standpoint because CXCL9 serves as a CXCR3 ligand, thus orthogonally connecting the 2 analytes. Compared with healthy donors and patients without GVHD, patients with GVHD had higher protein levels of CXCR3 expression and Th17-prone Tconv as assessed by flow cytometry. To explore the association of CXCR3 with GVHD in vivo and provide further confidence in this finding, the authors generated a major histocompatibility complex–matched murine HCT model and observed an increased number of CXCR3+ Tconv in the spleens and livers of mice who developed breakthrough aGVHD after PTCy. The identification of the CXCL9-CXCR3 axis represents a novel finding, potentially representing a predictive biomarker as well as a possibly exploitable therapeutic target.
McCurdy et al then used their data to investigate transplantation outcomes, and they identified quantitative NK-cell recovery deficits at day 28 after HCT as the major determinant of PFS and OS driven by increases in both relapse- and nonrelapse-related mortality. In the PTCy setting, NK-cell deficiency has been correlated with cytomegalovirus reactivation and an increased incidence of cGVHD among recipients of haploidentical HCT. Mechanistically, this was linked to a deficiency of NK-cell aldehyde-dehydrogenase expression and attendant protection from PTCy-mediated destruction with in vitro abrogation of deficiency mediated by administering the NK-proliferation signal interleukin-15 (IL-15).9,10 The McCurdy et al study provides further rationale for performing more studies that focus on NK-cell recovery across donor settings after PTCy, and there are ongoing trials investigating adoptive NK-cell therapy after HCT.
As a whole, the study by McCurdy et al provides an important initial map of immune reconstitution after haploidentical and matched HCT using PTCy. More studies are needed to explore the CXCL9-CXCR3 axis as a potential biomarker or therapeutic target for aGVHD. In addition, given that this cohort of patients received myeloablative conditioning and bone marrow grafts, it will be important to understand to what degree these findings are present in the settings of reduced intensity conditioning and peripheral blood progenitor grafts. Hopefully, such important investigations will identify actionable immunologic profiles for providers to follow serially after HCT. Along with sensitive viral load assays, individualized assessment of measurable residual disease, and mucosal injury GVHD biomarkers, these immunologic profiles can potentially allow us to act preemptively after HCT to modify calculated risks of clinical outcomes rather than wait to react to frank clinical manifestations.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal