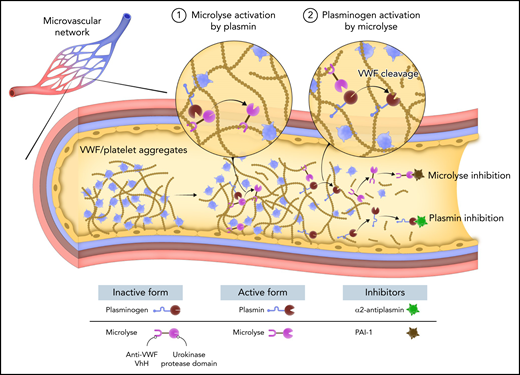
In this issue of Blood, de Maat et al1 report the development of Microlyse, a novel thrombolytic that directs plasminogen activation to von Willebrand factor (VWF). This agent is based on a fusion between the protease domain of urokinase and a VWF-targeted nanobody and has therapeutic potential to treat acute episodes of thrombotic thrombocytopenic purpura (TTP) (see figure).
VWF-targeted plasmin generation by Microlyse. TTP is a devastating thrombotic syndrome characterized by the deposition of VWF and platelets in the microvasculature. Plasmin generation occurs during acute episodes of TTP and can degrade VWF, suggesting that the fibrinolytic system may serve as a potential bypassing pathway for the treatment of TTP. Microlyse is a fusion between the protease domain of urokinase and a nanobody targeting VWF. Exogenous plasmin converts Microlyse into its active form. Activated Microlyse catalyzes plasminogen activation in the presence of VWF, leading to a burst of plasmin activity. Plasmin degrades VWF and dissolves the thrombus. Microlyse and plasmin are rapidly inhibited by PAI1 and α2-antiplasmin, respectively, thereby limiting systemic thrombolytic activity.
VWF-targeted plasmin generation by Microlyse. TTP is a devastating thrombotic syndrome characterized by the deposition of VWF and platelets in the microvasculature. Plasmin generation occurs during acute episodes of TTP and can degrade VWF, suggesting that the fibrinolytic system may serve as a potential bypassing pathway for the treatment of TTP. Microlyse is a fusion between the protease domain of urokinase and a nanobody targeting VWF. Exogenous plasmin converts Microlyse into its active form. Activated Microlyse catalyzes plasminogen activation in the presence of VWF, leading to a burst of plasmin activity. Plasmin degrades VWF and dissolves the thrombus. Microlyse and plasmin are rapidly inhibited by PAI1 and α2-antiplasmin, respectively, thereby limiting systemic thrombolytic activity.
VWF serves as a carrier for coagulation factor VIII and as an adhesive link between circulating platelets and injured blood vessels. VWF platelet-binding activity is proteolytically regulated by ADAMTS13. Congenital or acquired deficiency in ADAMTS13 causes TTP due to an increase in thrombogenic VWF multimer lengths. Illnesses associated with elevated VWF levels are often accompanied by reduced ADAMTS13 activity. These illnesses include obesity, peripheral and coronary arterial disease, ischemic stroke, myocardial infarction, preeclampsia, liver cirrhosis, inflammatory bowel disease, and sepsis.2 Therefore, excessive VWF may contribute to the pathophysiology of many diseases.
Pharmacological agents that block the interaction between VWF and platelets (including ARC1779, caplacizumab, AJW200, and anfibatide) can relieve symptoms of acute coronary syndromes, ischemic stroke, and TTP. However, their capacity to clear VWF/platelet aggregates is limited. Recombinant ADAMTS13 (BAX-930, TAK-755) is in clinical trials for the treatment of congenital TTP3 and may be examined in other diseases. Recombinant ADAMTS13 is an attractive thrombolytic agent because of its stability in circulation and its specificity for VWF. ADAMTS13 cleavage of VWF is dependent on fluid shear rates present at sites of vascular injury and during VWF secretion from endothelial cells (ECs), which prevents VWF degradation in circulation. This specificity distinguishes ADAMTS13 from other thrombolytic agents like tPA (and derivatives) that cause bleeding due to nonspecific fibrinogen degradation in circulation.4 These properties may explain the benefit of recombinant ADAMTS13 treatment in preclinical models of stroke, which have shown no increased risk of bleeding.5
Recombinant ADAMTS13 may not be effective in treating immune-TTP, in which patients have autoantibodies that neutralize endogenous ADAMTS13. Infusion of high doses of recombinant ADAMTS13 to overcome these autoantibodies risks amplifying this immune response to ADAMTS13. In sepsis, VWF knockout mice are protected from death, but ADAMTS13 infusion does not protect WT mice.6 The failure of recombinant ADAMTS13 in this model may reflect its inactivation by proteases generated during sepsis.
These observations led de Maat et al to examine alternative pathways to proteolytically regulate VWF. They previously detected plasmin activity in TTP patients and showed that plasmin efficiently cleaves VWF-platelet strings.7,8 While endogenous plasmin activity is not capable of clearing VWF-platelet complexes in TTP patients, therapeutic doses of plasminogen activators like streptokinase partially normalized platelet counts in a mouse model of TTP, suggesting the fibrinolytic system as an alternative pathway to treat TTP.7 However, the bleeding risk associated with systemic administration of plasminogen activators may limit their use in TTP.4
Therefore, de Maat et al developed Microlyse to restrict plasminogen activation to the VWF surface and prevent bleeding. Urokinase is an ideal plasminogen activator for these purposes because it exists in a zymogen state and is readily activated by plasmin.9 Fusing the protease domain of urokinase to a VWF-binding nanobody localizes its activity to VWF. Microlyse binds VWF with high affinity (KD ∼30 pM) via the CT/CK domain and accumulates on VWF/platelet strings. An earlier version, Microlyse#1, bound to the D’D3 domain of VWF. It was abandoned because it displaced FVIII and, thus, may cause unintended side effects. Microlyse can be activated by plasmin and can catalyze plasminogen activation, demonstrating the expected reciprocal feedback-activation loop. Importantly, Microlyse is rapidly inhibited by PAI-1. These biochemical properties suggest that Microlyse is equipped to localize plasminogen activation to VWF-rich thrombi. A fascinating (perhaps unexpected) observation is that VWF alone did not accelerate plasminogen activation by Microlyse in vitro. However, ristocetin accelerated VWF-dependent plasmin generation by Microlyse ∼3-fold. Therefore, the thrombolytic activity of Microlyse may be partially dependent on fluid shear stresses (similar to ADAMTS13) that minimize VWF degradation in circulation. Future studies are needed to fully characterize Microlyse and plasminogen binding to VWF.
The capacity of Microlyse to clear VWF/platelet strings was compared with caplacizumab, a nanobody targeting the VWF A1 domain approved for the treatment of TTP. Both Microlyse and caplacizumb cleared platelets from VWF strings in vitro. In ADAMTS13−/− mice administered a bolus of VWF to induce TTP, Microlyse partially normalized platelet counts and normalized LDH levels. High dose caplacizumab normalized platelet counts better than Microlyse, but was unable to normalize LDH levels, indicating persistent organ damage.
Interestingly, Microlyse was equally effective at all 3 doses (208 nM, 416 nM, and 832 nM), whereas caplacizumab exhibited a threshold response in which high dose (925 nM), but not low dose (416 nM), improved platelet counts in mice. These data illustrate the potential advantage of irreversible proteolytic regulation of VWF compared with antagonist-based therapies. Microlyse is expected to remove both VWF and platelets from the EC surface, whereas caplacizumab displaces platelets but otherwise leaves VWF intact. Additional studies are needed to examine the importance of removing VWF from the EC surface in TTP treatment.
Importantly, Microlyse did not prolong the tail-bleeding time 15 minutes after administration. In contrast, a subtherapeutic dose of caplacizumab was associated with prolonged tail-bleeding times. This promising preliminary result suggests that Microlyse exerts a thrombolytic effect at safe doses. However, bleeding risk should also be examined at 24 hours to align with platelet count and LDH measurements of thrombolytic activity. Furthermore, measuring the effect of Microlyse on markers of fibrinolysis such as d-dimer, plasmin-α2antiplasmin, fibrinogen, and prothrombin-time are needed to strengthen the authors’ conclusions on safety and bleeding risk.
Development of novel proteolytic regulators of VWF, like Microlyse, may be beneficial in the treatment of TTP and other cardiovascular diseases. In this report, de Maat et al did not compare Microlyse to recombinant ADAMTS13. Therefore, future studies are needed to test if targeted plasminogen activation on VWF is as effective and safe as recombinant ADAMTS13. Microlyse nonetheless signals a potential new strategy for the targeted regulation of VWF in thrombosis. Investigating the thrombolytic utility of Microlyse in more complex preclinical models of thrombosis, such as stroke, may reveal the synergistic benefits of plasmin to degrade both VWF and fibrin.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal