Key Points
The combination of ATRA and LD-RTX could achieve an 80% OR rate and a 61% SR rate.
The ATRA plus LD-RTX regimen is a promising treatment option for corticosteroid-resistant or relapsed ITP.
Abstract
The study aimed to compare the efficacy and safety of all-trans retinoic acid (ATRA) plus low-dose rituximab (LD-RTX) with LD-RTX monotherapy in corticosteroid-resistant or relapsed immune thrombocytopenia (ITP) patients. Recruited patients were randomized at a ratio of 2:1 into 2 groups: 112 patients received LD-RTX plus ATRA, and 56 patients received LD-RTX monotherapy. Overall response (OR), defined as achieving a platelet count of ≥30 × 109/L confirmed on ≥2 separate occasions (≥7 days apart), at least a doubling of the baseline platelet count without any other ITP-specific treatment, and the absence of bleeding within 1 year after enrollment, was observed in more patients in the LD-RTX plus ATRA group (80%) than in the LD-RTX monotherapy group (59%) (between-group difference, 0.22; 95% CI, 0.07-0.36). Sustained response (SR), defined as maintenance of a platelet count >30 × 109/L, an absence of bleeding, and no requirement for any other ITP-specific treatment for 6 consecutive months after achievement of OR during 1 year following enrollment, was achieved by 68 (61%) patients in the combination group and 23 (41%) patients in the monotherapy group (between-group difference, 0.20; 95% CI, 0.04-0.35). The 2 most common adverse events (AEs) for the combination group were dry skin and headache or dizziness. Our findings demonstrated that ATRA plus LD-RTX significantly increased the overall and sustained response, indicating a promising treatment option for corticosteroid-resistant or relapsed adult ITP. This study is registered at www.clinicaltrials.gov as #NCT03304288.
Medscape Continuing Medical Education online
In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and the American Society of Hematology. Medscape, LLC is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.00 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.0 MOC points in the American Board of Internal Medicine’s (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider’s responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 467.
Disclosures
Associate Editor Jorge A. Di Paola, CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, and the authors declare no competing financial interests.
Learning objectives
Upon completion of this activity, participants will:
- 1.
Compare responses to all-trans retinoic acid (ATRA) plus low-dose rituximab (LD-RTX) vs LD-RTX monotherapy in patients with corticosteroid-resistant or relapsed immune thrombocytopenia (ITP), according to a multicenter prospective, randomized controlled study
- 2.
Compare adverse events with ATRA plus LD-RTX vs LD-RTX monotherapy in patients with corticosteroid-resistant or relapsed ITP, according to a multicenter prospective, randomized controlled study
- 3.
Identify clinical implications of the comparative efficacy and safety of ATRA plus LD-RTX vs LD-RTX monotherapy in patients with corticosteroid-resistant or relapsed ITP, according to a multicenter prospective, randomized controlled study
Release date: January 20, 2022; Expiration date: January 20, 2023
Introduction
Primary immune thrombocytopenia (ITP) is an autoimmune disorder characterized by increased platelet destruction and insufficient platelet production.1-4 Corticosteroids and IV immunoglobulin (IVIg) are first-line treatments.5,6 Approximately one-third of ITP patients fail to achieve a response; in addition, most patients who respond to first-line treatment relapse and require further second-line therapies.5,6 However, the optimal second-line treatment remains uncertain.6
Rituximab (RTX), a chimeric anti-CD20 monoclonal antibody that exerts its treatment effects through the rapid depletion of CD20-positive B lymphocytes and modulation of T cells,7,8 has been frequently used in ITP treatment.5,6 There is not yet a gold standard regimen of RTX. Schedules have been proposed, including the standard dose (SD) (375 mg/m2 weekly for 4 administrations) and low dose (LD) (100 mg flat dose weekly for 4 administrations). Previous studies using SD-RTX demonstrated an overall response (OR) of nearly 60% and a sustained response (SR) of 30% to 40%.9-11 A meta-analysis of 9 studies on LD-RTX in ITP revealed a comparable OR with SD-RTX.12 Moreover, LD-RTX usually means significantly reduced expenditure (10 000 to 40 000 USD per 4-infusion course) and easier administration than SD-RTX.12,13 These advantages indicate that LD-RTX might be a promising treatment option for ITP. However, the reported median time to response (TTR) of monotherapy of LD-RTX was 30 days, significantly longer than that of SD-RTX (10.5 days).10 A lower SR of LD-RTX compared with SD-RTX was also revealed.10 Moreover, another study reported that for patients with corticosteroid-resistant or relapsed ITP, no difference between SD-RTX and placebo in OR and treatment failure rate was observed within 78 weeks in the treatment, indicating that a general recommendation for the use of SD-RTX monotherapy in corticosteroid-resistant or relapsed ITP could not be supported.14 It was indicated that increased doses of RTX might maintain the concentration and prolong the elimination of B cells according to the pharmacokinetic data of RTX.15,16 Additional doses of RTX were demonstrated to improve the efficacy of rheumatoid arthritis and lymphoma treatment.17-19
All-trans retinoic acid (ATRA), a key metabolite of vitamin A, is involved in cell proliferation and differentiation.20,21 Our preliminary studies indicated the efficacy of ATRA in the ITP model due to its effects on inducing megakaryocyte (MK) differentiation and maturation, as well as its immunomodulatory effects.22-25 We previously conducted a multicenter, randomized trial demonstrating that the combination of ATRA (10 mg twice daily) and danazol (200 mg twice daily) achieved an improvement in overall response (82% vs 44%; odds ratio [OdR] 5.95, 95% CI 2.29 to 15.43) and 12-month remission (62% vs 25%; OdR 4.94, 95% CI 2.03 to 12.02) compared with danazol monotherapy (200 mg twice daily).26 Dai et al also evaluated immunomodulatory therapy with ATRA (10 mg 3 times daily) and prednisone (10 mg twice daily) in 35 patients with chronic ITP, reporting an OR of 54.3%.27
Since RTX and ATRA share disparate mechanisms in treating ITP, a combination of LD-RTX (with 2 additional doses) and ATRA may work synergistically based on a “double-hit” mechanism targeting both platelet production and destruction, which may overcome the long TTR and improve the SR rate of LD-RTX. Therefore, our study aimed to determine the efficacy and safety of ATRA plus LD-RTX compared with LD-RTX monotherapy in patients with corticosteroid-resistant or relapsed ITP.
Methods
Study design
We conducted an open-label, investigator-initiated, randomized clinical study in 7 tertiary medical centers in China. Patient enrollment was conducted between October 2017 and January 2020. The study protocol was approved by the ethics committees or institutional review boards of all participating centers. Patients were recruited during routine medical assessments. Informed consent was obtained from each participant in accordance with the Declaration of Helsinki.
Patients
Adult patients with primary ITP diagnosed according to the 2011 American Society of Hematology guidelines28 and the consensus of Chinese experts on the diagnosis and treatment of adult ITP (version 2016)29 had a platelet count <30 × 109/L or with a platelet count ≥30 × 109/L and concomitant bleeding manifestations at enrollment, and did not achieve a sustained response to therapy with full-dose corticosteroids for a duration of at least 4 weeks or relapsed during the process of corticosteroid tapering or discontinuation were enrolled. Patients with other concomitant treatments (corticosteroids, ciclosporin, danazol, azathioprine, mycophenolate mofetil, and herbs) were also eligible when the dose of the concomitant treatments had been stable over the past 4 weeks, and the dose was expected to be stable until 4 weeks after the study.
Patients who had severe dysfunction of the heart, kidney, lung, or liver or primary immunodeficiency were excluded. Pregnant or lactating patients were also excluded. Moreover, patients were ineligible if they had previously received RTX.
Randomization and treatments
Statisticians randomly assigned eligible patients in a 2:1 ratio to receive LD-RTX plus ATRA or LD-RTX alone. A list of random numbers and the corresponding treatment assignments were centrally pregenerated at Peking University People’s Hospital (Beijing, China). The random numbers were kept in sealed envelopes with the corresponding sequential numbers written on the cover and sent to the investigating center when patients who met the enrollment criteria signed the informed consent form. Neither clinicians nor patients were masked to group assignments. All data were collected and analyzed by experts from Peking University, and the experts who participated in data collection and analysis were masked to group assignments. RTX was given at a fixed dose of 100 mg weekly for 6 weeks. For the combination group, ATRA was given concomitantly orally at 20 mg/m2 daily for 12 weeks (supplemental Figure 1, available on the Blood Web site). The treatment period lasted for 12 weeks for patients in the combination group and 6 weeks for patients in the monotherapy group unless otherwise specified (loss of follow-up, severe side effects, other interventions, etc).
Patients were withdrawn from the study if severe adverse events occurred or at the patients’ request. Treatment was discontinued if platelet counts in 2 consecutive tests ≥2 weeks apart were >300 × 109/L and resumed when the platelet count fell to <150 × 109/L.
Assessments were performed at baseline, weekly during the first 4 weeks, every 2 weeks until week 24, and every 4 weeks thereafter. At each study visit, we performed careful clinical examinations, recorded the bleeding score, concomitant medications were given, noted adverse events, and measured platelet counts. Bleeding score was graded in accordance with the World Health Organization's bleeding scale (no bleeding = grade 0; petechiae = grade 1; mild blood loss = grade 2; gross blood loss = grade 3; and debilitating blood loss = grade 4).30
Study endpoints
One thing we need to state is that due to the misunderstanding of the recording specifications of the trial registration and the interpretation of clinical trial data, we incorrectly added descriptions about the interim analysis and edited the endpoints at clinicaltrials.gov, which was not in accordance with the actual implementation process of our study. Since COVID-19 broke out and spread quickly during the study process, the follow-up time had to be amended to 1 year, which was the only amendment from the original protocol.
The definitions of endpoints are shown in Table 1. The primary endpoint of the study was OR, defined as achieving a platelet count of ≥30 × 109/L confirmed on at least 2 separate occasions (≥7 days apart), at least a doubling of the baseline platelet count without administration of any other ITP-specific treatment and the absence of bleeding within 1 year following enrollment.28 The primary endpoint could be achieved at any time during 1 year. Enrollment and initiation of the study drugs were performed on the same day.
Definitions of endpoints in the study
| Outcomes . | Definitions . |
|---|---|
| Primary outcome | |
| Overall response | Achieving a platelet count of ≥30 × 109/L confirmed on ≥2 separate occasions (≥7 d apart), at least a doubling of the baseline platelet count without administration of any other ITP-specific treatment, and the absence of bleeding within 1 y following enrollment. |
| Secondary outcomes | |
| Complete response | Platelet count ≥100 × 109/L measured on 2 occasions at least 7 d apart and the absence of bleeding within 1 y following enrollment. |
| Sustained response | Maintenance of a platelet count >30 × 109/L, an absence of bleeding, and no requirement for any other ITP-specific treatment of 6 consecutive mo after achievement of response during 1 y following enrollment. |
| Remission | A durable platelet count >30 × 109/L without bleeding up to 12 mo after enrollment. |
| Outcomes . | Definitions . |
|---|---|
| Primary outcome | |
| Overall response | Achieving a platelet count of ≥30 × 109/L confirmed on ≥2 separate occasions (≥7 d apart), at least a doubling of the baseline platelet count without administration of any other ITP-specific treatment, and the absence of bleeding within 1 y following enrollment. |
| Secondary outcomes | |
| Complete response | Platelet count ≥100 × 109/L measured on 2 occasions at least 7 d apart and the absence of bleeding within 1 y following enrollment. |
| Sustained response | Maintenance of a platelet count >30 × 109/L, an absence of bleeding, and no requirement for any other ITP-specific treatment of 6 consecutive mo after achievement of response during 1 y following enrollment. |
| Remission | A durable platelet count >30 × 109/L without bleeding up to 12 mo after enrollment. |
The prespecified secondary endpoints of the study included CR, TTR, duration of response, adverse events (AEs), and bleeding symptoms, and we added several post-hoc secondary endpoints, including SR, relapse-free survival (RFS), remission, and rescue treatment. CR was defined as a platelet count ≥100 × 109/L measured on 2 occasions at least 7 days apart and the absence of bleeding within 1 year following enrollment.28 SR was defined as maintenance of a platelet count >30 × 109/L, an absence of bleeding, and no requirement for any other ITP-specific treatment of 6 consecutive months after achievement of OR during 1 year following enrollment.31,32 Rescue therapy was defined as any new medical intervention taken to increase the platelet count or prevent bleeding events or an increase in the dose of concomitant treatments. If patients had bleeding manifestations or a platelet count <10 × 109/L, rescue treatment was started. If patients had a platelet count between 10 and 30 × 109/L, whether rescue treatment would be used was dependent on physicians’ assessment of bleeding predisposition, patient preferences, patient tolerance of side effects caused by specific treatment, lifestyle and activity, and patient expectations. Patients who received rescue treatment were considered nonresponse or loss of response for those who had achieved OR. As the median TTR was approximately 4 to 5 weeks in preliminary studies,10,33,34 we defined nonresponse or loss of response as patients who received rescue treatment from week 6 to the end of the study. Patients with platelet responses that occurred within 5 weeks after rescue drug administration were also regarded as nonresponse. TTR was defined as the time from starting treatment to the time a response was achieved.28 Duration of response was defined as time from OR until relapse or until the last follow-up visit.28 Relapse was defined as loss of OR, which was manifested by platelet counts <30 × 109/L or bleeding after achieving an OR. RFS was defined as the time interval between the achievement of OR and relapse or the end of the follow-up.35 Remission was defined as a durable platelet count >30 × 109/L without bleeding up to 12 months after enrollment. We graded adverse events in accordance with the Common Terminology Criteria for Adverse Events, version 5.0.36
Statistical analysis
Based on preliminary findings,10,33,34,37-43 the authors hypothesized that the probability of achieving an OR would be 90% with ATRA plus LD-RTX and 66% with LD-RTX monotherapy. With the assumption that 20% of patients would drop out before treatment failure, 125 patients for ATRA plus LD-RTX and 63 patients for LD-RTX were needed to have 90% power using a 2-sided test and a significance level of 0.05. However, 168 patients were finally included due to difficulties in enrollment and the term limit of funding.
The main analysis of the primary and secondary endpoints was performed in the intention-to-treat (ITT) population, including all randomized allocated patients. The safety population included all patients who were allocated. We also analyzed per-protocol populations, including all patients who had completed the treatment.
Patients’ baseline characteristics, concomitant medications, and worst bleeding score are provided as descriptive data: qualitative variables are expressed as counts and (or) proportions, and quantitative variables as medians with interquartile ranges (IQRs).
Patients who discontinued treatment for any reason were also considered nonresponse. Therefore, the main analysis of the primary and secondary endpoints was performed in the ITT population by imputing missing values (those who did not complete treatment) as failures. The between-group differences for the primary endpoint and secondary endpoints, including CR, SR, remission, and rescue treatment, were analyzed by using a χ2 test and the results were expressed as differences between proportions with 95% CI. We also performed sensitivity analysis of the primary endpoint first using multiple imputations by fully conditional specification to take missing values of those who did not complete treatment into account and then using complete cases. The results of the multiple imputations are also expressed as the difference between proportions with 95% CI estimated with a linear regression model. Remission was analyzed by imputing missing values as a failure and by using complete cases to take those who did not have a full-year follow-up into account. TTR and duration of response were analyzed using Mann-Whitney U tests. The Kaplan-Meier method and log-rank test were used to assess differences in the fraction of RFS. Due to previous studies that proposed several factors (eg, age, sex, child-bearing female, duration of response, etc) might have an effect on the efficacy of treatment regimens, including RTX or ATRA,9,26,34,44-46 and in the interests of investigators, logistic regression was used to determine factors associated with OR and SR in an exploratory, post-hoc analysis after data collection. SPSS Statistics version 24.0.0 (IBM SPSS Statistics, Armonk, NY), R for Windows, version 4.1.0 (R Foundation), and GraphPad Prism version 7 (GraphPad Software) were used for all analyses.
Results
Patient characteristics
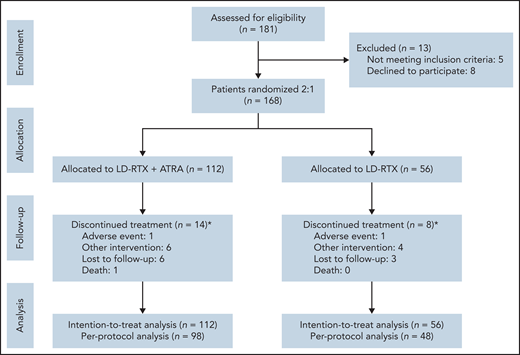
Patients were enrolled in the study from October 2017 to January 2020. During this period, 181 patients were screened for eligibility, and 168 were ultimately enrolled and subsequently randomized to receive the combination treatment of LD-RTX with ATRA (n = 112) or monotherapy of LD-RTX (n = 56) on the same day as enrollment. Fourteen patients in the combination group exited the study during treatment (1 adverse event, 6 needed other intervention, 6 lost to follow-up, and 1 death) and 8 patients in the LD-RTX monotherapy group withdrew (1 adverse event, 4 needed other intervention, and 3 lost to follow-up) (Figure 1).
Trial profile. *The timing of withdrawal occurred in week 2 (1 lost to follow-up), week 3 (2 lost to follow-up), week 4 (1 death, 1 lost to follow-up), week 6 (5 other interventions, 1 lost to follow-up), week 8 (1 other intervention, 1 AE), week 10 (1 lost to follow-up) for the combination treatment group and occurred in week 2 (1 lost to follow-up), week 3 (1 other intervention), week 4 (2 other interventions, 1 lost to follow-up), week 6 (1 AE, 1 other intervention, 1 lost to follow-up) for the monotherapy group.
Trial profile. *The timing of withdrawal occurred in week 2 (1 lost to follow-up), week 3 (2 lost to follow-up), week 4 (1 death, 1 lost to follow-up), week 6 (5 other interventions, 1 lost to follow-up), week 8 (1 other intervention, 1 AE), week 10 (1 lost to follow-up) for the combination treatment group and occurred in week 2 (1 lost to follow-up), week 3 (1 other intervention), week 4 (2 other interventions, 1 lost to follow-up), week 6 (1 AE, 1 other intervention, 1 lost to follow-up) for the monotherapy group.
Baseline characteristics of the 2 groups are compared in Table 2. Median age was 46.0 years and 44.5 years, and median duration from diagnosis was 20.0 and 20.5 months in the combination and monotherapy group, respectively. There were 59 (53%) males in the combination group and 24 (43%) male patients in the monotherapy group. All enrolled patients had failed first-line treatment with corticosteroids. The median numbers of previous therapies were 2.0 for the 2 groups. The median baseline platelet count was 17.0 × 109/L in the combination group and 19.0 × 109/L in the monotherapy group. Baseline bleeding scores were also shown in Table 2. Sixteen (14%) patients in the combination group and 10 (18%) patients in the monotherapy group were receiving maintenance medication at enrollment (supplemental Table 1). Regarding visit adherence after enrollment, 87% had a 44-week visit, 85% had a 48-week visit, and 75% had a 1-year visit.
Baseline characteristics of patients enrolled in the study
| Characteristics . | ATRA + LD-RTX (n = 112) . | LD-RTX (n = 56) . |
|---|---|---|
| Age (y), median (IQR) | 46.0 (36.0-55.8) | 44.5 (36.0-58.0) |
| Male, n (%) | 59 (52.7) | 24 (42.9) |
| Platelet count (109/L), median (IQR) | 17.0 (14.0-21.0) | 19.0 (14.0-25.0) |
| Duration of ITP (mo), median (IQR) | 20.0 (15.0-32.8) | 20.5 (15.0-27.0) |
| Previous treatments, n (%) | ||
| Corticosteroids | 112 (100.0) | 56 (100.0) |
| IVIG | 20 (17.9) | 14 (25.0) |
| Vincristine | 2 (1.8) | 3 (5.4) |
| rhTPO | 21 (18.8) | 8 (14.3) |
| Cyclosporin | 12 (10.7) | 9 (16.1) |
| Danazol | 21 (18.8) | 15 (26.8) |
| Azathioprine | 4 (3.6) | 3 (5.4) |
| Mycophenolate mofetil | 8 (7.1) | 8 (14.3) |
| Splenectomy | 1 (0.9) | 1 (1.8) |
| Herbs | 7 (6.3) | 9 (16.1) |
| Bleeding score, % (n) | ||
| 0 | 53 (47.3) | 31 (55.4) |
| 1 | 40 (35.7) | 17 (30.4) |
| 2 | 11 (9.8) | 5 (8.9) |
| 3 | 5 (4.5) | 2 (3.6) |
| 4 | 3 (2.7) | 1 (1.8) |
| Characteristics . | ATRA + LD-RTX (n = 112) . | LD-RTX (n = 56) . |
|---|---|---|
| Age (y), median (IQR) | 46.0 (36.0-55.8) | 44.5 (36.0-58.0) |
| Male, n (%) | 59 (52.7) | 24 (42.9) |
| Platelet count (109/L), median (IQR) | 17.0 (14.0-21.0) | 19.0 (14.0-25.0) |
| Duration of ITP (mo), median (IQR) | 20.0 (15.0-32.8) | 20.5 (15.0-27.0) |
| Previous treatments, n (%) | ||
| Corticosteroids | 112 (100.0) | 56 (100.0) |
| IVIG | 20 (17.9) | 14 (25.0) |
| Vincristine | 2 (1.8) | 3 (5.4) |
| rhTPO | 21 (18.8) | 8 (14.3) |
| Cyclosporin | 12 (10.7) | 9 (16.1) |
| Danazol | 21 (18.8) | 15 (26.8) |
| Azathioprine | 4 (3.6) | 3 (5.4) |
| Mycophenolate mofetil | 8 (7.1) | 8 (14.3) |
| Splenectomy | 1 (0.9) | 1 (1.8) |
| Herbs | 7 (6.3) | 9 (16.1) |
| Bleeding score, % (n) | ||
| 0 | 53 (47.3) | 31 (55.4) |
| 1 | 40 (35.7) | 17 (30.4) |
| 2 | 11 (9.8) | 5 (8.9) |
| 3 | 5 (4.5) | 2 (3.6) |
| 4 | 3 (2.7) | 1 (1.8) |
rhTPO, recombinant human thrombopoietin.
Responses
The main analysis of the primary and secondary endpoints was performed with the ITT population, and the results are shown in Table 3. OR was achieved in a total of 90 (80%) patients in the combination group compared with 33 (59%) patients in the LD-RTX monotherapy group (between-group difference, 0.22; 95% CI, 0.07-0.36) within 1 year after enrollment, indicating that the combination treatment of LD-RTX with ATRA increased the response rate. Sensitivity analyses with multiple imputation of missing values (between-group difference, 0.23; 95% CI, 0.11-0.35) and with complete cases (between-group difference, 0.23; 95% CI, 0.07-0.39) yielded similar results. Outcomes by site are shown in supplemental Table 2. Age (OdR, 0.993 [95% CI, 0.969-1.017]), male sex (OdR, 1.162 [95% CI, 0.586-2.302]), duration of ITP (OdR, 1.326 [95% CI, 0.503-3.500]), child-bearing female (OdR, 1.138 [95% CI, 0.529-2.449]), grade 3 or 4 bleeding at enrollment (OdR, 1.697 [95% CI, 0.352-8.173]), prior danazol use (OdR, 0.788 [95% CI, 0.351-1.771]), and the number of previous treatments (OdR, 0.855 [95% CI, 0.576-1.269]) were not associated with OR in an exploratory, post-hoc logistic regression analysis. Forty-five (40%) patients in the combination group and 14 (25%) patients in the monotherapy group achieved CR (between-group difference, 0.15; 95% CI, 0.01-0.30). To assess the potential effect of concomitant medication, we performed a sensitivity analysis by excluding patients receiving concomitant medication. As shown in supplemental Table 3, the sensitivity analysis yielded similar results as the ITT analysis, and an increased OR (between-group difference, 0.25; 95% CI, 0.07-0.43), CR (between-group difference, 0.18; 95% CI, 0.00-0.35) and SR (between-group difference, 0.19; 95% CI, 0.00-0.38) was observed in patients receiving combination treatment. Moreover, among the 16 patients who were receiving concomitant medication at the time of enrollment in the combination group, 14 (88%), 5 (31%), and 12 (75%) patients achieved OR, CR, and SR, respectively; 76 (79%) patients, 40 (42%), and 56 (58%) patients not receiving concomitant medication in the combination group achieved OR, CR, and SR, respectively. No difference was observed in the OR (between-group difference, 0.08; 95% CI, −0.13-0.30), CR (between-group difference, −0.10; 95% CI, −0.39-0.18), or SR (between-group difference, 0.17; 95% CI, −0.10-0.44) rate between patients who were or were not receiving concomitant medication in the combination group. Median platelet counts from 10 weeks to the end of follow-up were higher in the combination group (supplemental Figure 2).
Responses and outcomes in the ATRA + LD-RTX and LD-RTX groups
| . | ATRA + LD-RTX . | LD-RTX . | Difference between proportions (95% CI) . | ||
|---|---|---|---|---|---|
| Participants, n = 112 . | Proportions . | Participants, n = 56 . | Proportions . | ||
| Primary outcome | |||||
| OR | 90 | 0.80 | 33 | 0.59 | 0.22 (0.07-0.36) |
| Secondary outcomes | |||||
| CR | 45 | 0.40 | 14 | 0.25 | 0.15 (0.01-0.30) |
| SR | 68 | 0.61 | 23 | 0.41 | 0.20 (0.04-0.35) |
| . | ATRA + LD-RTX . | LD-RTX . | Difference between proportions (95% CI) . | ||
|---|---|---|---|---|---|
| Participants, n = 112 . | Proportions . | Participants, n = 56 . | Proportions . | ||
| Primary outcome | |||||
| OR | 90 | 0.80 | 33 | 0.59 | 0.22 (0.07-0.36) |
| Secondary outcomes | |||||
| CR | 45 | 0.40 | 14 | 0.25 | 0.15 (0.01-0.30) |
| SR | 68 | 0.61 | 23 | 0.41 | 0.20 (0.04-0.35) |
In patients who achieved a response, the median TTR was 28.0 (IQR: 21.0, 42.0) days in the combination group and 28.0 days (IQR: 28.0, 49.0) in the monotherapy group. SR was achieved by 68 (61%) patients in the combination group and 23 (41%) patients in the monotherapy group (between-group difference, 0.20; 95% CI, 0.04-0.35). It was demonstrated that CR (OR, 26.844 [95% CI, 8.999-80.074]) was associated with SR by a post-hoc multivariate logistic regression analysis adjusted for age. Additionally, 47 (42%) subjects in the combination group and 7 (13%) in the monotherapy group were still in remission at 12 months (between-group difference: 0.29 [95% CI, 0.16-0.43] by imputing missing values as failures and 0.32 [95% CI, 0.18-0.46] by complete cases).
During the follow-up period, 43 (48%) of 90 patients in the combination group and 26 (79%) of 33 patients in the monotherapy group relapsed. The median duration of response was 43.0 weeks in the combination group and 40.0 weeks in the LD-RTX monotherapy group. The RFS rate of the combination group was significantly higher than that of the monotherapy group (HR = 1.875 [95% CI, 1.082 to 3.248]; P = .007) (Figure 2).
Kaplan-Meier plot of time to relapse in patients achieving OR of the 2 groups. Kaplan-Meier estimates of patients with RFS. The frequency of RFS of the combination group was significantly higher than that of the monotherapy group (HR = 1.875 [95% CI 1.082-3.248]; P = .007). Data were obtained from patients who achieved OR. HR, hazard ratio.
Kaplan-Meier plot of time to relapse in patients achieving OR of the 2 groups. Kaplan-Meier estimates of patients with RFS. The frequency of RFS of the combination group was significantly higher than that of the monotherapy group (HR = 1.875 [95% CI 1.082-3.248]; P = .007). Data were obtained from patients who achieved OR. HR, hazard ratio.
All 11 patients with grade 3 or 4 bleeding at entry received rescue therapies within 5 weeks after the initiation of treatment, including IVIg, high-dose corticosteroids, TPO-RA or rhTPO, and platelet transfusion. Twenty-one (19%) patients given LD-RTX plus ATRA required rescue treatment, and 19 (34%) patients in the monotherapy group required rescue therapy from week 6 to the end of the study (between-group difference, −0.15; 95% CI, −0.31 to 0.01) (supplemental Table 4).
AEs
The occurrence of AEs in both groups is summarized in Table 4. The severity of AEs was primarily grade 1 to 2. The 2 most common AEs for the combination group were dry skin and headache or dizziness, with incidences of 40% (45/112) and 19% (21/112), respectively, which were considered manifestations caused by ATRA therapy. A total of 12 (21%) and 8 (14%) patients receiving monotherapy complained of fever and upper respiratory infections, respectively. One patient in the combination group discontinued the treatment due to insomnia, while 1 patient in the monotherapy group discontinued the treatment due to mood disorders. In addition, 1 patient in the combination group died of intracranial hemorrhage (ICH) 27 days after enrollment. The occurrence of ICH was mainly associated with a history of hypertension and severe thrombocytopenia resistant to treatments.
Adverse events recorded in the 2 groups
| Events . | ATRA + LD-RTX (n = 112) . | LD-RTX (n = 56) . |
|---|---|---|
| Death | 1 (0.9) | 0 (0.0) |
| Patients reporting AEs | ||
| Any AEs | 79 (70.5) | 30 (53.6) |
| Any grade 3 to 4 AEs* | 4 (3.6) | 1 (1.8) |
| AE leading to discontinuation of therapy | 1 (0.9)† | 1 (1.8)‡ |
| AEs occurring in ≥2% of participants overall | ||
| Dry skin | 45 (40.2) | 0 (0.0) |
| Headache or dizziness | 21 (18.8) | 6 (10.7) |
| Fever | 16 (14.3) | 12 (21.4) |
| Upper respiratory infection | 4 (3.6) | 8 (14.3) |
| Urinary tract infection | 5 (4.5) | 4 (7.1) |
| Gastrointestinal infections | 2 (1.8) | 2 (3.6) |
| Muscle/joint pain | 2 (1.8) | 4 (7.1) |
| Rash acneiform | 3 (2.7) | 3 (5.4) |
| Any grade 3-4 AEs | ||
| Dry skin | 2 (1.8) | 0 (0.0) |
| Insomnia | 2 (1.8) | 0 (0.0) |
| Mood disorder | 0 (0.0) | 1 (1.8) |
| Events . | ATRA + LD-RTX (n = 112) . | LD-RTX (n = 56) . |
|---|---|---|
| Death | 1 (0.9) | 0 (0.0) |
| Patients reporting AEs | ||
| Any AEs | 79 (70.5) | 30 (53.6) |
| Any grade 3 to 4 AEs* | 4 (3.6) | 1 (1.8) |
| AE leading to discontinuation of therapy | 1 (0.9)† | 1 (1.8)‡ |
| AEs occurring in ≥2% of participants overall | ||
| Dry skin | 45 (40.2) | 0 (0.0) |
| Headache or dizziness | 21 (18.8) | 6 (10.7) |
| Fever | 16 (14.3) | 12 (21.4) |
| Upper respiratory infection | 4 (3.6) | 8 (14.3) |
| Urinary tract infection | 5 (4.5) | 4 (7.1) |
| Gastrointestinal infections | 2 (1.8) | 2 (3.6) |
| Muscle/joint pain | 2 (1.8) | 4 (7.1) |
| Rash acneiform | 3 (2.7) | 3 (5.4) |
| Any grade 3-4 AEs | ||
| Dry skin | 2 (1.8) | 0 (0.0) |
| Insomnia | 2 (1.8) | 0 (0.0) |
| Mood disorder | 0 (0.0) | 1 (1.8) |
Values are presented as numbers (percentages) of participants experiencing the event at least once.
Defined as any AE with significant symptoms requiring hospitalization, invasive intervention, transfusion, elective interventional radiological procedure or therapeutic endoscopy or operation, or any life-threatening or disabling AEs which are complicated by acute, life-threatening metabolic or cardiovascular complications or have a need for intensive care, emergent invasive procedure, emergent interventional radiological procedure or therapeutic endoscopy or operation.
Insomnia.
Mood disorder.
Discussion
In this multicenter prospective randomized controlled study, we evaluated the efficacy and safety of combined treatment with LD-RTX plus ATRA in corticosteroid-resistant or relapsed ITP patients. The combination of LD-RTX plus ATRA yielded a significantly higher OR rate (between-group difference, 0.22; 95% CI, 0.07-0.36) and a higher SR rate (between-group difference, 0.20; 95% CI, 0.04-0.35) than LD-RTX alone. However, TTR between the 2 groups was similar (28.0 [IQR, 21.0, 42.0] for the combination group vs 28.0 [IQR, 28.0, 49.0] days for the monotherapy group).
The pathogenesis of ITP is heterogeneous, making the identification of the differences in pathobiological mechanisms between different patients challenging, such that the determination of treatment strategies becomes a “trial and error” process.47 For corticosteroid-resistant or relapsed ITP patients, there have been no guidelines to specify the optimal second-line therapy or the order of agents used. Miltiadous et al proposed that combination treatment using agents with different mechanisms of action is an important direction for patients who have a poor response to a single agent.47
ATRA exerts a broad range of immunological effects. We had the experience of using ATRA plus danazol in the treatment of corticosteroid-resistant or relapsed ITP, and we found that the combination of ATRA and danazol might achieve good efficacy.26 However, there is a lack of long-term data about the safety of danazol. It was suggested that androgen treatment might result in liver dysfunction, and patients should receive liver function tests every 3 to 6 months, the inconvenience of which might hinder the use of danazol.48 Virilization was also a major obstacle, especially for female patients, when selecting this agent.48 Therefore, it is important to investigate treatment regimens with good convenience and tolerability.
It was reported that LD-RTX failed to achieve a fast and long-term response.10 However, the combination of LD-RTX with another agent might overcome these shortcomings. Zhou et al demonstrated that the combination of LD-RTX and rhTPO increased the CR rate and shortened TTR in corticosteroid-resistant or relapsed ITP.34 In addition, Li et al used a regimen of 100mg RTX once weekly for 4 weeks followed by 2 additional doses of 100 mg to treat chronic inflammatory demyelinating polyneuropathy and confirmed a significant improvement in disease severity, indicating that prolonged RTX therapy might further improve the efficacy, particularly the duration, of response.49
Accordingly, we postulated that the combination of LD-RTX for 6 weeks plus ATRA might induce a better OR, SR, and a shorter TTR because of their synergistic effects. Our study showed that a combination of LD-RTX with ATRA significantly improved OR and SR but showed no beneficial effects on shortening TTR. Therefore, the combination treatment of LD-RTX with ATRA represents a promising treatment regimen for corticosteroid-resistant or relapsed ITP patients. It was proposed that LD-RTX might exert long-term treatment efficacy in ITP due to the depletion of B cells and the improvement of regulatory T cells (Tregs),34 whereas the 12-month remission rate was only 13% for patients with LD-RTX monotherapy. Adding ATRA to the LD-RTX regimen improved the 12-month remission rate by 29%; however, it seems that there was a decline in platelet counts in the combination group toward the end of the study, as shown in supplemental Figure 2A. We further investigated the platelet counts by study week in patients who achieved an OR in the combination group, including those who relapsed and those who achieved remission; we observed a more significant decline in platelet counts for patients who relapsed (supplemental Figure 2B). Moreover, we observed that the RFS rate of the combination group was significantly higher than that of the monotherapy group (HR = 1.875 [95% CI 1.082-3.248]; P = .007), but the conclusion may not be steady enough because of the small number of patients at risk. More clinical trials with longer follow-up times and larger sample sizes are required to further investigate the sustained efficacy and RFS of the combination of ATRA and LD-RTX. Furthermore, it was difficult to reach a conclusion about whether the combination regimen of ATRA plus LD-RTX or that of ATRA plus danazol was better because of the limitations relating to the small sample size and short follow-up period; therefore, more studies are required to determine the better regimen.
Notably, all 11 patients with grade 3 or 4 bleeding at entry received rescue therapies within 5 weeks; therefore, therapies that help increase the platelet count faster, including high-dose corticosteroids, IVIg, platelet transfusion, TPO-RA, or rhTPO, might be needed when considering treatment of LD-RTX with ATRA for patients with more severe bleeding.
Furthermore, this multicenter study suggested that the combination therapy of LD-RTX with ATRA was well tolerated. AEs were parallel with those previously reported.10,26,33 ATRA-related AEs primarily included dry skin and headache or dizziness, and the manifestations were mild and did not require additional treatment. ICH was reported to be the most common fatal bleeding event in ITP patients who had a low platelet count.50,51 In our study, 1 patient in the combination group died of ICH on the twenty-seventh day after the initiation of treatment. The death was considered not treatment-related.
As indicated in a network meta-analysis,52 TPO-RAs might have the best balance in short-term efficacy and AEs. Considering that TPO-RAs work by promoting platelet production and ATRA by immunomodulating effects, further studies should focus on the combination of these 2 treatments for their synergetic effects in the management of ITP.
Our study has some limitations. First, since clinicians and patients were not blinded to treatment assignment, medical care and follow-up may have differed, further affecting results. However, all data in the study were collected and analyzed by masked experts to minimize bias. Further studies including more patients with a placebo control group are required to validate the efficacy and safety of ATRA plus LD-RTX in the treatment of corticosteroid-resistant or relapsed ITP. Second, patients who completed the study were not followed continuously, so their long-term outcomes are unknown. Third, concomitant therapies were inevitable for the study. Although it is difficult to conclude whether interactions among concomitantly used drugs affected the outcomes, patients with other maintenance treatments were enrolled only when the treatment dose had been stable during the last 4 weeks and was expected to be stable throughout the entire study. In addition, we analyzed the potential effects of receiving concomitant medication. We found no significant difference between patients receiving concomitant medication and patients not receiving concomitant medication in terms of OR (between-group difference, 0.08; 95% CI, −0.13-0.30), CR (between-group difference, −0.10; 95% CI, −0.39-0.18) and SR (between-group difference, 0.17; 95% CI, −0.10-0.44) in the combination treatment group. However, there were only 16 patients receiving concomitant medication at the time of enrollment, and considering the small sample size, it was difficult to reach a conclusion about whether concomitant medication could help achieve a better response. Fourth, the ideal sample size was not reached, and 168 patients were ultimately included due to difficulties in enrollment and the term limit of the funding, which might have influenced the statistical power. Fifth, we used a randomization ratio of 2:1 to enhance the recruitment process, mitigate ethical concerns, and gain more information about the efficacy and safety of the regimens, even though this ratio might result in a slight loss of statistical power. Sixth, we only required a single platelet count <30 × 109/L at enrollment, although platelet counts may vary over time.
In summary, ATRA plus LD-RTX is suggested to be a promising combination therapy for treating corticosteroid-resistant or relapsed adult ITP patients. The combination showed beneficial effects on the overall and long-lasting response.
Acknowledgments
The authors thank the patients and medical staff who participated in this study. The authors thank Xiao Liu, Feng-Qi Liu, Qing-Yuan Qu, Yue Hou (all from the Department of Hematology, Peking University People’s Hospital, Peking, China), and Gong-Wen Liang (Department of Academic Research, Peking University People’s Hospital, Peking, China) for monitoring and data management of the study.
This work was supported by grants from the Beijing Municipal Science and Technology Commission (Z171100001017084). The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Authorship
Contribution: X.-H.Z., X.-J.H., Y.-J.W., and H.L. designed research, analyzed data, and wrote the paper; X.-H.Z., Q.-Z.Z., Y.L., J.-W.W., W.-S.W., J.F., and H.-B.Z. were responsible for performing research and acquisition of data; Q-.Z.Z., Y.L., J.-W.W., W.-S.W., J.F., H.-B.Z., Q.-S.H., Y.H., H.-X.F., X.-L.Z., Q.J., H.J., Y.-J.C., L.-P.X., and X.-J.H. were responsible for critical revision of the manuscript for important intellectual content; X.-H.Z. obtained funding; X.-H.Z. had full access to all the study data in the study after completion of the trial and had final responsibility for the decision to submit for publication; and all authors read and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xiao-Hui Zhang, No.11 Xizhimen South St, Xicheng District, Beijing, China; e-mail: zhangxh@bjmu.edu.cn; and Xiao-Jun Huang, No.11 Xizhimen South St, Xicheng District, Beijing, China; e-mail: xjhrm@medmail.com.cn.
For original data, please contact zhangxh@bjmu.edu.cn. The study protocol is included as a data supplement available with the online version of this article.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
REFERENCES
Author notes
These authors contributed equally to the paper.



![Kaplan-Meier plot of time to relapse in patients achieving OR of the 2 groups. Kaplan-Meier estimates of patients with RFS. The frequency of RFS of the combination group was significantly higher than that of the monotherapy group (HR = 1.875 [95% CI 1.082-3.248]; P = .007). Data were obtained from patients who achieved OR. HR, hazard ratio.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/139/3/10.1182_blood.2021013393/5/m_bloodbld2021013393f2.png?Expires=1767795084&Signature=C0N7GHCu93BbWPL7SPESesdwKJsqJeNCmvJ~pTMKLLhOGApnF6FjXE3sZJuyYnpUiFNz3rqQEEg1xDy~zWv2r71FCY2UVFjXN3zho22NIUuaFN15DC9a4~~L4~00998YHs4I3fSZMOZkjsiSKgw-~f5I9uMC~GKeF1BG01ZqpNZs58ZsNxNRf3eVp5iqosWuHgWIXfI~PKPxnI5cfJZTvC2Xvo1vXWXPTMvuvxfLgmVIl~CMFKTgWRpgT76Ed-XxIFtlN7HyK8zGUzt53G~TuX~mziDif30vdzABb1fUDvTC7o3sNYwpmEgPnY8K3iv4X4bLHjaeLQ-kje~GUcxrFg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal