TO THE EDITOR:
Cerebral venous thrombosis (CVT) is the most common and severe manifestation of vaccine-induced immune thrombotic thrombocytopenia (VITT), which is a rare side effect of the SARS-CoV-2 vaccine ChAdOx1 nCoV-19 (Vaxzevria, AstraZeneca/Oxford).1-4 The absolute risk of VITT and VITT-related CVT is estimated at 20 and 8 per million first doses of ChAdOx1 nCoV-19, respectively.5,6
So far, no definite VITT cases occurring after a second ChAdOx1 nCoV-19 vaccine dose have been reported, raising the question of whether VITT only occurs after a first dose. Two pharmacovigilance studies reported cases of thrombosis with thrombocytopenia after a second ChAdOx1 nCoV-19 dose, but because of lack of clinical data, none of these could be classified as VITT.7-9 Knowledge on whether VITT can occur after a second ChAdOx1 nCoV-19 dose is relevant for clinicians and policymakers, especially in low- and middle-income countries, which are currently the main users of adenovirus-based vaccines.10
We used data from the “CVT after SARS-CoV-2 vaccination” registry4,11 to identify VITT-related CVT cases occurring after a second ChAdOx1 nCoV-19 dose.
Details of this registry have been published.4 Briefly, this ongoing study collects data on patients with CVT with symptom onset 28 days from SARS-CoV-2 vaccination, regardless of the type and dose of vaccine. The study is endorsed by the European Academy of Neurology and the European Stroke Organization. Investigators are instructed to report consecutive cases from their hospitals. The ethical review board of the Academic Medical Centre issued a waiver of formal approval for this observational study. Each center obtained local permission to carry out the study and acquired informed consent for the use of pseudonymized care data according to national law.
We used the case definition criteria of the United Kingdom expert hematology panel to classify cases as definite, probable, possible, or unlikely VITT after ChAdOx1 nCoV-19 administration among CVT cases reported until 1 December 2021.9
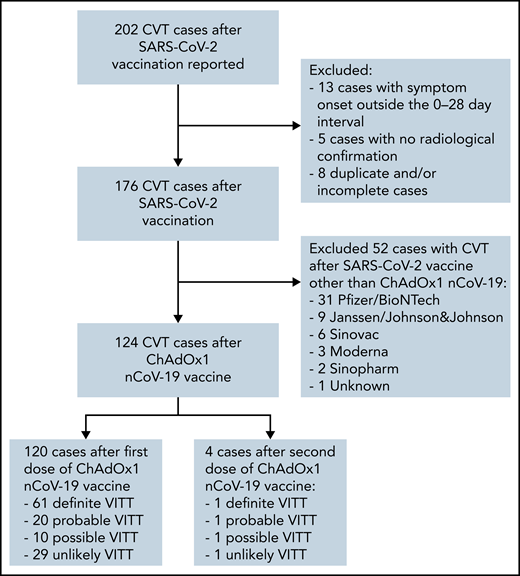
Within the study period, 202 CVT cases after SARS-CoV-2 vaccination were reported from 24 countries (Figure 1). Of the 124 patients with CVT following ChAdOx1 nCoV-19 vaccination, 120 were after a first dose, and 4 were after a second dose. There were 61 definite, 20 probable, 10 possible, and 29 unlikely VITT cases after a first ChAdOx1 nCoV-19 dose. Of the 4 cases after the second dose, 1 was definite, 1 was probable, 1 was possible, and 1 was an unlikely VITT. There were no possible, probable, or definite VITT cases after the second dose of any of the other vaccines.
Flowchart of patient selection. Out of 202 reported patients with CVT after SARS-CoV-2 vaccination, we excluded 13, 5, and 8 cases with symptom onset outside of the 0-28 day interval, with no radiological confirmation, and duplicate and/or incomplete cases, respectively. Out of the remaining 176 cases, 124 cases developed CVT after ChAdOx1 nCoV-19 vaccination. Of these, 120 developed CVT after a first dose (61 definite, 20 probable, 10 possible, and 29 unlikely VITT), and 4 after a second dose (1 definite, 1 probable, 1 possible, and 1 unlikely).
Flowchart of patient selection. Out of 202 reported patients with CVT after SARS-CoV-2 vaccination, we excluded 13, 5, and 8 cases with symptom onset outside of the 0-28 day interval, with no radiological confirmation, and duplicate and/or incomplete cases, respectively. Out of the remaining 176 cases, 124 cases developed CVT after ChAdOx1 nCoV-19 vaccination. Of these, 120 developed CVT after a first dose (61 definite, 20 probable, 10 possible, and 29 unlikely VITT), and 4 after a second dose (1 definite, 1 probable, 1 possible, and 1 unlikely).
Details of the 4 cases after a second ChAdOx1 nCoV-19 dose are provided in Table 1. A timeline of the clinical course of each of the cases is provided in supplemental Figures 1-4, available on the Blood Web site. None of the patients reported any symptoms after the first dose of ChAdOx1 nCoV-19. The patients (3 men, 1 woman) were between their forties and sixties. None had preexistent comorbidities. The interval between receiving the second vaccine dose and symptom onset varied between 1 and 6 days. The 2 patients who met the criteria for probable and definite VITT (patients 1 and 2) both died of brain herniation.
Clinical details of CVT cases after a second ChAdOx1 nCoV-19 dose
| . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . |
|---|---|---|---|---|
| VITT classification* | Probable | Definite | Possible | Unlikely |
| Demographics | ||||
| Age† | 60s | 50s | 40s | 60s |
| Sex | Male | Female | Male | Male |
| Medical history | Unremarkable | Thrombophilia | Unremarkable | Unremarkable |
| Prior COVID-19 infection at any time | No | No | No | No |
| Baseline characteristics | ||||
| Interval between first and second vaccination (d)‡ | 90 | 44 | 62 | 77 |
| Interval between second vaccination and symptom onset (d) | 5 | 6 | 1 | 4 |
| Interval between symptom onset and diagnosis (d) | 0 | 1 | 0 | 0 |
| Headache | No | Yes | Yes | No |
| Focal neurologic deficits | Yes | Yes | Yes | Yes |
| Coma | Yes | Yes | No | No |
| Seizure | No | No | Yes | Yes |
| Imaging findings | ||||
| Intracerebral hemorrhage | Yes | Yes | No | No |
| Location of CVT | Superior sagittal sinus | Superior sagittal sinus, left transverse and sigmoid sinus, straight sinus, left jugular vein | Right transverse and sigmoid sinuses | Superior sagittal sinus, right transverse and sigmoid sinus, right jugular vein |
| Laboratory values | ||||
| Platelet count at admission, ×109/L | 188 | 40 | 109 | 175 |
| Platelet count nadir, ×109/L | 55 | 14 | 55 | 124 |
| Anti-PF4 antibody ELISA | Negative | Positive | Negative | Negative |
| Type ELISA test | Lifecodes PF4 IgG from Immucor | PF4 IgG from Immucor | Lifecodes PF4 IgG from Immucor | ZYMUTEST HIA IgG, HYPHEN BIOMED |
| Optical density ELISA | 0.06 | 2.12§ | 0.12 | 0.03 |
| Optical density test threshold | ≥0.4 | ≥0.4 | ≥0.4 | ≥0.3 |
| Functional assay to detect platelet- activating PF4 antibodies | Positiveǁ | Not performed | Positiveǁ | Negative¶ |
| Type of functional assay | Modified HIPA | NA | Modified HIPA | Multiplate HIMEA |
| D-dimer, ug/L FEU | 35 200 | 29 100 | 2400 | 513 |
| Fibrinogen, g/L | 4.17 | 2.63 | 3.34 | 4.14 |
| ref <3.50 | ref <4.00 | ref <3.50 | ref <4.50 | |
| Treatment | ||||
| Anticoagulation | Argatroban | None# | Argatroban followed by dabigatran | Fondaparinux followed by dabigatran |
| IVIG | Yes | No | Yes | No |
| Decompressive hemicraniectomy | Yes | No | No | No |
| Complications and outcome | ||||
| Major bleeding during admission | Yes** | No | No | No |
| New VTE during admission | No | Yes, pelvic veins | No | No |
| Outcome at hospital discharge | Dead | Dead | No disability | No disability |
| Days between symptom onset and death | 2 | 3 | NA | NA |
| Cause of death | Brain herniation | Brain herniation | NA | NA |
| . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . |
|---|---|---|---|---|
| VITT classification* | Probable | Definite | Possible | Unlikely |
| Demographics | ||||
| Age† | 60s | 50s | 40s | 60s |
| Sex | Male | Female | Male | Male |
| Medical history | Unremarkable | Thrombophilia | Unremarkable | Unremarkable |
| Prior COVID-19 infection at any time | No | No | No | No |
| Baseline characteristics | ||||
| Interval between first and second vaccination (d)‡ | 90 | 44 | 62 | 77 |
| Interval between second vaccination and symptom onset (d) | 5 | 6 | 1 | 4 |
| Interval between symptom onset and diagnosis (d) | 0 | 1 | 0 | 0 |
| Headache | No | Yes | Yes | No |
| Focal neurologic deficits | Yes | Yes | Yes | Yes |
| Coma | Yes | Yes | No | No |
| Seizure | No | No | Yes | Yes |
| Imaging findings | ||||
| Intracerebral hemorrhage | Yes | Yes | No | No |
| Location of CVT | Superior sagittal sinus | Superior sagittal sinus, left transverse and sigmoid sinus, straight sinus, left jugular vein | Right transverse and sigmoid sinuses | Superior sagittal sinus, right transverse and sigmoid sinus, right jugular vein |
| Laboratory values | ||||
| Platelet count at admission, ×109/L | 188 | 40 | 109 | 175 |
| Platelet count nadir, ×109/L | 55 | 14 | 55 | 124 |
| Anti-PF4 antibody ELISA | Negative | Positive | Negative | Negative |
| Type ELISA test | Lifecodes PF4 IgG from Immucor | PF4 IgG from Immucor | Lifecodes PF4 IgG from Immucor | ZYMUTEST HIA IgG, HYPHEN BIOMED |
| Optical density ELISA | 0.06 | 2.12§ | 0.12 | 0.03 |
| Optical density test threshold | ≥0.4 | ≥0.4 | ≥0.4 | ≥0.3 |
| Functional assay to detect platelet- activating PF4 antibodies | Positiveǁ | Not performed | Positiveǁ | Negative¶ |
| Type of functional assay | Modified HIPA | NA | Modified HIPA | Multiplate HIMEA |
| D-dimer, ug/L FEU | 35 200 | 29 100 | 2400 | 513 |
| Fibrinogen, g/L | 4.17 | 2.63 | 3.34 | 4.14 |
| ref <3.50 | ref <4.00 | ref <3.50 | ref <4.50 | |
| Treatment | ||||
| Anticoagulation | Argatroban | None# | Argatroban followed by dabigatran | Fondaparinux followed by dabigatran |
| IVIG | Yes | No | Yes | No |
| Decompressive hemicraniectomy | Yes | No | No | No |
| Complications and outcome | ||||
| Major bleeding during admission | Yes** | No | No | No |
| New VTE during admission | No | Yes, pelvic veins | No | No |
| Outcome at hospital discharge | Dead | Dead | No disability | No disability |
| Days between symptom onset and death | 2 | 3 | NA | NA |
| Cause of death | Brain herniation | Brain herniation | NA | NA |
ELISA, enzyme-linked immunosorbent assay; FEU, fibrinogen equivalent units; HIMEA, heparin-induced multiple electrode aggregometry; HIPA, heparin-induced platelet aggregation; IVIG, intravenous immune globulin; NA, not applicable; VTE, venous thromboembolism.
According to the United Kingdom expert hematology panel.9
To avoid the possibility of patient identification, exact age has been removed.
In all cases, the first vaccination was ChAdOx1 nCoV-19.
Blood was drawn from the patient at admission, stored at 4°C for 1 wk, then stored at −20°C for 327 d before it was tested.
Modified HIPA assay was performed as previously described.15
HIMEA assay was performed as previously described.16
Reason: multiple intracerebral hemorrhages and diffuse subarachnoid hemorrhage.
Worsening of intracerebral hemorrhages.
In patient 3 with symptom onset on day 1, the rapid onset could be explained if circulating anti-PF4 antibodies were present after the first vaccination, suggesting immunological preconditioning similar to that described in heparin-induced thrombocytopenia.12
Of note, no specific events were observed after the first dose of this vaccine, suggesting that the development of VITT after the second dose of ChAdOx1 nCoV-19 cannot be predicted on clinical grounds. Although the numbers are small, the clinical severity appears comparable to CVT-VITT after a first ChAdOx1 nCoV-19 dose, as 2 patients had an intracerebral hemorrhage, 1 had a concurrent venous thrombosis, and 2 patients died during admission.4,5,13
Based on reported CVT cases to the registry, VITT appears to be much less common after a second ChAdOx1 nCoV-19 dose than after a first. However, since many countries, especially in Europe, restricted the use of the ChAdOx1 nCoV-19 vaccine after the emergence of VITT, the lower frequency of reported VITT after a second dose could partly be explained by the fact that fewer people received a second dose of ChAdOx1 nCoV-19 than a first dose. Even so, data from the European Centre for Disease Prevention and Control show that, until week 33 of 2021, 39 million first doses and 29 million second doses were administered in the European Economic Area.14 Therefore, this imbalance cannot fully explain the difference in the incidence of VITT. Still, due to the risk of reporting bias, data from our registry must be interpreted cautiously when concluding that VITT is much less common after a second than after a first dose.
In conclusion, CVT-VITT can occur after the second dose of the ChAdOx1 nCoV-19 vaccine but was reported less often than after a first vaccine dose. Symptom onset of VITT may be more rapid after a second than after a first dose, but the low number of cases precludes firm conclusions.
Acknowledgments
This work was supported by The Netherlands Organisation for Health Research and Development (ZonMw, grant number 10430072110005) (J.M.C.) and the Dr. C. J. Vaillant Foundation (J.M.C.). The sponsors of this study are public or nonprofit organizations that support science in general. They had no role in gathering, analyzing, or interpreting the data.
K.K., A.v.d.M., and M.S.v.K. are PhD candidates at the University of Amsterdam. This work is submitted in partial fulfillment of the requirement for the doctoral degree.
Authorship
Contribution: D.A.d.S. and J.M.C. conceptualized the study; K.K., A.v.d.M., and M.S.v.K. provided data curation; K.K. and A.v.d.M. provided formal analysis; K.K., A.v.d.M., J.Z., F.J.B., G.F., T.K., B.P., J.A.K.H., and M.S.v.K. provided investigation for the study; M.A., J.M.F., D.A.d.S., and J.M.C. are responsible for methodization; K.K., A.v.d.M., M.S.v.K., M.R.H., D.A.d.S., and J.M.C. provided project administration; M.A., J.M.F., D.A.d.S., and J.M.C. provided resources; D.A.d.S. and J.M.C. supervised the study; K.K. and A.v.d.M. contributed validation and visualization; K.K., D.A.d.S., and J.M.C. wrote the original draft of the manuscript; K.K., A.v.d.M., J.Z., F.J.B., G.F., T.K., B.P., M.S.v.K., J.A.K.H., M.A., M.R.H., J.M.F., D.A.d.S., and J.M.C. wrote, reviewed, and edited the manuscript; and K.K., A.v.d,M., and J.M.C. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict-of-interest disclosure: M.R.H. has received grants from the Swiss Heart Foundation and Bangerter Foundation; travel support from Bayer; personal fees for data safety monitoring board or advisory board participation from Amgen; and is a member of the European Stroke Organisation Board of Directors and European Stroke Organisation Education Committee. M.A. has received personal fees from AstraZeneca, Bayer, Bristol Myers Squibb, Covidien, Daiichi Sankyo, Medtronic, Novartis, Sanofi, Pfizer, and Amgen; and grants from the Swiss National Science Foundation and Swiss Heart Foundation. J.A.K.H. has received grants from Baxalta as well as personal fees paid to her institution from Shire, Ablynx, Roche, Sobi, and the Swiss Federal Office of Public Health. J.M.F. has received personal fees from Boehringer Ingelheim, Bayer, and Daiichi Sankyo as well as grants from Bayer. D.A.d.S. has received travel support from Boehringer Ingelheim; speaker fees from Bayer; and personal fees for advisory board participation from AstraZeneca. J.M.C. has received grants paid to his institution from Boehringer Ingelheim and Bayer and payments paid to his institution for data safety monitoring board participation by Bayer. The remaining authors declare no competing financial interests.
A complete list of the members of the Cerebral Venous Sinus Thrombosis With Thrombocytopenia Syndrome Study Group appears in the supplemental appendix.
Correspondence: Jonathan M. Coutinho, Department of Neurology, Amsterdam University Medical Centers, Location AMC, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; e-mail: j.coutinho@amsterdamumc.nl.
For original data, please contact j.coutinho@amsterdamumc.nl.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
REFERENCES
Author notes
D.A.d.S. and J.M.C. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal