In the current issue of Blood, Krzywicka et al1 convincingly show for the first time, that vaccine-induced immune thrombocytopenia and thrombosis (VITT) can occur after a second dose of the AstraZeneca/Oxford ChAdOx1 nCoV-19 vaccine.
ChAdOx1 nCoV-19 was one of the first vaccines to be developed and systematically rolled out in the United Kingdom and European countries and has reduced severe disease and death from COVID-19 infection. The vaccine was developed by researchers at Oxford University in collaboration with the pharmaceutical company AstraZeneca and uses a chimpanzee adenoviral vector containing the genomic sequence of the SARS-CoV-2 spike protein.
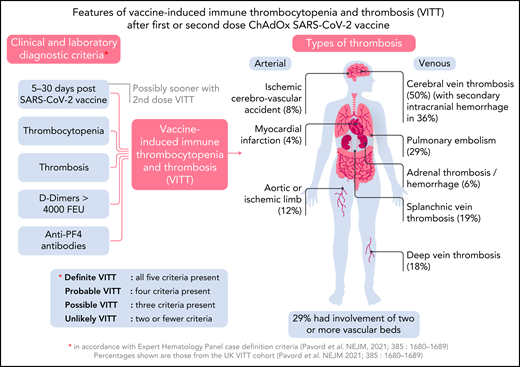
Soon after the start of the ChAdOx1 nCoV-19 vaccination program in Europe, hematologists began observing previously healthy young individuals present with severe, extensive thrombosis. Unlike most cases of thrombosis, there was associated thrombocytopenia, and no predisposing thrombotic risk factors.2 Furthermore, thrombosis occurred in many organs, affected both arteries and veins, and in half of the cases, it involved the cerebral venous circulation, often with secondary intracranial hemorrhage (see figure). Mortality in these early cases was >70%.2 Severe platelet activation mediated by anti-platelet factor 4 (anti-PF4) antibodies was identified as the underlying cause, leading to the description of the new syndrome of VITT, with 5 diagnostic features: presentation 5 to 30 days after SARS-CoV-2 vaccination, thrombocytopenia, thrombosis, markedly raised D-dimer, and presence of anti-PF4 antibodies2 (see figure).
National adverse event reporting systems record all cases of thrombosis and thrombocytopenia syndrome seen after SARS-CoV-2 vaccine. Not all these cases are VITT, and it is important to appreciate the overlap between the 2 syndromes.3 For example, Bhuyan and colleagues published a series that discussed patients with thrombosis and thrombocytopenia after a second AstraZeneca dose,4 but none of the cases fulfilled the criteria for VITT.5
In 2021, Krzywicka and colleagues set up an international registry to record episodes of cerebral vein thrombosis (CVT) within 28 days of any SARS-CoV-2 vaccination. One hundred twenty-four (70.5%) of the CVT cases were after AstraZeneca vaccination, and 4 (3.2%) of these were after the second dose. These 4 cases are described in detail in this journal, and according to the UK expert group classification,2 1 case is in each of the categories: definite, probable, possible, and unlikely VITT.
The most important of the cases reported is patient 2, who presented 6 days after the second dose of the AstraZeneca vaccine with CVT and met all the criteria for definite VITT. Cases 1 and 3 met the criteria for probable and possible VITT, respectively. It is interesting to note that all 3 cases presented sooner than would be expected for first-dose VITT, where the earliest time to presentation is 5 days, and the median time 14 days.2 Although case 2 presented at 6 days after vaccine, the advanced nature of her disease, with widespread thrombosis and severe thrombocytopenia, suggests onset before this time. This early presentation with second-dose VITT could be explained by sensitization after the first dose of ChAdOx1 nCoV-19, giving rise to a clinical event on rechallenge. This is seen with heparin-induced thrombocytopenia (HIT), another anti-PF4 antibody-mediated condition causing thrombosis and thrombocytopenia; initial presentation occurs at 5 or more days after first heparin exposure, but rechallenge with heparin causes an immediate reactivation of the condition.6 Rechallenge with heparin beyond 100 days of the initial event, when HIT anti-PF4 antibodies become undetectable, is not immediate and again takes 5 days for the immune response to manifest. VITT anti-PF4 antibody-mediated platelet activation has been shown to disappear by 12 weeks in 90% of cases,7 and rechallenge beyond this time might also be expected to take 5 days to develop the immune response. The few VITT patients who have received second-dose ChAdOx1 nCoV-19 in the United Kingdom were all after an interval of 12 weeks and had no signs of recurrence.8 The cases identified by Krzywicka and colleagues had no history of VITT after first-dose ChAdOx1 nCoV-19, but it is possible subclinical disease occurred, with profound reactivation on rechallenge with the vaccine.
Another important matter highlighted by the cases presented by Krzywicka and colleagues relates to anti-PF4 antibody tests. Although these antibodies can occur in up to 8% of patients after SARS-CoV-2 vaccination,9 the optical densities are only just above the normal range and not as high as the 2.12 seen in patient 2, a level similar to those observed in the UK VITT cohort. It is also notable that enzyme-linked immunosorbent assays (ELISAs) may vary in their sensitivity to different anti-PF4 antibodies,10 which may explain the negative ELISA but positive platelet activation assays seen in cases 1 and 3. Hence, the presence of anti-PF4 antibodies is not considered a confirmatory test in itself, but one of 5 equally important diagnostic features.2
In summary, Krzywicka and colleagues have shown that VITT may occur after a second dose of the ChAdOx1 nCoV-19 vaccine and can present earlier than after the first dose. ChAdOx1 nCoV-19 is being widely used in low- and middle-income countries, and clinicians should be alert for symptoms occurring after both first and second doses, so that diagnosis and intervention can be implemented rapidly.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal