Key Points
The combination of brentuximab vedotin and lenalidomide is active in relapsed/refractory DLBCL with a favorable safety profile.
A phase 3 study is currently enrolling patients to investigate the efficacy of this regimen in combination with rituximab.
Abstract
New therapies are needed for patients with relapsed/refractory (rel/ref) diffuse large B-cell lymphoma (DLBCL) who do not benefit from or are ineligible for stem cell transplant and chimeric antigen receptor therapy. The CD30-targeted, antibody-drug conjugate brentuximab vedotin (BV) and the immunomodulator lenalidomide (Len) have demonstrated promising activity as single agents in this population. We report the results of a phase 1/dose expansion trial evaluating the combination of BV/Len in rel/ref DLBCL. Thirty-seven patients received BV every 21 days, with Len administered continuously for a maximum of 16 cycles. The maximum tolerated dose of the combination was 1.2 mg/kg BV with 20 mg/d Len. BV/Len was well tolerated with a toxicity profile consistent with their use as single agents. Most patients required granulocyte colony-stimulating factor support because of neutropenia. The overall response rate was 57% (95% CI, 39.6-72.5), complete response rate, 35% (95% CI, 20.7-52.6); median duration of response, 13.1 months; median progression-free survival, 10.2 months (95% CI, 5.5-13.7); and median overall survival, 14.3 months (95% CI, 10.2-35.6). Response rates were highest in patients with CD30+ DLBCL (73%), but they did not differ according to cell of origin (P = .96). NK cell expansion and phenotypic changes in CD8+ T-cell subsets in nonresponders were identified by mass cytometry. BV/Len represents a potential treatment option for patients with rel/ref DLBCL. This combination is being further explored in a phase 3 study (registered on https://clinicaltrials.org as NCT04404283). This trial was registered on https://clinicaltrials.gov as NCT02086604.
Introduction
Diffuse large B-cell lymphoma (DLBCL) accounts for 25% to 30% of non-Hodgkin lymphomas (NHLs).1 It is a heterogeneous disease and is classified histologically, as well as molecularly, into multiple subtypes.2 Although response rates and overall survival (OS) have improved in the rituximab era, ∼30% of patients are refractory to or relapse after first-line chemotherapy.3 A minority of these relapsed/refractory (rel/ref) patients respond to salvage chemotherapy, autologous stem cell transplant (ASCT), or chimeric antigen receptor (CAR) T cells. New treatments are needed for patients who are ineligible for or in whom current therapies failed.4
Brentuximab vedotin (BV) is a CD30-directed antibody-drug conjugate approved for use in select Hodgkin, anaplastic large-cell, and CD30+ peripheral T-cell lymphomas. Although the primary mechanism of action of BV is through directing the tubulin-disrupting agent MMAE to CD30-expressing cells, BV-mediated cytotoxicity also appears to be augmented by immune cell stimulation and bystander effects.5,6 A phase 2 trial of BV in rel/ref DLBCL reported an overall response rate (ORR) of 44%.7,8 Responses occurred in patients with variable levels of CD30 expression, with no correlation between CD30 expression and response.7,9 BV has been explored in combination with R-CHP (rituximab with cyclophosphamide and doxorubicin) as the frontline treatment for patients with CD30+ DLBCL, demonstrating an ORR of 100% (86% complete response [CR]) and a 2-year median progression-free survival (PFS) of 85%.10
Lenalidomide (Len) is an immunomodulating agent approved for mantle cell, follicular, and marginal zone lymphomas and is believed to work by interacting with the ubiquitin E3 ligase cereblon and degrading Ikaros family transcription factors.11-13 A phase 2 trial of Len in rel/ref DLBCL demonstrated an ORR of 28%.14 Clinical responses were significantly higher in non-germinal center B-cell (non-GCB) phenotype compared with GCB (52.9% vs 8.7% respectively).15 A phase 2/3 trial in heavily pretreated rel/ref DLBCL reported an ORR of 27.5% (non-GCB 45.5%, GCB 21.4%) for Len compared with 11.8% for investigator choice.16
Based on the single-agent activity of Len and BV in rel/ref DLBCL, favorable safety profiles, unique mechanisms of action, and low likelihood of antagonistic interaction because of the modest effects of BV on the immune system, we studied this combination in an investigator-initiated, multicenter, open-label, phase 1/dose expansion study in rel/ref DLBCL.
Methods
Eligibility
Eligible patients were aged ≥18 years, had histologically confirmed rel/ref de novo or transformed DLBCL after at least 1 prior therapy, Eastern Cooperative Oncology Group (ECOG) performance status ≤2, and adequate organ function as defined in supplemental Methods (available on the Blood Web site). All patients had measurable disease, CD30 immunohistochemical staining (BerH2 clone) on the most recent biopsy and had received or were ineligible for ASCT. Patients treated with prior Len or refractory to prior BV were excluded.
Study design and treatment
This multicenter, open-label, phase 1 study of BV in combination with Len in rel/ref DLBCL was conducted in accordance with the Declaration of Helsinki and approved by the institutional review boards of each participating site. All patients provided written informed consent. All authors had access to the primary clinical trial data.
The treatment cycle was 21 days with intravenous BV administered on day 1 and Len administered on days 1 to 21 for a maximum of 16 cycles. Continuous dosing of Len was chosen to maintain a cycle of 3-week intervals and based on institutional experience in ref/ref Hodgkin lymphoma that demonstrated a similar frequency of adverse events compared with standard interrupted dosing on days 1 to 21 of a 28-day schedule (17 and submitted manuscript). The dose-escalation phase was a standard 3 + 3 design with starting doses of BV 1.2 mg/kg and Len 20 mg (Table 1). Dose limiting toxicities (DLTs) were defined as any of the following occurring during cycle 1: grade 4 neutropenia lasting >7 days, grade 4 infection with grade 3 or 4 neutropenia, grade 4 thrombocytopenia associated with life-threatening bleeding or requiring >1 transfusion, treatment delays >14 days because of hematologic toxicity, or grade 3 or 4 nonhematologic toxicities. Len dose reductions were allowed in 5-mg increments to the lowest dose of 5 mg daily. No dose re-escalation, apart from the cycle 2 dose of Len, which could be re-escalated with use of granulocyte colony-stimulating factor (G-CSF) if the cycle 1 dose was reduced because of neutropenia, was allowed. ANC ≥1×109/L, platelet count ≥50×109/L, and resolution of nonhematologic treatment-related toxicities to ≤grade 1 were necessary to initiate a new cycle. BV dose reductions were allowed for adverse events (AEs), in 0.3-mg/kg increments to a lowest dose of 0.9 mg/kg, with no dose re-escalation permitted. Patients used aspirin, warfarin, or low-molecular-weight heparin for prophylactic anticoagulation. AEs were coded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 4.0.
Study dose-escalation schedule
| Dose level . | BV dose (mg/kg) . | Len dose (mg) . |
|---|---|---|
| Level −2 | 1.2 | 15 (D1-14 only) |
| Level −1 | 1.2 | 15 |
| Level 0 (starting dose) | 1.2 | 20 |
| Level 1 | 1.2 | 25 |
| Level 2 | 1.8 | 25 |
| Dose level . | BV dose (mg/kg) . | Len dose (mg) . |
|---|---|---|
| Level −2 | 1.2 | 15 (D1-14 only) |
| Level −1 | 1.2 | 15 |
| Level 0 (starting dose) | 1.2 | 20 |
| Level 1 | 1.2 | 25 |
| Level 2 | 1.8 | 25 |
The primary objective was to determine the safety and maximum tolerated dose (MTD) of BV in combination with Len in patients with rel/ref DLBCL. Secondary objectives included ORR (CR + PR), duration of response (DOR), and PFS. Exploratory objectives included assessment of recurrently mutated genes by next-generation sequencing and a longitudinal analysis of peripheral blood immune cell populations by mass cytometry.
Response criteria and assessments
Response was assessed according to the 2007 Revised Response Criteria for Malignant Lymphoma.18 CT or positron emission tomography–computed tomography (PET/CT) assessments were performed after cycles 2, 4, 6, and 9 and then every 6 months for 2 years. PET/CT scans were graded visually by the Deauville criteria, with scores of 4 or 5 considered positive and scores of 1, 2, or 3 considered negative.19 The Siteman Cancer Center Imaging Response Assessment Core determined responses in collaboration with the principal investigator.
Lymph node samples, sequencing, and determination of cell of origin
All patients provided written informed consent for sequencing of their tissue as part of the Washington University School of Medicine (WUSM) Lymphoma Banking Program (IRB approvals 201108251 and 201104048). Sample processing, sequencing, and gene expression analyses are described in the supplemental Methods.
Read alignment, variant calling, annotation, and filtering
Sequence analysis was performed using the Genome Modeling System.20 Paired-end reads were aligned to the human reference genome sequence GRCh38. Variants were identified by using 4 independent callers, annotated using the Ensembl variant effect predictor tool (Ensembl v93), and filtered to distinguish somatic from germline variants and pipeline artifacts. Filtering made use of custom cutoffs, the gnomAD database,21 and a panel of 34 normal samples from patients with previously sequenced follicular lymphoma.22,23 Variants that passed all filtering were manually reviewed by 1 of 5 experienced reviewers at WUSM.24 Somatic variant analysis was further limited to the 150 recurrently putative driver genes identified by Reddy et al25 and described in supplemental Methods. Figures were produced using GenVisR26 and ggplot2.27
Mass cytometry
Mass cytometry was performed on thawed peripheral blood mononuclear cells (PBMCs) stained with a custom immune panel (supplemental Table 1) and sample processing, acquisition, and analysis were performed as described (supplemental Methods).28
Statistical analyses
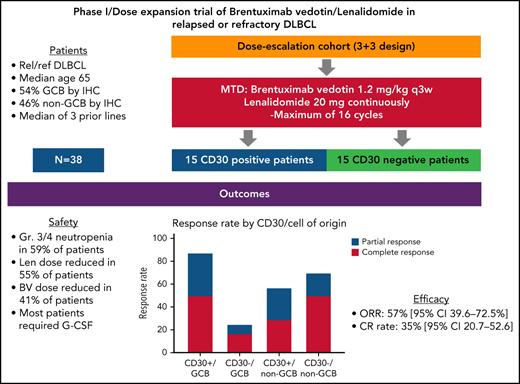
This phase 1 open-label study had no formal statistical hypothesis for the primary efficacy or secondary end points. Secondary end points were analyzed to provide supporting evidence for the overall clinical benefit of BV/Len. Descriptive statistics (mean, median, standard deviation, minimum, and maximum) were used to describe continuous variables. Frequencies and percentages were used to describe categorical variables. The phase 1 dose-escalation portion adopted a 3 + 3 design, along 3 dose levels (Table 1) with a minimum of 6 and a maximum of 18 patients. The dose-expansion cohort treated at the MTD enrolled a total of 15 CD30+ and 15 CD30− patients, including the patients treated at the MTD in the dose-escalation cohort. CD30+ was defined as ≥1% of tumor cells expressing CD30 by immunohistochemistry. No statistical comparisons between the groups defined by CD30 status were preplanned.
Genes mutated in 3 or more individuals were included in statistical analyses. Time-to-event analyses were performed using a log-rank test to identify significant PFS differences between individuals who were either mutated or wild-type for a particular gene. Additionally, the χ2 or Fisher’s exact test (for expected values ≤5) was performed to examine the association between gene mutation status and treatment response. The Benjamini-Hochberg method was used to correct all P-values for multiple tests. Time-to-event analysis was performed using R (v3.5.2) with the “survival,” “multtest” and “stat” packages.
Results
Patients
From September 2014 through March 2017, 38 patients were enrolled, with 37 patients evaluable for toxicity and efficacy. One patient could not obtain insurance approval and never started therapy. No patients with known double- or triple-hit DLBCL were enrolled, although fluorescence in situ hybridization testing was not available for 5 patients. Baseline patient characteristics are presented in Table 2.
Baseline patient characteristics
| Characteristic . | BV/Len (n = 37) . | |
|---|---|---|
| . | n . | % . |
| Median age, y (range) | 65 (51-79) | |
| Baseline ECOG performance status | ||
| 0-1 | 28 | 76 |
| 2 | 8 | 21 |
| Unknown | 1 | 3 |
| IPI score at study entry | ||
| 0 | 3 | 8 |
| 1 | 3 | 8 |
| 2 | 17 | 46 |
| 3 | 8 | 22 |
| 4 | 4 | 11 |
| 5 | 2 | 5 |
| Transformed disease | 9 | 24 |
| Elevated LDH | 20 | 54 |
| Cell of origin by IHC | ||
| GCB | 20 | 54 |
| Non-GCB | 17 | 46 |
| Time from initial diagnosis, months | ||
| Median (range) | 14 (4-138) | |
| Stage at study screening | ||
| Stages I and II | 9 | 24 |
| Stages III and IV | 28 | 76 |
| Median prior therapies (range) | 3 (1-6) | |
| Primary refractory | 17 | 46 |
| Refractory to most recent treatment | 20 | 54 |
| Patients with prior rituximab exposure | 37 | 100 |
| Bulky disease (≥7.5 cm) | 13 | 35 |
| Prior stem cell transplant | 10 | 27 |
| Reason why ineligible for ASCT | ||
| Age | 9 | 33 |
| Comorbidities | 1 | 3 |
| Inadequate response to salvage | 17 | 64 |
| Characteristic . | BV/Len (n = 37) . | |
|---|---|---|
| . | n . | % . |
| Median age, y (range) | 65 (51-79) | |
| Baseline ECOG performance status | ||
| 0-1 | 28 | 76 |
| 2 | 8 | 21 |
| Unknown | 1 | 3 |
| IPI score at study entry | ||
| 0 | 3 | 8 |
| 1 | 3 | 8 |
| 2 | 17 | 46 |
| 3 | 8 | 22 |
| 4 | 4 | 11 |
| 5 | 2 | 5 |
| Transformed disease | 9 | 24 |
| Elevated LDH | 20 | 54 |
| Cell of origin by IHC | ||
| GCB | 20 | 54 |
| Non-GCB | 17 | 46 |
| Time from initial diagnosis, months | ||
| Median (range) | 14 (4-138) | |
| Stage at study screening | ||
| Stages I and II | 9 | 24 |
| Stages III and IV | 28 | 76 |
| Median prior therapies (range) | 3 (1-6) | |
| Primary refractory | 17 | 46 |
| Refractory to most recent treatment | 20 | 54 |
| Patients with prior rituximab exposure | 37 | 100 |
| Bulky disease (≥7.5 cm) | 13 | 35 |
| Prior stem cell transplant | 10 | 27 |
| Reason why ineligible for ASCT | ||
| Age | 9 | 33 |
| Comorbidities | 1 | 3 |
| Inadequate response to salvage | 17 | 64 |
ECOG, Eastern Cooperative Oncology Group; IHC, immunohistochemistry; LDH, lactate dehydrogenase.
MTD and safety
The MTD of the combination was dose level (DL) 0 (BV 1.2 mg/kg and Len 20 mg), with 28 patients initiating therapy on this dose level. Six patients were treated at DL1 (BV 1.2 mg/kg, Len 25 mg), with no DLTs, and 3 patients at DL2 (BV 1.8 mg/kg, Len 25 mg) with 2 DLTs, both treatment delays >14 days because of prolonged Gr3-4 neutropenia. Because most patients on DL1 required Len dose reductions within the first few cycles due to cytopenias, the decision was made to expand DL0 instead of DL1. The median number of cycles administered was 4 (range, 1-16 cycles); 4 patients completed all 16 cycles.
Reasons for withdrawal included disease progression (n = 23, 62%), patient decision (n = 2), and AEs (n = 4). AEs leading to discontinuation of treatment included 1 each of neutropenia (cycle 8), thrombocytopenia (cycle 2), bicytopenia (cycle 12), and rash (cycle 2). Table 3 lists grade 1 and 2 AEs occurring in >10% of patients and all ≥grade 3 AEs. Of all enrolled patients, 94% experienced at least 1 grade 3 or 4 AE, with neutropenia (59%), lymphocytopenia (43%), leukopenia (32%), hypokalemia (27%), thrombocytopenia (27%), anemia (24%), maculopapular rash (24%), febrile neutropenia (8%), thromboembolism (8%), diarrhea (5%), bacteremia (5%), sepsis (5%), and syncope (5%), each occurring in more than 1 patient; 73% required at least 1 dose reduction. Len dose reductions occurred in 21 (55%) patients, at a median of cycle 3 (range, 2-9) and most commonly for grade 3 or 4 neutropenia (20 of 21), grade 3 or 4 thrombocytopenia (6 of 21), or grade 3 rash (5 of 21), with some patients experiencing more than 1 simultaneous reason for dose reduction. BV dose reductions occurred in 15 (41%) patients, most commonly because of grade 3 to 4 neutropenia (11 of 15), sensory neuropathy (6 of 15), or thrombocytopenia (3 of 15), and occurred at a median of cycle 5 (range, 2-14). Len was discontinued for toxicity in 3 patients (1 patient each with neutropenia, thrombocytopenia, or both) despite dose reduction to 5 mg. BV was discontinued in 1 patient for thrombocytopenia. Complete data on dose reductions and delays of Len and BV are detailed in supplemental Table 2. One potentially treatment-related death, related to presumed sepsis, occurred during cycle 1 in the setting of profound cytopenias and rapidly progressing disease. G-CSF was administered to 31 patients (83.8%); 17 patients (45.9%) initiated G-CSF prophylactically, with patients in cycle 1 and 14 received G-CSF after experiencing a dose delay for neutropenia or febrile neutropenia. Of the 28 patients treated at the MTD, 23 (82.1%) received G-CSF.
Grades 1 and 2 AEs occurring in >10% of patients and all grade 3-4 AEs
| . | All attributions (N = 37) . | |||
|---|---|---|---|---|
| Grade 1-2 . | Grade 3-4 . | |||
| AE . | n . | % . | n . | % . |
| Blood and lymphatic system | ||||
| Neutropenia | 24 | 65 | 22 | 59 |
| Lymphocytopenia | 22 | 59 | 16 | 43 |
| Leukopenia | 27 | 73 | 12 | 32 |
| Thrombocytopenia | 26 | 70 | 10 | 27 |
| Anemia | 26 | 70 | 9 | 24 |
| Leukocytosis | 1 | 3 | 7 | 19 |
| Febrile neutropenia | 0 | 0 | 3 | 8 |
| Cardiac disorders | ||||
| Pericardial effusion | 0 | 0 | 1 | 3 |
| Others | 0 | 0 | 1 | 3 |
| Gastrointestinal | ||||
| Diarrhea | 10 | 27 | 2 | 5 |
| Nausea | 13 | 35 | 1 | 3 |
| Vomiting | 9 | 24 | 1 | 3 |
| Abdominal pain | 6 | 16 | 1 | 3 |
| Stomach pain | 2 | 5 | 1 | 3 |
| Constipation | 5 | 14 | 0 | 0 |
| Infection | ||||
| Bacteremia | 0 | 0 | 2 | 5 |
| Sepsis | 0 | 0 | 2 | 5 |
| Upper respiratory infection | 6 | 16 | 1 | 3 |
| Tooth infection | 2 | 5 | 1 | 3 |
| Urinary tract infection | 2 | 5 | 1 | 3 |
| Lung infection | 0 | 0 | 1 | 3 |
| Laboratory | ||||
| Increased alkaline phosphatase | 13 | 35 | 1 | 3 |
| Increased ALT | 12 | 32 | 1 | 3 |
| Increased bilirubin | 8 | 22 | 1 | 3 |
| Increased INR | 4 | 11 | 1 | 3 |
| Prolonged pTT | 2 | 5 | 1 | 3 |
| Increased AST | 15 | 41 | 0 | 0 |
| Increased creatinine | 9 | 24 | 0 | 0 |
| Metabolic | ||||
| Hypokalemia | 20 | 54 | 10 | 27 |
| Hypoalbuminemia | 22 | 59 | 3 | 8 |
| Hypophosphatemia | 3 | 8 | 2 | 5 |
| Hypocalcemia | 23 | 62 | 1 | 3 |
| Hyponatremia | 9 | 24 | 0 | 0 |
| Hypercalcemia | 6 | 16 | 0 | 0 |
| Hypomagnesemia | 5 | 14 | 0 | 0 |
| Hyperkalemia | 5 | 14 | 0 | 0 |
| Neurological | ||||
| Syncope | 0 | 0 | 2 | 5 |
| Peripheral sensory neuropathy | 15 | 41 | 1 | 3 |
| Dizziness | 8 | 22 | 0 | 0 |
| Dysgeusia | 4 | 11 | 0 | 0 |
| Renal | ||||
| Dyspnea | 14 | 38 | 1 | 3 |
| Acute kidney disease | 2 | 5 | 1 | 3 |
| Chronic kidney disease | 2 | 5 | 1 | 3 |
| Urinary tract obstruction | 0 | 0 | 1 | 3 |
| Hematuria | 4 | 11 | 0 | 0 |
| Pulmonary | ||||
| COPD | 1 | 3 | 1 | 3 |
| Hypoxia | 0 | 0 | 1 | 3 |
| Stridor | 0 | 0 | 1 | 3 |
| Cough | 9 | 24 | 0 | 0 |
| Skin | ||||
| Maculopapular rash | 17 | 46 | 9 | 24 |
| Cellulitis | 0 | 0 | 1 | 3 |
| Pruritus | 5 | 14 | 0 | 0 |
| Vascular | ||||
| Thromboembolism | 0 | 0 | 3 | 8 |
| Hypotension | 3 | 8 | 1 | 3 |
| Others | ||||
| Fatigue | 15 | 41 | 3 | 8 |
| Anorexia | 12 | 32 | 1 | 3 |
| Dehydration | 5 | 14 | 1 | 3 |
| Pain | 5 | 14 | 1 | 3 |
| Neck pain | 2 | 5 | 1 | 3 |
| Flank pain | 2 | 5 | 1 | 3 |
| Arthritis | 0 | 0 | 1 | 3 |
| Noncardiac pain | 1 | 3 | 1 | 3 |
| Multiorgan failure | 0 | 0 | 1 | 3 |
| Fever | 13 | 35 | 0 | 0 |
| Weight loss | 10 | 27 | 0 | 0 |
| Edema (limb) | 5 | 14 | 0 | 0 |
| Arthralgia | 4 | 11 | 0 | 0 |
| . | All attributions (N = 37) . | |||
|---|---|---|---|---|
| Grade 1-2 . | Grade 3-4 . | |||
| AE . | n . | % . | n . | % . |
| Blood and lymphatic system | ||||
| Neutropenia | 24 | 65 | 22 | 59 |
| Lymphocytopenia | 22 | 59 | 16 | 43 |
| Leukopenia | 27 | 73 | 12 | 32 |
| Thrombocytopenia | 26 | 70 | 10 | 27 |
| Anemia | 26 | 70 | 9 | 24 |
| Leukocytosis | 1 | 3 | 7 | 19 |
| Febrile neutropenia | 0 | 0 | 3 | 8 |
| Cardiac disorders | ||||
| Pericardial effusion | 0 | 0 | 1 | 3 |
| Others | 0 | 0 | 1 | 3 |
| Gastrointestinal | ||||
| Diarrhea | 10 | 27 | 2 | 5 |
| Nausea | 13 | 35 | 1 | 3 |
| Vomiting | 9 | 24 | 1 | 3 |
| Abdominal pain | 6 | 16 | 1 | 3 |
| Stomach pain | 2 | 5 | 1 | 3 |
| Constipation | 5 | 14 | 0 | 0 |
| Infection | ||||
| Bacteremia | 0 | 0 | 2 | 5 |
| Sepsis | 0 | 0 | 2 | 5 |
| Upper respiratory infection | 6 | 16 | 1 | 3 |
| Tooth infection | 2 | 5 | 1 | 3 |
| Urinary tract infection | 2 | 5 | 1 | 3 |
| Lung infection | 0 | 0 | 1 | 3 |
| Laboratory | ||||
| Increased alkaline phosphatase | 13 | 35 | 1 | 3 |
| Increased ALT | 12 | 32 | 1 | 3 |
| Increased bilirubin | 8 | 22 | 1 | 3 |
| Increased INR | 4 | 11 | 1 | 3 |
| Prolonged pTT | 2 | 5 | 1 | 3 |
| Increased AST | 15 | 41 | 0 | 0 |
| Increased creatinine | 9 | 24 | 0 | 0 |
| Metabolic | ||||
| Hypokalemia | 20 | 54 | 10 | 27 |
| Hypoalbuminemia | 22 | 59 | 3 | 8 |
| Hypophosphatemia | 3 | 8 | 2 | 5 |
| Hypocalcemia | 23 | 62 | 1 | 3 |
| Hyponatremia | 9 | 24 | 0 | 0 |
| Hypercalcemia | 6 | 16 | 0 | 0 |
| Hypomagnesemia | 5 | 14 | 0 | 0 |
| Hyperkalemia | 5 | 14 | 0 | 0 |
| Neurological | ||||
| Syncope | 0 | 0 | 2 | 5 |
| Peripheral sensory neuropathy | 15 | 41 | 1 | 3 |
| Dizziness | 8 | 22 | 0 | 0 |
| Dysgeusia | 4 | 11 | 0 | 0 |
| Renal | ||||
| Dyspnea | 14 | 38 | 1 | 3 |
| Acute kidney disease | 2 | 5 | 1 | 3 |
| Chronic kidney disease | 2 | 5 | 1 | 3 |
| Urinary tract obstruction | 0 | 0 | 1 | 3 |
| Hematuria | 4 | 11 | 0 | 0 |
| Pulmonary | ||||
| COPD | 1 | 3 | 1 | 3 |
| Hypoxia | 0 | 0 | 1 | 3 |
| Stridor | 0 | 0 | 1 | 3 |
| Cough | 9 | 24 | 0 | 0 |
| Skin | ||||
| Maculopapular rash | 17 | 46 | 9 | 24 |
| Cellulitis | 0 | 0 | 1 | 3 |
| Pruritus | 5 | 14 | 0 | 0 |
| Vascular | ||||
| Thromboembolism | 0 | 0 | 3 | 8 |
| Hypotension | 3 | 8 | 1 | 3 |
| Others | ||||
| Fatigue | 15 | 41 | 3 | 8 |
| Anorexia | 12 | 32 | 1 | 3 |
| Dehydration | 5 | 14 | 1 | 3 |
| Pain | 5 | 14 | 1 | 3 |
| Neck pain | 2 | 5 | 1 | 3 |
| Flank pain | 2 | 5 | 1 | 3 |
| Arthritis | 0 | 0 | 1 | 3 |
| Noncardiac pain | 1 | 3 | 1 | 3 |
| Multiorgan failure | 0 | 0 | 1 | 3 |
| Fever | 13 | 35 | 0 | 0 |
| Weight loss | 10 | 27 | 0 | 0 |
| Edema (limb) | 5 | 14 | 0 | 0 |
| Arthralgia | 4 | 11 | 0 | 0 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; COPD, chronic obstructive pulmonary disease; INR, international normalized ratio; pTT, partial thromboplastin time.
Efficacy
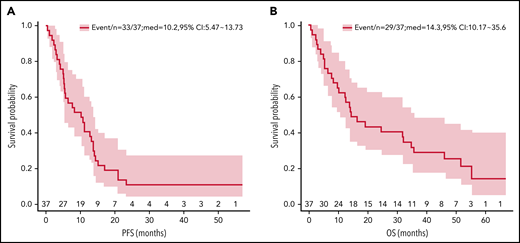
The ORR was 57% (95% CI, 39.6-72.5), with 13 patients (35%; 95% CI, 20.7-52.6) confirmed by PET/CT to have achieved CR and 8 (22%, 95% CI, 10.4-38.7) a PR. Seven CRs occurred after cycle 2 at the time of first restaging, 3 after cycle 4, 1 after cycle 6, and 1 after cycle 8. The median time to best response was 1.4 months (range, 0.6-8 months). Median follow-up was 14.3 months (range, 0.5-66.6 months). Remission is ongoing in 3 responders, with durations of 36.6 to 56.8 months. Twenty-nine patients have died; 25 from progressive disease, and 1 each from pneumonia secondary to septicemia, septic shock (possibly treatment-related), small-cell lung cancer, and an unknown cause. The median PFS was 10.2 months (95% CI, 5.5-13.7 months) and median OS, 14.3 months (95% CI, 10.2-35.6; Figure 1).
Kaplan-Meier PFS and OS estimates. The survival analysis included 37 patients with evaluable disease. Median PFS was 10.2 months (95% CI 5.5-13.7 mo) (A); median OS was 14.3 months (95% CI 10.2-35.6) (B).
Kaplan-Meier PFS and OS estimates. The survival analysis included 37 patients with evaluable disease. Median PFS was 10.2 months (95% CI 5.5-13.7 mo) (A); median OS was 14.3 months (95% CI 10.2-35.6) (B).
The median DOR was 13.1 months for all responders, 15.1 months in patients achieving CR, and 8.4 months for patients with a PR (supplemental Table 3). The DOR was 13.5 months (interquartile range, 7.3-20.2) in the 15 patients with at least 1 Len dose reduction and 7 months (interquartile range, 3.9-10.8) in the 6 patients who did not require dose reduction. This difference was not statistically significant (Wilcoxon rank sum test P = .23). An exploratory analysis of ORR by baseline characteristics is included in supplemental Table 4. ORRs were similar across the subgroups including patients with high International Prognostic Index (IPI) and refractoriness to prior therapy.
Clinical responses according to DLBCL subtype
The cell of origin (COO) was GCB in 20 patients and non-GCB in 17 patients using Hans’ criteria.29 ORR was 50% for GCB patients, and 65% for non-GCB (P = .957). There were 6 CRs and 4 PRs among the GCB cohort and 7 CRs and 4 PRs among the non-GCB cohort. Defining ≥1% CD30 expression on neoplastic cells as positive, 15 (41%) patients were CD30+, and 22 (59%) CD30−. Among CD30+ patients, the ORR was 73% with 6 CRs (40%). Table 4 summarizes response rates by CD30 expression, COO, and combined subsets by CD30 and COO. The lowest response rates were among the subset of patients with CD30−, GCB DLBCL (25%).
Response to BV/Len treatment based on patient’s CD30 status (positive or negative) and DLBCL subtype by Hans’ criteria
| . | CD30 status/Hans’ criteria . | |||||||
|---|---|---|---|---|---|---|---|---|
| CD30+/GCB (n = 8) . | CD30−/GCB (n = 12) . | CD30+/non-GCB (n = 7) . | CD30−/non-GCB (n = 10) . | |||||
| Response . | n . | % . | n . | % . | n . | % . | n . | % . |
| CR | 4 | 50 | 2 | 17 | 2 | 29 | 5 | 50 |
| PR | 3 | 37.5 | 1 | 8 | 2 | 29 | 2 | 20 |
| SD | 1 | 12.5 | 4 | 33 | 0 | 0 | 0 | 0 |
| PD | 0 | 0 | 5 | 42 | 3 | 43 | 3 | 30 |
| ORR (CR+PR) | 7 | 87.5 | 3 | 25 | 4 | 57 | 7 | 70 |
| . | CD30 status/Hans’ criteria . | |||||||
|---|---|---|---|---|---|---|---|---|
| CD30+/GCB (n = 8) . | CD30−/GCB (n = 12) . | CD30+/non-GCB (n = 7) . | CD30−/non-GCB (n = 10) . | |||||
| Response . | n . | % . | n . | % . | n . | % . | n . | % . |
| CR | 4 | 50 | 2 | 17 | 2 | 29 | 5 | 50 |
| PR | 3 | 37.5 | 1 | 8 | 2 | 29 | 2 | 20 |
| SD | 1 | 12.5 | 4 | 33 | 0 | 0 | 0 | 0 |
| PD | 0 | 0 | 5 | 42 | 3 | 43 | 3 | 30 |
| ORR (CR+PR) | 7 | 87.5 | 3 | 25 | 4 | 57 | 7 | 70 |
PD, progressive disease; SD, stable disease.
Biomarker analyses
Molecular confirmation of DLBCL assignment by Nanostring analysis
Given previous reports that responses to Len are predominantly seen in the non-GCB subset and the number of responses we observed in the GCB group assigned according to Hans’ criteria, we evaluated the subtype categorization of 24 patients with available tissue by Nanostring analysis. In these 24 samples, 14 were GCB, 8 ABC (activated B-cell), and 2 unclassifiable. In 3 cases, the classification changed from non-GCB to GCB, whereas in 2 cases the classification changed from GCB to ABC. Both unclassified samples were previously called non-GCB by Hans’ criteria. The Nanostring analysis confirmed the observation of clinical responses in both subtypes. For the 14 patients classified as GCB using Nanostring analysis, the ORR was 57%, with 5 CRs and 3 PRs, whereas, for the 8 patients classified as ABC by Nanostring analysis, the ORR was 75%, with 5 CRs and 1 PR.
Analysis of recurrent DLBCL mutations
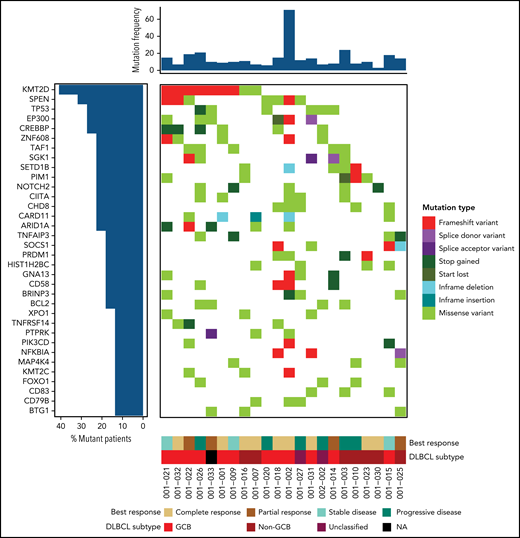
We analyzed mutations of genes previously associated with clinical outcomes in DLBCL.25 Of the 150 genetic drivers assessed by Reddy et al, 92 genes were mutated in our patients and included in these analyses (supplemental Table 5). The most commonly mutated genes included KMT2D (41%), SPEN (32%), TP53 (27%), EP300 (27%), CREBBP (27%), and ZNF608 (23%) (Figure 2). Similar to Reddy et al, we found that CREBBP, GNA13, TNFRSF14, SGK1, EZH2, BCL7A, and SOCS1 are enriched within the GCB subtype and TBL1XR, CD79B, PIM1, and ETV6 are enriched in non-GCB patients. However, some genes (eg, BCL2, IRF8, B2M, and STAT; supplemental Figure 2) were classified in the other subtype compared with the original associations in the DLBCL cohort described by Reddy et al.25 While limited due to small numbers of patients with each mutation, significant associations of alterations in BCL2, FOXO1, TP53, TAF1, and SGK1 with shorter PFS and TNFAIP3 and FOXO1 with a lack of response to therapy were observed (supplemental Tables 6 and 7).
Recurrently mutated genes in DLBCL are associated with treatment response to BV plus Len. Genomic landscape of trial patients. Genes were mutated in 3 or more patients (34 of 92 mutated genes for 22 of 37 patients) among the 150 recurrently mutated putative driver genes previously identified.
Recurrently mutated genes in DLBCL are associated with treatment response to BV plus Len. Genomic landscape of trial patients. Genes were mutated in 3 or more patients (34 of 92 mutated genes for 22 of 37 patients) among the 150 recurrently mutated putative driver genes previously identified.
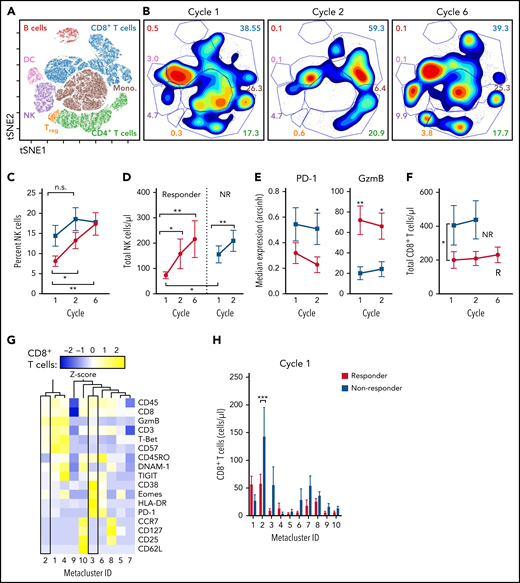
Changes in peripheral blood immune subsets induced by BV/Len
As both BV and Len have been shown to have pleiotropic effects on multiple cellular immune subsets,30,31 we used mass cytometry for multidimensional profiling of PBMCs collected during therapy (Figure 3; supplemental Figure 1; supplemental Table 1). viSNE was used to differentiate the major circulating immune populations based on expression of 12 markers and to visualize dynamic changes over time (Figure 3A-B). Although the number of NK cells increased over time in both responders and nonresponders (Figure 3C-D), the difference at cycle 2 was not significant. Consistent with augmented cytotoxicity, NK cells from responders expressed significantly higher levels of granzyme B and reduced levels of the immune checkpoint PD-1 (Figure 3E; supplemental Figure 1D). Whereas the percentage of NK cells expressing the inhibitory KIR family members KIR2DL1, KIR2DL2/2DL3, and KIR3DL1 was unchanged during treatment, there was a significant increase in NK cells expressing the transcription factor EOMES (supplemental Figure 1D). Despite cell surface expression of CD30, the total number of Tregs (defined as cells expressing FOXP3) increased during treatment (supplemental Figure 1H). In responders, a significant reduction in CD19+ B-cells was observed by cycle 6 (supplemental Figure 1B). Although the total number of CD4+ T cells was not altered during BV/Len therapy (supplemental Figure 1C), we unexpectedly noted that a significantly greater number of CD8+ T cells were present in nonresponders compared with responders (Figure 3F). Using unsupervised clustering analysis, we segregated CD8+ T cells into 10 defined metaclusters based on a panel of 17 markers (Figure 3G-H). CD8+ T cells from nonresponders significantly localized to metacluster 2, which has an effector/effector memory phenotype, and numerically to metacluster 3, which has a terminally exhausted CD45RO+ T-betlo Eomeshi CD38hi HLA-DRhi PD-1hi GzmBlo phenotype (Figure 3G). We did not observe any metacluster skewing in the CD8+ T-cell compartment of responders.
Mass cytometry defines differences in lymphocyte populations between responders and nonresponders, before treatment. Patient PBMC samples were assessed before cycles 1, 2, and 6 by mass cytometry. Using viSNE analysis, CD45+ cells were clustered and lymphocyte populations defined by lineage-marker–informed manual gating. (A) Representative viSNE plot depicting the major lymphocyte populations identified. (B) Representative density plot of 1 patient, over time. Numbers indicate the frequency of cells within the gate. (C) Summary data showing the frequency of NK cells between responders (R, n = 20; red) and nonresponders (NR, n = 13; blue). (D) Summary of total NK cells as determined by ALC and percentage of NK cells. (E) Summary expression of PD-1 and GzmB on NK cells from R (red) vs NR (blue). (F) Summary data of total CD8+ T cells from R (red) vs NR (blue). (G-H) FlowSOM was performed on CD8+ CD3+ T cells; 10 metaclusters were identified. (G) Heat map depicting the relative expression of the markers used to generate the FlowSOM clusters. Metaclusters 2 and 3 are within boxes. (H) Total CD8+ T cells within each Metacluster from R (red) vs NR (blue) at cycle 1. Data were compared in a type 3 mixed-effects model (C-F) or by 2-way analysis of variance (H). *P ≤ .05; **P ≤ .01; ***P ≤ .001. n.s. not significant.
Mass cytometry defines differences in lymphocyte populations between responders and nonresponders, before treatment. Patient PBMC samples were assessed before cycles 1, 2, and 6 by mass cytometry. Using viSNE analysis, CD45+ cells were clustered and lymphocyte populations defined by lineage-marker–informed manual gating. (A) Representative viSNE plot depicting the major lymphocyte populations identified. (B) Representative density plot of 1 patient, over time. Numbers indicate the frequency of cells within the gate. (C) Summary data showing the frequency of NK cells between responders (R, n = 20; red) and nonresponders (NR, n = 13; blue). (D) Summary of total NK cells as determined by ALC and percentage of NK cells. (E) Summary expression of PD-1 and GzmB on NK cells from R (red) vs NR (blue). (F) Summary data of total CD8+ T cells from R (red) vs NR (blue). (G-H) FlowSOM was performed on CD8+ CD3+ T cells; 10 metaclusters were identified. (G) Heat map depicting the relative expression of the markers used to generate the FlowSOM clusters. Metaclusters 2 and 3 are within boxes. (H) Total CD8+ T cells within each Metacluster from R (red) vs NR (blue) at cycle 1. Data were compared in a type 3 mixed-effects model (C-F) or by 2-way analysis of variance (H). *P ≤ .05; **P ≤ .01; ***P ≤ .001. n.s. not significant.
Discussion
BV/Len was well-tolerated in this population of heavily pretreated patients with rel/ref DLBCL, with the major DLTs being neutropenia and thrombocytopenia and most patients requiring G-CSF. The MTD of the combination was determined to be 1.2 mg/kg of BV and 20 mg of Len given continuously. ORR was 57%, with 35% CR; median PFS, 10.2 months (95% CI, 5.5-13.7), median OS, 14.3 months (95% CI 10.2-35.6), and median DOR, 13.1 months. Three patients had durable ongoing responses more than 3 years after completing therapy.
BV/Len compares favorably to other Len-containing combinations in patients with rel/ref DLBCL. In the LEGEND trial, the combination of rituximab, methylprednisolone, gemcitabine, and Len (R-GEM-L) did not reach a prespecified CR rate of 40% compared with the control of R-GEM plus cisplatin.32 Promising results have been reported for Len in combination with R-ICE (rituximab, ifosfamide, carboplatin, and etoposide; ORR 73%),33 R-ESHAP (rituximab, etoposide, methylprednisolone, cytarabine, and cisplatin; ORR 68%)34 and ibrutinib plus rituximab (ORR 44%).35 In the L-MIND trial, Len in combination with the anti-CD19 monoclonal antibody tafasitamab followed by tafasitamab maintenance demonstrated an ORR of 60%,36 which is similar to that in our study. Both trials enrolled patients with rel/ref DLBCL who were ineligible for ASCT, although L-MIND allowed no more than 3 prior lines of therapy compared with 1 to 6 prior therapies (median, 3) in those receiving BV/Len. Compared with L-MIND, our study also included more patients with primary refractory disease (46% vs 19%), and more patients refractory to their most recent treatment (54% vs 44%). Our study allowed a maximum of 16 cycles of treatment, compared with L-MIND, which allowed continued maintenance tafasitamab every 14 days until progression.
Consistent with the mechanism of action of BV, more patients with CD30+ DLBCL responded to BV/Len (ORR, 73%; 40% CR) than CD30− DLBCL (ORR, 45%; 32% CR). The activity of BV/Len in the GCB subset (ORR 50%) was unanticipated. Further stratification using both CD30 status and COO suggests that the addition of Len to BV may be particularly beneficial for the CD30+/GCB subset, as 7 of 8 patients responded with 4 CRs (ORR, 87.5%; 50% CR). As Len-containing therapies have consistently demonstrated activity in the non-GCB subtype, we verified the immunohistochemistry-based classification using Hans’ criteria in 24 available samples using Nanostring analysis. This analysis changed the original DLBCL subtype in 5 patients, but verified the finding that responses were seen in both molecular subtypes (GCB ORR, 57%; non-GCB ORR, 75%). Although limited by the low number of patients, these results suggest that both CD30 expression and COO may be predictive biomarkers of response to the BV/Len combination.
To assess for recurrent genomic alterations present in our patient cohort, we evaluated a panel of mutated genes that have previously been associated with clinical outcomes in DLBCL.25 Although they were present in a small subset of patients, the mutations in BCL2 (n = 4) and FOXO1 (n = 3) showed an association with shorter PFS in patients treated in this study, consistent with prior reports.37-39BCL2 contributes to lymphomagenesis primarily through translocation events that result in constitutive expression, thereby promoting the survival of germinal center B-cells.40 Point mutations in BCL2 can affect its function and stability.41 Three of the 6 BCL2 (50%) mutations identified are in the flexible loop domain (AA32-87) and are predicted to affect BCL2’s putative p53 binding domain.42,43 These mutations may influence the ability of p53 to regulate apoptosis through BCL2. FOXO proteins are a family of transcription factors involved in multiple signaling pathways and most likely play pathological roles in some cancers by promoting cell-cycle arrest and apoptosis.39,44,45 AKT phosphorylates FOXOs, causing their translocation and sequestration from the nucleus to the cytoplasm where transcriptional activities are inactive.45,46 Mutations in FOXO1 are reported in 8.6% of DLBCL, with most localized to the first exon, consistent with our findings. Prior studies have identified a hotspot at the T24 AKT phosphorylation site and mutations within the first exon diminish phosphorylation at T24.47,48 We also observed mutations in this area of FOXO1, which are likely to have a similar mechanistic impact (T24, P26). However, mutations at S72 and N377 are outside of the AKT recognition motif and downstream of the first exon, and further experimentation is necessary to establish whether these mutations also affect AKT phosphorylation of FOXO1. FOXO1 mutations were previously shown to be associated with decreased OS in DLBCL after R-CHOP, independent of COO and IPI.39 Larger patient cohorts are needed to establish whether any of the specific somatic alterations that we observed are associated with response to the BV/Len combination.
CD30 is expressed on activated B and T cells, as well as on regulatory T cells.49 We therefore carried out a comprehensive analysis of peripheral blood immune subsets before and during therapy. NK cells expanded from the start of therapy to cycle 6 (Figure 3D), generating the hypothesis that NK-cell–mediated cytotoxicity may be critical to the mechanism of action of the BV/Len combination. Additional clinical strategies to augment NK cell/lymphoma recognition (eg, anti-CD20 monoclonal antibodies) or enhance their functionality may be of interest for future combination studies. We hypothesized that the number of regulatory T cells would be reduced by BV therapy, but instead identified an increase in the total number of Treg cells over time (supplemental Figure 1H). In a recently reported study of the combination of BV and nivolumab in patients with Hodgkin lymphoma, Tregs decreased after the first dose of BV, but increased after the addition of nivolumab.50 Whether the addition of Len was protective of Tregs in this study is unknown. The finding that nonresponders had more CD8+ T cells than responders was also unexpected, and we pursued additional phenotypic characterization of this population. Interestingly, nonresponders were found to have significantly more CD8+ T cells with an effector/effector memory phenotype at the initiation of therapy. Whether the presence of defined CD8+ T-cell populations could be used to predict response to BV/Len or could be rescued with PD-1 directed therapy are questions that could be evaluated in future studies.
In summary, BV/Len was well tolerated, with responses in both GCB and non-GCB DLBCL, regardless of CD30 expression. ORRs were similar across subgroups with poor prognosis, with or without refractory disease and IPI score. AEs were generally consistent with the known toxicity profiles of BV and Len when given as single agents. Although definitive conclusions on the utility of BV/Len in patients with rel/ref DLBCL are limited by the small size of our study and the lack of a comparator arm, the safety profile and response rate potentially make this an encouraging salvage regimen for patients who are not candidates for or have relapsed after ASCT or CAR T-cell therapy. Our observations that distinct mutations were seen more frequently in patients with reduced PFS are hypothesis generating because of the low number of patients and require validation in larger patient cohorts. Based on these results, an international, randomized phase 3 study of BV or placebo in combination with Len and rituximab has been initiated (ECHELON-3) to further explore the utility of this combination (registered on https://clinicaltrials.gov as NCT04404283).
Acknowledgments
The authors thank the nursing and research staff involved in this study and all the participants and their families.
This work was supported by the Siteman Cancer Center Investment Program Team Science Award (National Institutes of Health, National Cancer Institute grant P30CA091842). The authors recognize the support of the Biostatistics Core, Clinical Trials Core, Tumor Procurement Core, and Imaging Response Assessment Team funded via the NIH, NCI Comprehensive Cancer Center support grant P30CA091842. The REDCap database used for managing clinical data were supported by National Center for Advancing Translational Sciences Clinical and Translational Science Award (CTSA) Grant UL1TR000448 and NCI grant P30CA091842. Lenalidomide was provided by Celgene Corporation through the Revlimid REMS program, and brentuximab vedotin was provided by Seagen. N.L.B. thanks the Barnes-Jewish Hospital Foundation for support. T.A.F. was supported by NIH, NCI grant R01CA205239, and a Lymphoma Team Science award from the Siteman Cancer Center (NCI grant P30CA091842). J.P.W. was supported by NCI grant K08CA245215. Seagen and Celgene provided research funding to Washington University to perform the investigator-initiated clinical trial.
Authorship
Contribution: J.P.W., T.A.F., and N.L.B. provided the study design, contributed patients, collected and analyzed data, and prepared the manuscript; M.M.B.-E., F.G., M.B.-H., M.M., and M.G., and O.L.G., contributed to the study design, data generation and analysis, and manuscript preparation; J.L. contributed to study design and performed statistical analysis; A.F.C., N.D.W.-J., and K.M. contributed patients and data; M.F., K.K., A.S., Z.L.S., and S.D. contributed to data generation and analysis; M.P.W. contributed to data collection and analysis and manuscript preparation; and A.F. contributed to study design and data collection; all authors reviewed, edited, and approved the final manuscript.
Conflict-of-interest disclosure: M.M.B.-E. has served as a consultant for Wugen and has equity in and patents licensed to Wugen. M.B.-H. holds patent US20100159594A1. N.D.W.-J. has received research funding from Roche/Genentech, Regeneron, ADC Therapeutics, JUNO, and ASTEX and has been on advisory boards for Karyopharm, Seagen, Grunenthal, Epizyme, ADC Therapeutics, and Regeron. K.M. has consulted for Pharmacyclics, Morphosys, Celgene, Gilead, Karyopharm, ADC Therapeutics, and Seagen. T.A.F. has served as a consultant for Wugen and Gamida Cell, has equity interest in Indapta, Kiadis, Orca Biosystems, and Wugen, and received research funding from Wugen, Affimed, HCW Biologics, Compass Therapeutics, and ImmunityBio, as well as possible royalties from patents licensed by Washington University to Wugen. N.L.B. has research funding from ADC Therapeutics, Affimed, Autolus, Bristol-Myers Squibb, Celgene, Forty Seven, Immune Design, Janssen, Kite Pharma/Gilead, Merck, Millennium, Pharmacyclics, Pfizer, Roche/Genentech, and Seagen, and been on advisory boards for ADC Therapeutics, Roche/Genentech, and Seagen. The remaining authors declare no competing financial interests.
Correspondence: Jeffrey P. Ward, Washington University School of Medicine, 660 South Euclid, Box 8056, St. Louis, MO 63110; e-mail: jward2@wustl.edu.
Presented in abstract form at the 57th annual meeting of the American Society of Hematology, Orlando, FL, 5 December 2015.
Individual participant data will not be shared. Requests for other original data may be made to the corresponding author.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
REFERENCES
Author notes
T.A.F. and N.L.B. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal