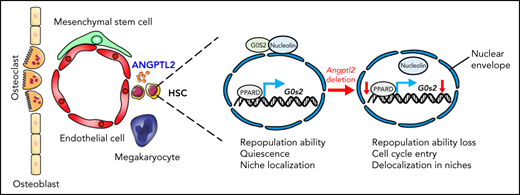
Key Points
Endothelial cell-derived ANGPTL2 is important for the maintenance of HSC activities in bone marrow niches.
ANGPTL2-mediated signaling pathways enhance PPARδ expression to transactivate G0s2 to sustain HSC activities.
Abstract
Bone marrow niche cells have been reported to fine-tune hematopoietic stem cell (HSC) stemness via direct interaction or secreted components. Nevertheless, how niche cells control HSC activities remains largely unknown. We previously showed that angiopoietin-like protein 2 (ANGPTL2) can support the ex vivo expansion of HSCs by binding to human leukocyte immunoglobulin-like receptor B2. However, how ANGPTL2 from specific niche cell types regulates HSC activities under physiological conditions is still not clear. Herein, we generated an Angptl2-flox/flox transgenic mouse line and conditionally deleted Angptl2 expression in several niche cells, including Cdh5+ or Tie2+ endothelial cells, Prx1+ mesenchymal stem cells, and Pf4+ megakaryocytes, to evaluate its role in the regulation of HSC fate. Interestingly, we demonstrated that only endothelial cell-derived ANGPTL2 and not ANGPTL2 from other niche cell types plays important roles in supporting repopulation capacity, quiescent status, and niche localization. Mechanistically, ANGPTL2 enhances peroxisome-proliferator-activated receptor D (PPARD) expression to transactivate G0s2 to sustain the perinuclear localization of nucleolin to prevent HSCs from entering the cell cycle. These findings reveal that endothelial cell-derived ANGPTL2 serves as a critical niche component to maintain HSC stemness, which may benefit the understanding of stem cell biology in bone marrow niches and the development of a unique strategy for the ex vivo expansion of HSCs.
Introduction
Hematopoietic stem cells (HSCs) can self-renew to maintain the stem cell pool and generate all downstream progenitors and terminally differentiated lineages. In adults, HSCs reside in a specific bone marrow (BM) niche (or microenvironment) containing many types of both nonhematopoietic and hematopoietic cells, including mesenchymal stem cells, endothelial cells, osteoblast cells, adipocytes, megakaryocytes, and other cells.1-3 In addition to their direct interaction with HSCs, these niche cells may also secrete many types of growth factors, cytokines, or other niche components to maintain HSC stemness.4 For example, several niche components, including stem cell factor (SCF),5 stromal cells-derived factor-1,6 thrombopoietin,7 Wnt,8,9 transforming growth factor-β,9,10 FGF1/2,11,12 and angiopoietin-like proteins (ANGPTLs),13 have been reported to tightly regulate the differentiation, self-renewal, quiescence, and niche localization of HSCs. However, whether other unknown niche cell types/secreted factors exist and how these niche components determine the different fates of HSCs remain largely unknown.
ANGPTLs are secreted glycoproteins with similar sequence homology to angiopoietins that have an N-terminal coiled-coil domain and a C-terminal fibrinogen-like domain.14,15 Currently, 8 ANGTPLs, including ANGPTL1-8, have been identified.16,17 ANGPTLs have been found to be expressed in many cell types, such as fat, liver, muscle, endothelium, and some endocrine organs.18,19 In contrast to angiopoietins, ANGPTLs do not bind Tie-1 or Tie-2.14 Many studies have shown that ANGPTLs play important roles in angiogenesis, lipid metabolism, and inflammation.14,20,21 In particular, ANGPTL2 signaling has been reported to be important for angiogenesis, chronic inflammation, metabolic disease, atherosclerotic diseases, and cancers.22-24 For example, ANGPTL2 can enhance the migration and chemotactic ability of endothelial cells through the Rac1 signaling pathway to induce vascularization in a murine cornea model.25 ANGPTL2 binds to integrin α5β1 to enhance cell motility and extracellular matrix remodeling, leading to tissue repair.26 ANGPTL2 is able to directly interact with CD146 to promote obesity via regulation of lipid metabolism.27 ANGPTL2 is highly upregulated in different cancer cells, such as breast and lung cancers, and is a critical facilitator of inflammatory tumorigenesis and metastasis.28,29 Nevertheless, how ANGPTLs, such as ANGPTL2, affect HSC fate still awaits further investigation.
We previously showed that several ANGPTLs, including ANGPTL2 and ANGPTL3, can inhibit differentiation and support HSC activities both in vitro and in vivo.30,31 We also demonstrated that human leukocyte immunoglobulin-like receptor B2 (LILRB2) or its mouse ortholog paired immunoglobulin-like receptor (PIRB) serves as the surface receptor for ANGPTL2 and plays an important role in the regulation of HSC stemness and leukemia development.13 Recently, we also showed that ANGPTL2 exists in exosomes and plays important roles in the maintenance of HSC activities.32 However, little is known about how ANGPTL2 derived from specific niche cell types regulates HSC activities under physiological conditions.
Methods
Mice
Angptl2fl/fl transgenic mice were generated by using a homologous combination knockout strategy, in which exon 2 was flanked with 2 loxP sites. The targeting construct was electroporated into mouse embryonic stem cells with a C57BL/6 genetic background. Heterozygous mice were obtained following standard procedures with in vivo Cre-mediated excision of the loxP-neo-loxP cassette. The Angptl2 and/or loxP insert was amplified by PCR using the following primers: 5′ATCCTAATGTCCCTCTTGGC3′ and 5′-CAGGCTGTGAACAGGTTAGTCAT C3′. Several specific niche cell Cre transgenic mice, including Tie2-Cre (004128), Cdh5-Cre (006137), Prx1-Cre (005584), Pf4-Cre (008535), and CD45.1 (002014) mice, were purchased from the Jackson Laboratory and Scl-Cre-ERT mouse line was kindly provided by Joachim R. Gothert at University Hospital Essen (Germany). These mice were used for cross breeding with Angptl2fl/fl transgenic mice to conditionally delete the Angptl2 gene. C57BL/6 mice were purchased from the Shanghai SLAC Laboratory Animal Co., Ltd., and bred at the Animal Core Facility. For all experiments, 6- to 8-week-old C57BL/6 CD45.1 and CD45.2 mice were used unless otherwise indicated. Animal experiments were evaluated and approved by our institution and performed under the Guideline for Animal Care at Shanghai Jiao Tong University School of Medicine.
Competitive reconstitution analysis
For the analysis of the function of endothelial cell-derived ANGPTL2 in HSC activities, a serial transplantation experiment was performed with Cdh5-Cre;Angptl2fl/fl or Tie2-Cre;Angptl2fl/fl transgenic mice because Cdh5 or Tie2 has been indicated to be expressed in a small population of HSCs.33,34 In brief, a total of 1 × 106 BM cells from CD45.1 mice (without competitor cells) were transplanted into lethally irradiated Angptl2fl/fl and Cdh5-Cre;Angptl2fl/fl or Tie2-Cre;Angptl2fl/fl recipient mice, followed by secondary transplantation 6 months later. BM cells (7 × 105) were freshly harvested from these primary recipient mice, and 3 × 105 CD45.2 competitor cells were transplanted into each of the lethally irradiated secondary recipient mice via retroorbital injection. These mice were analyzed for the repopulation of CD45.1 donor cells at 4 to 16 weeks after transplantation. For the analysis of the role of ANGPTL2 derived from other niche cells or HSCs in HSC activities, BM donor cells (CD45.2) from Angptl2fl/fl and Prx1-Cre;Angptl2fl/fl or Pf4-Cre;Angptl2fl/fl or Scl-Cre-ERT; Angptl2fl/fl (pretreated with tamoxifen for 3 weeks) mice were freshly harvested, mixed with competitor cells (CD45.1) at a ratio of 1:1, and transplanted into lethally irradiated recipient mice via retroorbital injection. Peripheral blood was collected to measure the repopulation changes at the indicated times after transplantation. In some cases, Lin−Sca-1+Kit+Flk2−CD34− immunophenotypic LT-HSCs (500) from Cdh5-Cre;Angptl2fl/fl or Tie2-Cre;Angptl2fl/fl and Angptl2fl/fl mice were injected into the lethally recipient mice along with 1 × 105 CD45.1 competitor cells. These mice were analyzed for the repopulation of CD45.2 donor cells at 4 to 16 weeks after transplantation.
Statistical analysis
Statistical analysis was performed using GraphPad and SPSS (version 19.0) software programs. Data are represented as the mean ± SD. Data were analyzed with Student t test (2-tailed), 1-way analysis of variance with Tukey’s multiple comparison test, or 2-way analysis of variance with Sidak multiple comparison test according to the experimental design, and statistical significance was set at P < .05 (*P < .05; **P < .01; ***P < .001).
All the other experimental details can be found in supplemental Methods (available on the Blood Web site).
Results
ANGPTL2 is highly expressed in endothelial cells and important for HSC stemness maintenance
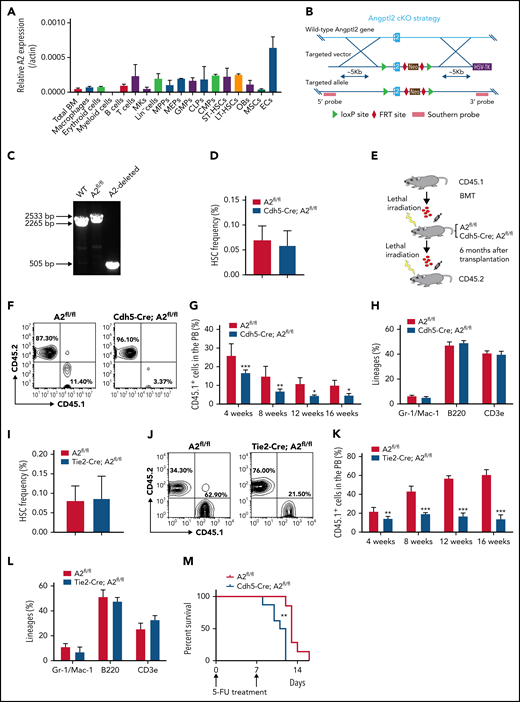
To determine the role of ANGPTL2 in HSC activities, we first analyzed the expression pattern of Angptl2 in hematopoietic cells and different types of niche cells by quantitative reverse transcriptase polymerase chain reaction (qRT-PCR). As shown in Figure 1A, Angptl2 was expressed in some hematopoietic cells (macrophages, erythroid cells, B cells, T cells, multipotent progenitors, megakaryocyte-erythroid progenitors, granulocyte-monocyte progenitors, lymphoid progenitors, common myeloid progenitors, short-term HSCs [ST-HSCs], and long-term HSCs [LT-HSCs]) and several BM niche cells (mesenchymal stem cells [MSCs], megakaryocytes [MKs], osteoblasts, and endothelial cells). Interestingly, endothelial cells had the highest messenger RNA (mRNA) expression level of Angptl2, which was ∼14.4-fold greater than that in total BM cells. We further purified BM sinusoidal endothelial cells (Lin−CD31+VE-cad+EMCN+Sca1lo), type H vessels (Lin−CD31+VE-cad+EMCNhiSca-1+), and arterioles (Lin−CD31+VE-cad+EMCN−Sca-1+) and examined mRNA levels of Angptl2 in these subtypes. Interestingly, Angptl2 was expressed in all 3 subtypes and their expression levels were comparable (supplemental Figure 1A-B), suggesting that all 3 subtypes of endothelial cells are able to provide ANGPTL2 to support HSC activities in BM. We further generated an Angptl2 conditional knockout murine strain (cKO) with exon 2 floxed by 2 loxP sites (Angptl2fl/fl; Figure 1B). The insertion of loxP and the deleted allele in Angptl2fl/fl mice were confirmed by PCR (Figure 1C). To determine the role of ANGPTL2 from endothelial cells in HSC stemness maintenance, we first crossed Angptl2fl/fl mice with transgenic Cdh5-Cre mice expressing Cre recombinase under the control of the cadherin 5 promoter, which deletes floxed genes in the endothelium of all organs. There was an almost 100% deletion in Angptl2 expression in Ter119−CD31+ endothelial cells isolated from the BM of Cdh5-Cre;Angptl2fl/fl mice (supplemental Figure 1C).
ANGPTL2 is highly expressed in endothelial cells and important for HSC stemness maintenance. (A) The relative mRNA levels of Angptl2 were measured in total bone marrow (BM), macrophages, erythroid cells, B cells, T cells, megakaryocytes (MKs), multipotent progenitors (MPPs), megakaryocyte-erythroid progenitors (MEPs), common myeloid progenitors (CMPs), granulocyte-monocyte progenitors (GMPs), lymphoid progenitors (CLPs), osteoblasts (OBs), mesenchymal stem cells (MSCs), and endothelial cells (ECs) by qRT-PCR (n = 3). (B) Schematic diagram for the strategy for the construction of the Angptl2 conditional knockout mouse lines. (C) Representative electrophoretic images for the genotyping of WT allele, Angptl2fl/fl allele (A2fl/fl) and Angptl2fl/fl deleted allele (A2-deleted) transgenic mice. (D) The frequencies of immunophenotypic Lin−Sca-1+c-Kit+CD34−CD135− LT-HSCs in the BM of Angptl2fl/fl and Cdh5-Cre; Angptl2fl/fl mice as measured by flow cytometric analysis (n = 7). (E) Schematic diagram for the strategy for a serial BM transplantation. (F-G) Representative flow cytometric analyses (F) and quantification data (G) for the competitive reconstitution assay with donor cells isolated from Angptl2fl/fl and Cdh5-Cre; Angptl2fl/fl recipient mice receiving CD45.1 BM cells 6 months after transplantation. The repopulation of CD45.1 donor cells was measured 4 to 16 weeks after transplantation (n = 5). (H) The multilineage contribution of donor cells to T cells (CD3), B cells (B220), and myeloid cells (Mac-1 and Gr-1) in peripheral blood was quantitated by flow cytometric analysis at 16 weeks posttransplantation (n = 5). (I) The frequencies of LT-HSCs in the BM of Angptl2fl/fl and Tie2-Cre; Angptl2fl/fl mice as measured by flow cytometric analysis (n = 7). (J-L) The repopulation ability of donor cells isolated from primary Angptl2fl/fl and Tie2-Cre; Angptl2fl/fl recipient mice was determined by flow cytometric analyses at the indicated time points (J) using a serial transplantation strategy (K). The multilineage contribution of donor cells in peripheral blood was quantified at 16 weeks after transplantation (L; n = 5). (M) Angptl2fl/fl and Cdh5-Cre; Angptl2fl/fl mice were treated with 5-fluorouracil and overall survival was analyzed (log-rank test, n = 8). *P < .05; **P < .01; ***P < .001.
ANGPTL2 is highly expressed in endothelial cells and important for HSC stemness maintenance. (A) The relative mRNA levels of Angptl2 were measured in total bone marrow (BM), macrophages, erythroid cells, B cells, T cells, megakaryocytes (MKs), multipotent progenitors (MPPs), megakaryocyte-erythroid progenitors (MEPs), common myeloid progenitors (CMPs), granulocyte-monocyte progenitors (GMPs), lymphoid progenitors (CLPs), osteoblasts (OBs), mesenchymal stem cells (MSCs), and endothelial cells (ECs) by qRT-PCR (n = 3). (B) Schematic diagram for the strategy for the construction of the Angptl2 conditional knockout mouse lines. (C) Representative electrophoretic images for the genotyping of WT allele, Angptl2fl/fl allele (A2fl/fl) and Angptl2fl/fl deleted allele (A2-deleted) transgenic mice. (D) The frequencies of immunophenotypic Lin−Sca-1+c-Kit+CD34−CD135− LT-HSCs in the BM of Angptl2fl/fl and Cdh5-Cre; Angptl2fl/fl mice as measured by flow cytometric analysis (n = 7). (E) Schematic diagram for the strategy for a serial BM transplantation. (F-G) Representative flow cytometric analyses (F) and quantification data (G) for the competitive reconstitution assay with donor cells isolated from Angptl2fl/fl and Cdh5-Cre; Angptl2fl/fl recipient mice receiving CD45.1 BM cells 6 months after transplantation. The repopulation of CD45.1 donor cells was measured 4 to 16 weeks after transplantation (n = 5). (H) The multilineage contribution of donor cells to T cells (CD3), B cells (B220), and myeloid cells (Mac-1 and Gr-1) in peripheral blood was quantitated by flow cytometric analysis at 16 weeks posttransplantation (n = 5). (I) The frequencies of LT-HSCs in the BM of Angptl2fl/fl and Tie2-Cre; Angptl2fl/fl mice as measured by flow cytometric analysis (n = 7). (J-L) The repopulation ability of donor cells isolated from primary Angptl2fl/fl and Tie2-Cre; Angptl2fl/fl recipient mice was determined by flow cytometric analyses at the indicated time points (J) using a serial transplantation strategy (K). The multilineage contribution of donor cells in peripheral blood was quantified at 16 weeks after transplantation (L; n = 5). (M) Angptl2fl/fl and Cdh5-Cre; Angptl2fl/fl mice were treated with 5-fluorouracil and overall survival was analyzed (log-rank test, n = 8). *P < .05; **P < .01; ***P < .001.
To evaluate whether endothelial cell-derived ANGPTL2 was important for HSC activities, we further examined the lineage distributions in peripheral blood and HSC frequencies in the BM from Cdh5-Cre;Angptl2fl/fl and Angptl2fl/fl control mice. However, the frequency and absolute number of Lin−Sca-1−Kit−Flk2−CD34− immunophenotypic LT-HSCs was not changed in the BM of Cdh5-Cre;Angptl2fl/fl mice compared with that of Angptl2fl/fl control mice (Figure 1D; supplemental Figure 1D-E ). No significant changes in lineage distributions in peripheral blood were found between Cdh5-Cre;Angptl2fl/fl and Angptl2fl/fl mice (supplemental Figure 1F-G). The cell percentages of granulocyte-monocyte progenitors, common myeloid progenitors, megakaryocyte-erythroid progenitors, and lymphoid progenitors remained unaltered in the BM of Cdh5-Cre;Angptl2fl/fl mice (supplemental Figure 1H). Consistently, a functional colony assay revealed that colony numbers of primitive myeloid progenitors, granulocyte-monocyte progenitors, erythroid progenitors (burst-forming unit-E and colony-forming unit-E), and B-cell progenitors (pre-B) were not changed between Cdh5-Cre;Angptl2fl/fl and Angptl2fl/fl mice (supplemental Figure 1I).
Because several studies have implied that Cdh5 may also be expressed in a small population of HSCs,33 we used a serial transplantation strategy to evaluate the function of endothelial cell-derived ANGPTL2 in HSC activities. BM cells from CD45.1 mice were transplanted into lethally irradiated Cdh5-Cre;Angptl2fl/fl or Angptl2fl/fl recipients without competitors for 6 months. Then, a secondary transplantation was performed to determine the repopulation capacities of HSCs (Figure 1E). Interestingly, the donor CD45.1+ cells from the primary Cdh5-Cre;Angptl2fl/fl recipients had markedly lower repopulation ability than those from the Angptl2fl/fl recipients at 4, 8, 12, and 16 weeks after transplantation (Figure 1F-G). However, lineage differentiation in myeloid cells, B cells, and T cells in peripheral blood (Figure 1H) and the BM HSC frequency (supplemental Figure 1J) from Cdh5-Cre;Angptl2fl/fl and Angptl2fl/fl donor recipients was similar.
We further examined the role of endothelial cell-derived ANGPTL2 in HSC activities using another endothelial cell-specific Cre, Tie2-Cre, to conditionally knock out Angptl2 in mice. The deletion efficiency in endothelial cells isolated from the BM of Tie2-Cre;Angptl2fl/fl mice was confirmed by quantitative RT-PCR (supplemental Figure 1K). Similar to the strategy used for the Cdh5-Cre;Angptl2fl/fl mice (Figure 1E), we transplanted CD45.1 BM cells into lethally irradiated Tie2-Cre;Angptl2fl/fl and Angptl2fl/fl recipients without competitors, followed by secondary transplantation. Although the BM HSC frequency (Figure 1I), absolute number, and lineages in peripheral blood (supplemental Figure 1L-M) were not changed between Tie2-Cre;Angptl2fl/fl and Angptl2fl/fl mice, the repopulation ability of HSCs from the primary Tie2-Cre;Angptl2fl/fl recipients was dramatically decreased to 30% of that from the Angptl2fl/fl recipients (Figure 1K-L). Consistent with these results, endothelial cell–derived ANGPTL2 had no effect on lineage differentiation (Figure 1L) and the HSC frequency (supplemental Figure 1N). Furthermore, to evaluate ANGPTL2 function on HSC activities under a stress condition, we treated Cdh5-Cre;Angptl2fl/fl or Tie2-Cre;Angptl2fl/fl and Angptl2fl/fl mice with 5-fluorouracil that causes the entry of cell cycle and exhaustion of HSCs. Consistently, Cdh5-Cre;Angptl2fl/fl or Tie2-Cre;Angptl2fl/fl mice exhibited markedly reduced overall survival compared with Angptl2fl/fl controls (Figure 1M; supplemental Figure 10), indicating that the function of HSCs is impaired under such stress condition.
ANGPTL2 derived from other niche cells or HSCs has no effect on HSC activities
Because ANGPTL2 was also expressed at relatively low levels in MSCs and MKs, we generated Prx1-Cre;Angptl2fl/fl and Pf4-Cre;Angptl2fl/fl mice to block ANGPTL2 secretion from MSCs and MKs in the BM, respectively. Donor BM cells were isolated from Prx1-Cre;Angptl2fl/fl and Pf4-Cre;Angptl2fl/fl mice and transplanted into lethally irradiated CD45.1 recipients together with CD45.1 competitor cells (1:1 ratio). However, no significant differences in HSC repopulation ability and lineage distribution were found in donor HSCs from Prx1-Cre;Angptl2fl/fl (Figures 2A-C) or Pf4-Cre;Angptl2fl/fl mice (Figure 2D-F).
ANGPTL2 derived from other niche cells or HSCs has no effect on HSC activities. (A-B) The repopulation ability of donor cells isolated from primary Angptl2fl/fl or Prx1-Cre; Angptl2fl/fl recipient mice was determined by flow cytometric analyses (A) at the indicated time points after transplantation (B; n = 5). (C) The multilineage contribution of donor cells in peripheral blood was quantified at 16 weeks after transplantation (n = 5). (D-E) The repopulation ability of donor cells isolated from primary Angptl2fl/fl or Pf4-Cre; Angptl2fl/fl recipient mice was determined by flow cytometric analyses (D) at the indicated time points after transplantation (E; n = 5). (F) The multilineage contribution of donor cells in peripheral blood was quantified at 16 weeks after transplantation (n = 5).
ANGPTL2 derived from other niche cells or HSCs has no effect on HSC activities. (A-B) The repopulation ability of donor cells isolated from primary Angptl2fl/fl or Prx1-Cre; Angptl2fl/fl recipient mice was determined by flow cytometric analyses (A) at the indicated time points after transplantation (B; n = 5). (C) The multilineage contribution of donor cells in peripheral blood was quantified at 16 weeks after transplantation (n = 5). (D-E) The repopulation ability of donor cells isolated from primary Angptl2fl/fl or Pf4-Cre; Angptl2fl/fl recipient mice was determined by flow cytometric analyses (D) at the indicated time points after transplantation (E; n = 5). (F) The multilineage contribution of donor cells in peripheral blood was quantified at 16 weeks after transplantation (n = 5).
Previous studies have implied that Cdh5 or Tie may also be expressed in hemogenic endothelium during embryonic development, which leads to the deletion of Angptl2 in HSCs while using Cdh5-Cre or Tie-Cre mice.33,34 To exclude the cell-autonomous effect of ANGPTL2 on HSCs in maintaining stemness, we specifically deleted Angptl2 in HSCs by using Scl-cre-ERT;Angptl2fl/fl mice35 and demonstrated that there was no difference in HSC frequencies, repopulation capacities, and lineage distribution (supplemental Figure 2A-C). To further directly confirm the effect on HSCs of endothelial cell-derived ANGPTL2, Lin−Sca-1+Kit+Flk2−CD34− immunophenotypic HSCs freshly harvested from Cdh5-Cre;Angptl2fl/fl or Tie2-Cre;Angptl2fl/fl and Angptl2fl/fl mice were injected into recipient mice along with CD45.1 competitor cells. Consistently, we also found that the repopulating activities of HSCs derived from the Cdh5-Cre;Angptl2fl/fl or Tie2-Cre;Angptl2fl/fl recipients were significantly decreased compared with those from the Angptl2fl/fl recipients (supplemental Figure 2D-F), whereas lineage distribution was not altered (supplemental Figure 2E-G). These results suggest that ANGPTL2 derived from endothelial cells, but not that from other niche cells or HSC themselves, may make a major contribution to the maintenance of the HSC pool in the BM.
ANGPTL2-producing endothelium supports the localization of HSCs in BM niches
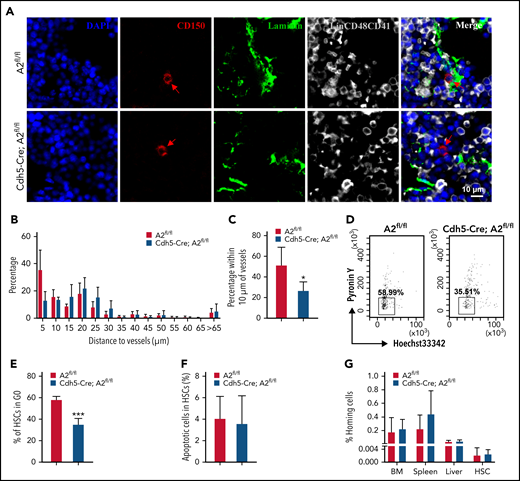
The fates of HSCs are tightly orchestrated via self-renewal, differentiation, quiescence, apoptosis, and localization in BM niches. We have confirmed that ANGPTL2 deletion in endothelial cells leads to a severe impairment of HSC repopulation ability. Previous studies have also reported that HSCs are mainly located in the vascular niches, where endothelial cells play an important role in maintaining HSC activities.36 By using immunofluorescence staining, we found that >50% of HSCs resided in the region adjacent to (within 10 μm of) laminin+ endothelial cells in the BM of Angptl2fl/fl mice (Figure 3A-C). In contrast, only 26.70% of HSCs localized close to laminin+ endothelial cells in the BM of Cdh5-Cre;Angptl2fl/fl mice, which is significantly lower than that in the BM of Angptl2fl/fl mice. The HSC localization pattern was also altered in the vascular niche in the BM of Tie2-Cre;Angptl2fl/fl mice (supplemental Figure 3A-C). These data suggest that ANGPTL2 produced by endothelial cells contributes to the localization of HSCs in BM niches, which may be critical for the stemness of HSCs.
ANGPTL2-producing endothelium supports the localization of HSCs in BM niches. (A) Representative images of the localization of LT-HSCs in the BM of Angptl2fl/fl or Cdh5-Cre; Angptl2fl/fl mice. BM sections were stained to reveal Lin−CD150+CD48−CD41− LT-HSCs (red, with arrowhead), laminin-positive vessels (green), and Lin+CD41+CD48+ differentiated hematopoietic cells (gray). Scale bar, 10 μm. (B) Quantification of the frequencies of LT-HSCs localized at the indicated distance to vessels. n = 225-256 cells per group. (C) The percentages of LT-HSCs in the BM of Angptl2fl/fl and Cdh5-Cre; Angptl2fl/fl mice adjacent to laminin-positive endothelial cells (within 10 μm) are shown. A total of 225 to 256 LT-HSCs were counted (n = 4). (D) The cell cycle status of LT-HSCs from Angptl2fl/fl and Cdh5-Cre; Angptl2fl/fl mice was determined by staining with Hoechst 33342 and pyronin Y. (E) The percentages of the G0 fraction in panel D are shown (n = 4). (F) The apoptotic status of LT-HSCs from Angptl2fl/fl or Cdh5-Cre; Angptl2fl/fl mice was measured by using Annexin V and 7-AAD staining (n = 4). (G) BM cells from Angptl2fl/fl and Cdh5-Cre; Angptl2fl/fl mice were labeled with CFSE and transplanted into lethally irradiated recipient mice. The percentage of CFSE+ cells in the BM, spleen and liver or CFSE+ LSK cells in the BM was determined by flow cytometric analysis 16 hours later (n = 5. *P < .05; ***P < .001.
ANGPTL2-producing endothelium supports the localization of HSCs in BM niches. (A) Representative images of the localization of LT-HSCs in the BM of Angptl2fl/fl or Cdh5-Cre; Angptl2fl/fl mice. BM sections were stained to reveal Lin−CD150+CD48−CD41− LT-HSCs (red, with arrowhead), laminin-positive vessels (green), and Lin+CD41+CD48+ differentiated hematopoietic cells (gray). Scale bar, 10 μm. (B) Quantification of the frequencies of LT-HSCs localized at the indicated distance to vessels. n = 225-256 cells per group. (C) The percentages of LT-HSCs in the BM of Angptl2fl/fl and Cdh5-Cre; Angptl2fl/fl mice adjacent to laminin-positive endothelial cells (within 10 μm) are shown. A total of 225 to 256 LT-HSCs were counted (n = 4). (D) The cell cycle status of LT-HSCs from Angptl2fl/fl and Cdh5-Cre; Angptl2fl/fl mice was determined by staining with Hoechst 33342 and pyronin Y. (E) The percentages of the G0 fraction in panel D are shown (n = 4). (F) The apoptotic status of LT-HSCs from Angptl2fl/fl or Cdh5-Cre; Angptl2fl/fl mice was measured by using Annexin V and 7-AAD staining (n = 4). (G) BM cells from Angptl2fl/fl and Cdh5-Cre; Angptl2fl/fl mice were labeled with CFSE and transplanted into lethally irradiated recipient mice. The percentage of CFSE+ cells in the BM, spleen and liver or CFSE+ LSK cells in the BM was determined by flow cytometric analysis 16 hours later (n = 5. *P < .05; ***P < .001.
Next, we evaluated the changes in the cell-cycle/quiescence, apoptosis, and homing abilities of HSCs from Angptl2fl/fl and Cdh5-Cre;Angptl2fl/fl or Tie2-Cre;Angptl2fl/fl mice. The frequency of cells in G0 among Lin−Sca-1−Kit−Flk2−CD34− immunophenotypic LT-HSCs was analyzed by Hoechst 33342 and pyronin Y staining. As shown in Figure 3D-E, ∼58.38% of Angptl2fl/fl HSCs were in G0, which was significantly higher than that of Cdh5-Cre;Angptl2fl/fl HSCs (35.33%). We also observed a similar reduction in the frequency of HSCs in the G0 fraction of Tie2-Cre;Angptl2fl/fl mice compared with Angptl2fl/fl control mice (64.93% vs 33.93%; supplemental Figure 3D-E). In addition, we did not observe a difference in the apoptosis of HSCs between Angptl2fl/fl and Cdh5-Cre;Angptl2fl/fl or Tie2-Cre;Angptl2fl/fl mice (Figure 3F; supplemental Figure 3F). No difference in homing capacity was found between Angptl2fl/fl and Cdh5-Cre;Angptl2fl/fl or Tie2-Cre;Angptl2fl/fl HSCs (Figure 3G; supplemental Figure 3G). Consistently, the CXCR4 protein levels were similar in HSCs from both cKO and their control mice as determined by flow cytometric analysis (supplemental Figure 3H,J). Meanwhile, we also evaluated the mRNA expression levels of other molecules related to the migration in HSCs37-40 and demonstrated that the mRNA levels of CD49d, Egr1, Mmp2, and Mmp9 were markedly decreased in both cKO mice (supplemental Figure 3I,K), although how ANGPTL2 controls their expression awaits further investigation. Therefore, our data suggest that the lack of ANGPTL2 in endothelial cells may lead to disrupted localization and loss of quiescence of HSCs in BM niches, which may further contribute to their decreased repopulation abilities.
ANGPTL2 sustains the level of G0S2 to support HSC activities
To identify the intracellular targets of ANGPTL2 that control HSC stemness, human cord blood CD34+ hematopoietic stem/progenitor cells were stimulated with ANGPTL2 for 24 hours and subjected to RNA-sequencing analyses (GSE169107; supplemental Tables 2 and 3). Because we previously showed that ANGPTL2 binds to LILRB2 to mediate downstream signaling and that ANGPTL2 deficiency led to the loss of quiescence and repopulation ability in HSCs, we then focused on the changes in several signaling pathways (KEGG analysis) that might be involved in ligand-receptor interactions and cell-cycle regulation, such as “cytokine-cytokine receptor interaction” and “chemokine signaling pathway” (supplemental Figure 4A). Interestingly, several potential candidate genes were notably changed in human hematopoietic stem/progenitor cells upon treatment with ANGPTL2, such as RORA (involved in transcriptional regulation), RAB20 (involved in vesicular trafficking pathways), PIK3R2 (a heterodimer of the catalytic subunit in growth signaling pathways), ISG15 (involved in cell-to-cell signaling), G0S2 (involved in proliferation, metabolism and inflammation), and peroxisome-proliferator-activated receptor D (PPARD) (involved in metabolic pathways; supplemental Figure 4B). Moreover, we have used Lin−Sca-1+Kit+Flk2−CD34− immunophenotypic LT-HSCs from Cdh5-Cre;Angptl2fl/fl and Angptl2fl/fl mice to perform the RNA-sequencing analysis (GSE186454). We also found and G0s2 and Ppard were downregulated in Cdh5-Cre;Angptl2fl/fl HSCs (supplemental Figure 4C).
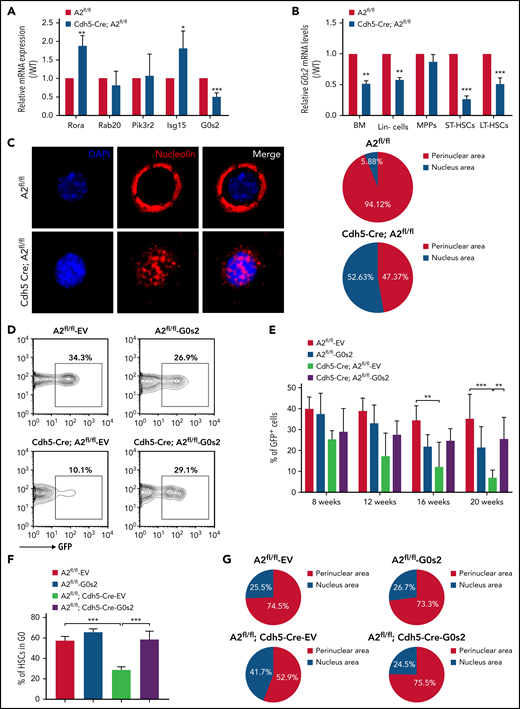
We then measured the mRNA expression of these potential candidates in HSCs purified from Angptl2fl/fl and Cdh5-Cre;Angptl2fl/fl or Tie2-Cre;Angptl2fl/fl mice and demonstrated that G0s2 was markedly decreased in both cKO mice (Figure 4A; supplemental Figure 4E). It has been reported that G0S2 is a multifaceted protein with disparate roles in proliferation, metabolism, inflammation, and carcinogenesis.41-43 G0S2 also regulates the cell cycle by either suspending cells in G0 phase or enhancing the G1-S switch under different physiological conditions.44 For example, Takeshi et al showed that G0S2 may sustain the quiescence of HSCs in the BM niche.45,46 In addition to LT-HSCs, we also observed that the G0s2 expression level was significantly reduced in total BM cells, Lin− cells and ST-HSCs from Cdh5-Cre;Angptl2fl/fl and Tie2-Cre;Angptl2fl/fl mice compared with their Angptl2fl/fl counterparts (Figure 4B; supplemental Figure 4E). These results imply that G0S2 may be a potential downstream target for ANGPTL2-mediated signaling.
ANGPTL2 supports HSC function by sustaining the level of G0S2. (A) Relative mRNA expression of potential candidates (Rora, Rab20, Pik3r2, Isg15, and G0s2) in LT-HSCs purified from Angptl2fl/fl and Cdh5-Cre; Angptl2fl/fl mice as measured by quantitative RT-PCR (n = 3). (B) Relative mRNA expression of G0s2 in total BM cells, Lin− cells, MPPs, short-term HSCs, and LT-HSCs from Angptl2fl/fl and Cdh5-Cre; Angptl2fl/fl mice as measured by qRT-PCR (n = 3). (C) Nucleolin expression (red) in LT-HSCs from Angptl2fl/fl and Cdh5-Cre; Angptl2fl/fl mice was examined by immunofluorescence staining. Representative images are shown on the left. A total of 17 to 19 LT-HSCs were evaluated in the Angptl2fl/fl and Cdh5-Cre; Angptl2fl/fl groups (right, n = 3). (D) Representative flow cytometric analyses for the repopulation of HSCs from Angptl2fl/fl and Cdh5-Cre; Angptl2fl/fl mice with/without G0s2 overexpression (GFP+ cells) 16 weeks after transplantation. (E) The quantification data of the donor contribution at the indicated time points after transplantation are shown (n = 5). (F) The cell-cycle status of LT-HSCs from Angptl2fl/fl and Cdh5-Cre; Angptl2fl/fl mice with/without G0s2 overexpression was determined by staining with Hoechst 33342 and pyronin Y. The percentages of the G0 fraction are shown (n = 3). (G) Nucleolin localization in LT-HSCs from Angptl2fl/fl and Cdh5-Cre; Angptl2fl/fl mice with/without G0s2 overexpression was examined by immunofluorescence staining. A total of 45 to 55 LT-HSCs were analyzed. *P < .05; **P < .01; ***P < .001.
ANGPTL2 supports HSC function by sustaining the level of G0S2. (A) Relative mRNA expression of potential candidates (Rora, Rab20, Pik3r2, Isg15, and G0s2) in LT-HSCs purified from Angptl2fl/fl and Cdh5-Cre; Angptl2fl/fl mice as measured by quantitative RT-PCR (n = 3). (B) Relative mRNA expression of G0s2 in total BM cells, Lin− cells, MPPs, short-term HSCs, and LT-HSCs from Angptl2fl/fl and Cdh5-Cre; Angptl2fl/fl mice as measured by qRT-PCR (n = 3). (C) Nucleolin expression (red) in LT-HSCs from Angptl2fl/fl and Cdh5-Cre; Angptl2fl/fl mice was examined by immunofluorescence staining. Representative images are shown on the left. A total of 17 to 19 LT-HSCs were evaluated in the Angptl2fl/fl and Cdh5-Cre; Angptl2fl/fl groups (right, n = 3). (D) Representative flow cytometric analyses for the repopulation of HSCs from Angptl2fl/fl and Cdh5-Cre; Angptl2fl/fl mice with/without G0s2 overexpression (GFP+ cells) 16 weeks after transplantation. (E) The quantification data of the donor contribution at the indicated time points after transplantation are shown (n = 5). (F) The cell-cycle status of LT-HSCs from Angptl2fl/fl and Cdh5-Cre; Angptl2fl/fl mice with/without G0s2 overexpression was determined by staining with Hoechst 33342 and pyronin Y. The percentages of the G0 fraction are shown (n = 3). (G) Nucleolin localization in LT-HSCs from Angptl2fl/fl and Cdh5-Cre; Angptl2fl/fl mice with/without G0s2 overexpression was examined by immunofluorescence staining. A total of 45 to 55 LT-HSCs were analyzed. *P < .05; **P < .01; ***P < .001.
It has been reported that elevated G0S2 expression can sequester nucleolin in the cytosol and preclude its pro-proliferative functions in the nucleolus, thus sustaining the G0 phase of HSCs.45 To investigate whether ANGPTL2 deficiency inhibits nucleolin trafficking between the cytosol and nucleolus in HSCs, nucleolin levels were determined in HSCs isolated from Cdh5-Cre;Angptl2fl/fl and Angptl2fl/fl mice by immunofluorescence staining (Figure 4C). Indeed, 94.12% of Angptl2fl/fl HSCs had perinuclear-distributed nucleolin, which was approximately twofold more than that in HSCs purified from Cdh5-Cre;Angptl2fl/fl mice (47.37%). Importantly, overexpression of G0s2 in HSCs isolated from Cdh5-Cre;Angptl2fl/fl mice partially reversed the reduction in repopulation ability (Figure 4D-E). The levels of ectopically expressed G0s2 were confirmed by qRT-PCR (supplemental Figure 4F). Meanwhile, the overexpression of G0s2 in HSCs isolated from either Cdh5-Cre;Angptl2fl/fl or Tie2-Cre;Angptl2fl/fl mice indeed could reverse their loss of quiescence and intracellular distribution of nucleolin (Figure 4F-G; supplemental Figure 4G-H). Therefore, these data reveal that endothelial cell–derived ANGPTL2 maintained HSC quiescence by upregulating G0s2 levels.
ANGPTL2 sustains the level of G0S2 via the PPARD pathway
To identify the upstream pathway targeting G0S2, we screened several potential candidates of transcription factors that may bind to the promoter of G0s2. Interestingly, the Ppard level was ∼50% lower in HSCs isolated from Cdh5-Cre;Angptl2fl/fl or Tie2-Cre;Angptl2fl/fl mice than in HSCs isolated from Angptl2fl/fl mice (Figure 5A-B). Meanwhile, as shown in supplemental Figure 5A, ANGPTL2 could upregulate the Ppard and G0s2 expression in a dose-dependent manner. PPARs, such as PPARA, PPARD, and PPARG, are members of the nuclear receptor superfamily of transcription factors that control nutrient sensing and metabolic pathways,47 especially fatty acid transportation and fatty acid oxidation.48,49 It has been reported that G0S2 is a novel target of PPARA that may be involved in adipocyte differentiation.50 The PML-PPARD-fatty acid oxidation pathway is also important for the maintenance of HSC activities.51
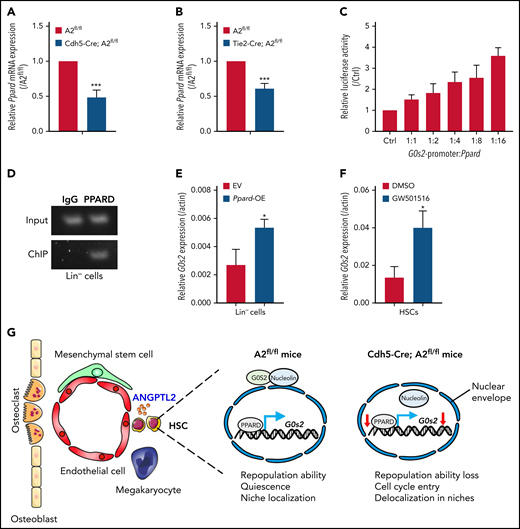
ANGPTL2 regulates G0s2 expression through Ppard. (A) Relative Ppard mRNA expression in LT-HSCs from Angptl2fl/fl and Cdh5-Cre; Angptl2fl/fl mice as measured by quantitative RT-PCR (n = 3). (B) Relative Ppard mRNA expression in LT-HSCs from Angptl2fl/fl and Tie2-Cre; Angptl2fl/fl mice as measured by quantitative RT-PCR (n = 3). (C) Relative luciferase activities were determined in 32D cells cotransfected with the Ppard plasmid and luciferase reporter containing the G0s2 promoter (n = 3). (D) Chromatin immunoprecipitation (ChIP) assays were performed in Lin− BM cells. The G0s2 promoter regions bound to endogenous PPARD were measured by PCR. (E) The G0s2 mRNA expression levels in Ppard-overexpressing (Ppard-OE) Lin− BM cells were detected by qRT-PCR (n = 3). (F) The G0s2 mRNA expression levels were measured in LT-HSCs upon the treatment with PPARD agonist (GW501516) for 24 hours by qRT-PCR (n = 3). (G) Schematic diagram of the working model for the role of endothelial cell-derived ANGPTL2 in HSC stemness. *P < .05; ***P < .001.
ANGPTL2 regulates G0s2 expression through Ppard. (A) Relative Ppard mRNA expression in LT-HSCs from Angptl2fl/fl and Cdh5-Cre; Angptl2fl/fl mice as measured by quantitative RT-PCR (n = 3). (B) Relative Ppard mRNA expression in LT-HSCs from Angptl2fl/fl and Tie2-Cre; Angptl2fl/fl mice as measured by quantitative RT-PCR (n = 3). (C) Relative luciferase activities were determined in 32D cells cotransfected with the Ppard plasmid and luciferase reporter containing the G0s2 promoter (n = 3). (D) Chromatin immunoprecipitation (ChIP) assays were performed in Lin− BM cells. The G0s2 promoter regions bound to endogenous PPARD were measured by PCR. (E) The G0s2 mRNA expression levels in Ppard-overexpressing (Ppard-OE) Lin− BM cells were detected by qRT-PCR (n = 3). (F) The G0s2 mRNA expression levels were measured in LT-HSCs upon the treatment with PPARD agonist (GW501516) for 24 hours by qRT-PCR (n = 3). (G) Schematic diagram of the working model for the role of endothelial cell-derived ANGPTL2 in HSC stemness. *P < .05; ***P < .001.
Therefore, we constructed a luciferase reporter with the G0s2 promoter and measured luciferase activities in Ppard-overexpressing 32D cells (a myeloblast-like cell line derived from long-term cultures of bone marrow cells), which showed that Ppard dramatically increased the luciferase activities compared with the control vector (Figure 5C). Furthermore, a chromatin immunoprecipitation assay in 32D cells and Lin− BM cells revealed that the endogenous PPARD could directly bind to the promoter of G0s2 (Figure 5D; supplemental Figure 5B). We also demonstrated that the expression levels of G0s2 were significantly increased in both 32D cells and Lin− BM cells upon Ppard overexpression (Figure 5E; supplemental Figure 5C). We then further treated both 32D cells and LT-HSCs with GW501516 (a PPARD agonist) for 24 hours and showed that G0s2 mRNA expression was also upregulated as determined by quantitative RT-PCR (Figure 5F; supplemental Figure 5D). These data show that PPARD may transactivate G0s2 expression to maintain the quiescent status of HSCs. Previously, we have revealed ANGPTLs may be the ligands of PIRB that activate SHP2/CAMKIV to maintain HSC activity in vivo. To find out the relationship between SHP2/CAMKIV and PPARD/G0S2, we further overexpressed SHP2 or CAMKIV in Lin− BM cells and demonstrated that either SHP2 or CAMKIV could upregulate the expression of PPARD and G0S2 (supplemental Figure 5E-F), suggesting that SHP2/CAMK may be the upstream signals controlling Ppard/G0s2 transcriptional activities. In addition, Lin− BM cells were purified and subjected to the treatment with ANGPTL2 protein for 24 or 48 hours, followed by the measurement of protein expression levels of SHP2, CAMKIV, PPARD, and G0S2 by western blot. The protein levels of SHP2, CAMKIV, PPARD, and G0S2 were also upregulated by ANGPTL2 at 48 hours after stimulation (supplemental Figure 5G), indicating that SHP2/CAMK/PPARD/G0S2 signaling maintains HSC activities upon the ANGPTL2 binding with its receptor of PIRB. However, the detailed underlying mechanisms related to how ANGPTL2 regulates G0S2 expression and cellular localization via PPARD awaits further investigation. In summary, we herein reveal that ANGPTL2 derived from endothelial cells but not from MSCs or MKs sustains the repopulation capacities, quiescent status and niche localization of HSCs in the BM, and this effect is tightly controlled by the PPARD/G0S2 pathway (Figure 5G).
Discussion
We previously showed that several ANGPTLs, including ANGPTL2, support ex vivo expansion of HSCs by binding to the receptors LILRB2 and PIRB expressed on human and mouse HSCs, respectively,13 but the underlying mechanisms have not been fully clarified. Herein, we used 4 niche cell type-specific mouse Cre lines to conditionally delete Angptl2 in endothelial cells, MSCs, and MKs and demonstrated that only endothelial cells serve as the major niche cell type to support HSC activities. Consistent with this result, endothelial cells had the highest expression level of Angptl2 compared with other niche cells. However, we also believe that other niche cell types,52 including osteoblasts, adipocytes and CXCL12-abundant reticular cells, may also be involved in the secretion of ANGPTL2 to support HSC activities. In particular, some studies have also reported that ANGPTL2 is highly expressed in adipocytes and important for lipid metabolism via various pathways, such as CD146-mediated signaling.27 The function of bone marrow adipocytes in sustaining HSC stemness is still controversial because several studies have shown that adipocytes can either sustain or suppress HSC stemness under physiological or stress conditions.53,54 Therefore, more efforts are required to delineate the role of ANGPTL2 from other niche cells in supporting HSC activities.
In addition, several ANGPTL family members, including ANGPTL2, ANGPTL3, ANGPTL5, and ANGPTL7, have been reported to be important for the ex vivo expansion of HSCs.30,31,55 Similarly, Angptl3-deficient mice also have decreased repopulation abilities in either an autonomous or niche-dependent manner.30 However, how these members act together with other growth factors to support HSC activities or their ex vivo expansion is still unknown.30 It is possible that there is a compensatory effect from the remaining ANGPTL members following the deletion of certain members (such as ANGPTL2). Interestingly, deletion of Angptl2 in endothelial cells leads to a marked decrease in HSC activities, indicating that some types of ANGPTLs may play a major role in HSC stemness maintenance under physiological conditions. Therefore, it is important to delineate the distinct impact of individual ANGPTLs in specific BM niches by using cKO mice. It will also be critical to understand how these ANGPTLs function together to maintain HSC stemness in vivo.
HSCs have been reported to localize in the vascular niches throughout the BM,56,57 which also was confirmed by our observation that more than 50% of HSCs localized adjacent to (within 10 μm of) laminin-positive endothelial cells. Recent studies also showed that proliferating HSCs may mainly reside in the CD144+ or CD105+ sinusoid vascular niche,58 whereas quiescent HSCs are preferentially sustained in the aSMA+ arteriolar vascular niche.59 Consistent with these results, we also noticed that deletion of Angptl2 in endothelial cells resulted in a notable reduction in the number of HSCs that resided close to laminin-postitive endothelial cells.
Perivascular stromal cells and endothelial cells can secrete high levels of CXCL12 and other factors to regulate HSC homeostasis.60,61 The factors secreted by these niche-supporting cells can directly bind to HSCs via cell surface receptors or indirectly affect the surrounding cell types to exert their effects on HSC activities. For example, it has been reported that conditional deletion of these factors from endothelial cells or MSCs may affect the secretion of SCF and CXCL12, which can further directly and indirectly impair HSC fates.1 We previously showed that ANGPTL2 can support HSC activities by directly binding to LILRB2 or PIRB receptors.13 Studies from other groups have also shown that ANGPTL2 is a positive regulator of osteoblast differentiation and bone metabolism.62 Because osteoblasts express several growth factors, including SCF, CXCL12, angiopoietin 1, and thrombopoietin, to enhance the maintenance of the HSC pool,63-67 it is possible that ANGPTL2 may regulate HSC fates in an indirect manner, which awaits further exploration.
ANGPTL2 also is known to be involved in angiogenesis, lipid metabolism, inflammation, and cancer development.68 In the current study, we found that endothelial cell–derived ANGPTL2 can serve as a critical growth factor involved in HSC stemness maintenance. ANGPTL2 can enhance PPARD expression to directly transactivate the G0s2 level to maintain the quiescent status of HSCs. PPARs represent a group of nuclear receptors that play pivotal roles in the regulation of nutrient and energy metabolism.69-71 PPARs can be activated by different ligands, such as unsaturated fatty acids.71,72 Interestingly, it seems that the PPARD pathway can also be activated by ANGPTL2 in HSCs, indicating that the lipid metabolic pathway may also be important for HSC stemness maintenance. However, how ANGPTL2/PIRB/SHP2/CAMK enhances PPARD/G0S2 expression remains unknown.
In summary, in this study, we showed that endothelial cell-derived ANGPTL2 in the BM niche plays an important role in sustaining HSC fates, including HSC repopulation capacity, quiescence, and localization in the vascular niche. These studies provide a unique angle for understanding how niche-specific factors determine the biological behavior of HSCs or other cell types of stem cells.
Acknowledgments
This work was supported by grants from the National Basic Research Program of China (2019YFA0801800, 2018YFA0107000), National Natural Science Foundation of China (81825001, 32030030, 31971052, 81570093, 81900147, 32100906, 82170175, 8200147), Innovative Group of the National Natural Science Foundation of China (81721004), Natural Science Foundation of Shanghai (20JC1410100, 20ZR1430900, 17ZR1415500, 20204Y0008), Shanghai Science and Technology Commission (19XD1422100), CAMS Innovation Fund for Medical Sciences (CIFMS) (2019-I2M-5-051), Shanghai Municipal Commission of Health and Family Planning (20204Y0008), Shanghai Frontiers Science Center of Cellular Homeostasis and Human Diseases, and the Fundamental Research Funds for the Central Universities.
Authorship
Contribution: Z.Y., W.Y., L.X., Y.Z., and J.Z. designed the experiments, performed the experiments, analyzed data, and wrote the paper; X.H., C.C., W.L., L.Z., and L.L. performed the experiments; and J.L. helped with the design of experiments and writing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Zhuo Yu, 280 South Chongqing Road, Shanghai 200025, China; e-mail: yuzhuo78@aliyun.com; Li Xie, 280 South Chongqing Road, Shanghai 200025, China; e-mail: xieli100@126.com; Yaping Zhang, 280 South Chongqing Rd, Shanghai 200025, China; e-mail: yapingzhang1117@126.com; and Junke Zheng, 280 South Chongqing Rd, Shanghai 200025, China; e-mail: zhengjunke@shsmu.edu.cn.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
REFERENCES
Author notes
Z.Y. and W.Y. contributed equally to this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal