In this issue of Blood, Liebers et al1 and Enßle et al2 address the gap in knowledge on vaccine-induced T-cell immune responses in patients with lymphoma or myeloma whose B-cell immunity is often therapeutically depleted.
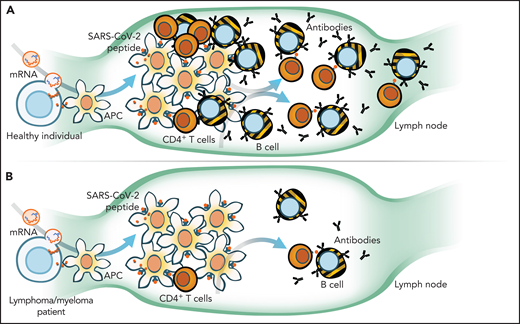
Natural or vaccine-acquired immunity to viruses, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), relies on the presence of antigen-presenting cells (APCs), T cells, and B cells that become plasma cells. The virus or vaccine-expressed antigen is picked up by APCs, which then traffic to lymph nodes where they interact with T and B cells (see figure, panel A) to generate an immune response that is amplified further by SARS-CoV-2–specific CD4+ T cells. Indeed, in healthy vaccinated individuals, the interplay between humoral (B) and cellular (T) responses results in “seroconversion” rates of >95% and the induction of higher neutralizing antibody titers than those who have been infected with SARS-CoV-2.3,4 In direct correlation with seroconversion status, ∼80% to 96% of vaccinated individuals mount potent SARS-CoV-2–specific T-cell responses.5
When compared with healthy individuals (A), Liebers et al and Enßle et al in this issue of Blood demonstrate that myeloma and lymphoma patients (B) have reduced total numbers and function of T cells and even fewer B cells to mount an immune response to COVID-19 vaccination. mRNA, messenger RNA. Illustration by Walter Mejia.
When compared with healthy individuals (A), Liebers et al and Enßle et al in this issue of Blood demonstrate that myeloma and lymphoma patients (B) have reduced total numbers and function of T cells and even fewer B cells to mount an immune response to COVID-19 vaccination. mRNA, messenger RNA. Illustration by Walter Mejia.
Patients with lymphoma and myeloma are among those at highest risk for hospitalization and death from COVID-196 but were excluded from pivotal trials establishing the effectiveness of COVID-19 vaccinations. Unfortunately, several studies have now shown that patients with myeloma and lymphoma mount abysmal humoral responses to COVID-19 vaccinations due in large part to their cancer-targeted therapies,7 which eliminate the very cells (B and plasma cells) required to generate protective antibodies. Thus, despite being “fully” vaccinated, patients and their oncologists may be disappointed by a lack of seroconversion and low neutralizing antibody titers. To date, there has been little focus on the protection offered by vaccine-induced SARS-CoV-2–specific T cells in such patients. Virus-targeted T cells are a formidable line of defense against viral diseases and should be relatively spared after B- or plasma-cell–directed therapies.
Not surprisingly, both reports confirm previously published findings that lymphoma and myeloma patients have poor SARS-CoV-2 antibody responses after vaccination (41% of lymphoma patients and 53% of those with myeloma seroconvert) and those that are actively receiving anti-CD20 or myeloma therapy have the worst seroconversion rates (9% and 19.5%, respectively).6,7 They go on to demonstrate that independent of humoral responses or ongoing therapies, a substantial proportion (34% to 58%) of vaccinated patients mount detectable SARS-CoV-2–specific T-cell responses. Although this rate is inferior to T-cell responses observed in their own healthy control group (71% responders in both studies) or those in previously published reports (86%),3 it is reassuring that even without seroconversion, the vaccine may induce protective T-cell immunity in patients. When looking for correlates of response, by contrast with healthy individuals, the emergence of SARS-CoV-2–specific T cells was not related to antibody status. However, in both studies, the quantity of total CD4+ T cells did correlate with antibody titers, although both were considerably lower than healthy controls.
Any perturbation of the adaptive immune axis, such as loss of APCs, as seen after a myeloablative allogeneic transplant, CD4 immunodeficiency (as in HIV infection), or therapies targeting B or plasma cells (such as anti-CD20/anti-CD38 antibodies, proteasome inhibition, or CD19/BCMA-directed chimeric antigen receptor T cells) will diminish vaccine responsiveness. Existing treatment approaches for lymphoma and myeloma are not “tumor-selective” and lead to large-scale ablation of normal B and plasma cells. Therefore, it is not surprising that antibody titers and seroconversion rates are significantly lower in the aftermath of recent anti-CD20 or combination myeloma therapy compared with those who are off therapy or healthy controls (panel B).
Liebers et al and Enßle et al extend our knowledge on COVID-19 immunity by describing the quantity and quality of SARS-CoV-2–specific T-cell responses in patients vs healthy individuals. Their key finding is that T-cell responses are more frequent than B-cell responses among patients even while on active therapy, although they remain inferior to healthy individuals. The latter is perhaps expected because most lymphoma and myeloma therapies, although primarily depleting B and plasma cells, also reduce total T-cell numbers (eg, corticosteroids) or narrow the T-cell repertoire (eg, cyclophosphamide). Specifically, both studies observed direct correlations between the frequency of CD4+ T cells and seroconversion status, demonstrating that even slight reductions in total CD4+ T cells as seen after anticancer treatments could greatly dampen the cell-mediated and humoral immune response to COVID-19.
Results presented in these 2 letters must be interpreted with the caveats that each paper presents single-center data, and each site had their own method to assess protective antibody titers. Although both studies used enzyme-linked immunospot assays to measure functional T-cell responses, each had a different cutoff threshold based on their laboratory standards that were disparate. Moreover, the healthy donor arms in each study were not statistically powered to demonstrate significant differences but supply a context upon which to base their conclusions. Although others have reported higher infection, hospitalization, and death rates in fully vaccinated patients with hematological malignancies,8 there remains the void of correlating individual B- and T-cell responses with clinical outcomes.
Thus, although most patients on active therapy will not seroconvert, some of those that do not may still mount T-cell responses. As with other SARS coronaviruses, memory T-cell responses are expected to be long lived and offer protection from severe disease or death, thus reaffirming our commitment to continue vaccinating our patients with the vaccines and dosing schemas we currently have. Finally, we look forward to results from ongoing trials of COVID-19 vaccinations in immunocompromised patients (NCT 04895982, OCTAVE-DUO, BMT-CTN 2101 trial, etc) that may inform timing and dosage of vaccination in relation to ongoing therapy or benefit from heterologous vaccinations for patients with lymphoma or myeloma.
Conflict-of-interest disclosure: P.D.L. has served on the advisory board for Karyopharm. L.C.H. has served on the advisory board for Incyte.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal