Abstract
Background: The Spanish Myeloma Group (GEM) demonstrated that post-transplant maintenance with thalidomide plus bortezomib was superior to thalidomide alone, although this combination was associated with a high rate of peripheral neuropathy. Lenalidomide is currently the standard postransplant maintenance treatment, and its association with ixazomib, an oral proteasome inhibitor that does not cause peripheral neuropathy, could be of interest.
Aim: To assess the potential benefit of postransplant maintenance therapy with Ixazomib /lenalidomide/dexamethasone (IRd) over lenalidomide/dexamethasone (Rd).
Patients: Patients who where at least with stable disease after the GEM2012menos65 trial that included VRD-GEM induction, autologous hematopoietic stem cell transplantation conditioned with either melphalan-200 or intravenous busulfan together with melphalan-140 and consolidation with VRD-GEM were randomized to receive maintenance treatment with IRd versus Rd. Each cycle lasted 28 days. Rd arm consisted of lenalidomide 15 mg/d on days 1-21 and 20mg of dexamethasone administered orally on days 1-4 and 9-12. In IRd arm ixazomib 4 mg/day on days 1, 8 and 15 of the cycle was added.
At two years, patients with negative MRD discontinued maintenance treatment. Patients with positive MRD continued with Rd for 3 additional years. In this case, 20 mg of dexamethasone was only administered on days 1-4 of the cycle. From November 24, 2014 to May 18, 2017, 161 patients were allocated to Rd arm and 171 to IRd arm. Patient characteristics at screening and prognostic factors such as ISS, cytogenetics and plasmacytomas, as well as response status, were similarly distributed in the two arms. Overall, 22% of the patients had high-risk cytogenetics [t(4;14), t(14;16) and/or 17p deletion]. MRD was analyzed by using next-generation flow at a sensitivity level of 3x10 -6.
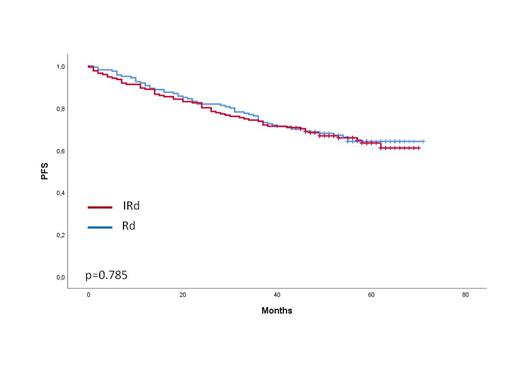
Results: After a median follow-up of 56 months, there was no difference in PFS between the two maintenance arms (median not reached, PFS at 5 years: 62% vs. 63% with IRd and Rd, respectively, p=0.785) (figure 1). In the overall series, there were no significant differences in PFS or OS among patients with standard (SR) or high-risk (HR) cytogenetics. Median PFS had not been reached in patients with SR in both arms (PFS at 5 years: 66% with IRD vs. 62% with Rd, p=0.633). In patients with HR the median PFS was 62 months with IRd vs. not reached with Rd (p=0.636). No significant differences across subgroups (ISS, conditioning, cytogenetics or MRD) were observed between IRd and Rd.
Negative MRD at screening overcomes the bad prognosis of cytogenetics (PFS at 5 years 78% for SR and 80% for HR; OS at 5 years was 90% for both, SR and HR). Patients with SR who had negative MRD at screening had a significantly longer PFS (PFS at 5 years 78% vs. 50%, p<0.0001) and a significantly longer OS (OS at 5 years; 90% vs. 80%, p=0.042) than patients with positive MRD. Patients with HR and positive MRD at screening had a dismal prognosis compared with patients with negative MRD (median PFS of 37 months vs. not reached, p<0.0001; OS at 5 years:70% vs. 90%, p=0.03). Patients with negative MRD at 2 years discontinued therapy with a low impact on relapse while patients with positive MRD had a higher rate of relapse despite the fact that they were receiving maintenance for 3 additional years. 73% and 65% of the patients were in sCR/CR at the time of screening and the sCR/CR rate was upgraded by 16% with IRd and 19% with Rd. Grade 3-4 neutropenia was similar in both arms (IRd: 37%, Rd:39%). However, grade 3-4 thrombocytopenia (16% vs. 7%, p=0.01) and grade 3-4 gastrointestinal toxicity (15% vs. 2%, p<0.0001) was significantly higher with IRd. 31% of the patients needed dose reductions of ixazomib and 9% discontinued the drug. There was a trend to a higher need of dose reductions of lenalidomide with IRd (IRd: 29% vs. Rd: 21%) while the need for dose reduction of dexamethasone was identical in both arms (21%).
Conclusions: Maintenance therapy with lenalidomide and dexamethasone in patients homogeneously treated with VRD-GEM induction, ASCT and VRD-GEM consolidation resulted in a long PFS of 63% at 5 years from the start of maintenance. The addition of ixazomib did not result in a PFS benefit. This could be partially explained by the higher toxicity leading to dose reductions or discontinuation of ixazomib in the IRd arm.
Rosinol: Janssen, Celgene, Amgen and Takeda: Honoraria. Oriol: Oncopeptides: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS/Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Jarque: AbbVie: Consultancy, Speakers Bureau; Alexion: Consultancy, Speakers Bureau; Amgen: Consultancy, Speakers Bureau; Apellis: Consultancy; AstraZeneca: Consultancy, Speakers Bureau; Beigene: Consultancy; CellTrion: Consultancy; Eusa: Consultancy; Gilead: Consultancy, Speakers Bureau; Grifols: Consultancy; Incyte: Consultancy; Janssen: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Pfizer: Consultancy, Speakers Bureau; Roche: Consultancy, Speakers Bureau; Servier: Speakers Bureau; Shionogi: Consultancy; Sobi: Consultancy; Takeda: Consultancy, Speakers Bureau. Moraleda: Pfizer: Other: Educational Grants, Research Funding; Sanofi: Other: Educational Grants, Research Funding; MSD: Other: Educational Grants, Research Funding; ROCHE: Consultancy, Honoraria, Other: Educational Grants, Research Funding; Takeda: Consultancy, Honoraria, Other: Educational Grants, Research Funding; Sandoz: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Other: Educational Grants, Research Funding; Gilead: Consultancy, Honoraria, Other: Educational Grants, Research Funding; Jazz Pharmaceuticals: Consultancy, Honoraria, Other: Educational Grants, Research Funding; NovoNordisk: Other: Educational Grants, Research Funding; Janssen: Other: Educational Grants, Research Funding; Celgene: Other: Educational Grants, Research Funding; Amgen: Other: Educational Grants, Research Funding. Sureda: Mundipharma: Consultancy; Bluebird: Membership on an entity's Board of Directors or advisory committees; Roche: Other: Support for attending meetings and/or travel; GSK: Consultancy, Honoraria, Speakers Bureau; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kite, a Gilead Company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; MSD: Consultancy, Honoraria, Speakers Bureau; BMS/Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings and/or travel, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings and/or travel, Research Funding, Speakers Bureau. Martínez-López: Roche, Novartis, Incyte, Astellas, BMS: Research Funding; Janssen, BMS, Novartis, Incyte, Roche, GSK, Pfizer: Consultancy. De Arriba: BMS-Celgene: Consultancy, Honoraria, Speakers Bureau; Glaxo Smith Kline: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Speakers Bureau. Mateos: Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene - Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bluebird bio: Honoraria; GSK: Honoraria; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sea-Gen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Regeneron: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria; Oncopeptides: Honoraria; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees. San-Miguel: AbbVie, Amgen, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Karyopharm, Merck Sharpe & Dohme, Novartis, Regeneron, Roche, Sanofi, SecuraBio, and Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees. Lahuerta: Celgene: Other: Travel accomodations and expenses; Celgene, Takeda, Amgen, Janssen and Sanofi: Consultancy. Bladé Creixenti: Janssen, Celgene, Takeda, Amgen and Oncopeptides: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal