TO THE EDITOR:
Tyrosine kinase inhibitors (TKIs) have profoundly affected the outcome of Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ ALL).1-7 In the recent front-line GIMEMA LAL2116 protocol for adult patients with Ph+ ALL, with no upper age limit, induction of dasatinib was followed by a consolidation with 2 to 5 cycles of the bispecific monoclonal antibody blinatumomab.8,9
With this targeted and immunotherapeutic induction-consolidation strategy devoid of systemic chemotherapy, a complete hematologic response was observed in 98.4% of cases and a molecular response, identified by the number of BCR/ABL1 copies in 60% of patients after 2 cycles of blinatumomab, the primary end point of the study.10 Molecular responses further increased after additional blinatumomab cycles. Overall survival and disease-free survival were 95.2% and 88.3%, respectively, at a median follow-up of 18 months.10 We hereby report extensive in vivo monitoring of the host immune modulation that occurs after repeated cycles of blinatumomab.
All patients were enrolled in the GIMEMA LAL2116 protocol, and all provided written informed consent. The overall data are summarized in supplemental Table 1 (available on the Blood Web site). Time point 0 (T0) represents the absolute cell counts or percentages of cells at the initiation of blinatumomab treatment, and T1 to T5 represent those measurements at the end of treatment cycles 1 to 5. In the 43 patients evaluated, we observed an increase in peripheral lymphocytes that became significant after cycle 3. The median count of lymphocytes was 1.400 × 109/L at T0 vs 1.660 × 109/L at T1, 1.800 × 109/L at T2, 2.120 × 109/L at T3 (P = .008), 2.080 × 109/L at T4 (P = .005), and 1.920 × 109/L at T5 (P = .039) (supplemental Figure 1A). These results were further confirmed by the increase in CD3+ cells: 0.879 × 109/L at T0 vs 1.199 × 109/L at T1, 0.997 × 109/L at T2, 1.222 × 109/L (P = .016) at T3, 1.255 × 109/L (P = .037) at T4, and 1.257 × 109/L at T5 (supplemental Figure 1B). With regard to the T-lymphocyte subset distribution, no difference was noted in the percentage of CD3/CD4+ lymphocytes (Figure 1A), whereas a progressive and significant increase in the percentage of CD3/CD8+ (Figure 1B) lymphocytes was recorded beginning with the first blinatumomab cycle: 22.2% at T0 vs 25.4% at T1 (P = .028), 25.1% at T2 (P = .040), 27.1% at T3 (P = .026), 29.2% at T4 (P = .023), and 29.4% at T5 (P = .011).
Median percentages of CD3+CD4+and CD3+CD8+ lymphocytes and the median numbers of CD8+CD45RO+ and CD8+CD45RA+ lymphocytes in 43 patients with Ph+ ALL enrolled in the GIMEMA LAL2116 D-ALBA protocol during blinatumomab administration. PB samples were tested for the percentage of CD3+CD4+ (A) and CD3+CD8+ (B) lymphocytes and the median count of PB CD8+CD45RO+ (C) and CD8+CD45RA+ (D) lymphocytes. Samples were collected before starting blinatumomab (T0) and after the first (T1), second (T2), third (T3), fourth (T4), and fifth (T5) cycles. Box plots represent the median (line), interquartile range (box), and minimum and maximum values (whiskers). °Outliers. *P < .05; **P < .01. PB, peripheral blood.
Median percentages of CD3+CD4+and CD3+CD8+ lymphocytes and the median numbers of CD8+CD45RO+ and CD8+CD45RA+ lymphocytes in 43 patients with Ph+ ALL enrolled in the GIMEMA LAL2116 D-ALBA protocol during blinatumomab administration. PB samples were tested for the percentage of CD3+CD4+ (A) and CD3+CD8+ (B) lymphocytes and the median count of PB CD8+CD45RO+ (C) and CD8+CD45RA+ (D) lymphocytes. Samples were collected before starting blinatumomab (T0) and after the first (T1), second (T2), third (T3), fourth (T4), and fifth (T5) cycles. Box plots represent the median (line), interquartile range (box), and minimum and maximum values (whiskers). °Outliers. *P < .05; **P < .01. PB, peripheral blood.
A significant increase in CD.8/CD45RO+ lymphocytes was documented after all blinatumomab cycles: the median count was 0.0866 × 109/L at T0 vs 0.1791 × 109/L at T1 (P = .010), 0.1988 × 109/L at T2 (P = .027), 0.1775 × 109/L at T3 (P = .003), 0.2075 × 109/L at T4 (P = .005), and 0.2215 × 109/L at T5 (P = .014) (Figure 1C). The CD8/CD45RA+ lymphocyte population increased significantly from the third blinatumomab cycle: the median count at T0 was 0.1997 × 109/L vs 0.3120 × 109/L at T1, 0.2257 × 109/L at T2, 0.3423 × 109/L at T3 (P = .010), 0.3770 × 109/L at T4 (P = .008), and 0.371 × 109/L at T5 (P = .005) (Figure 1D). CD4/CD45RO+ lymphocytes also increased significantly: the median count was 0.2541 × 109/L at T0 vs 0.3165 × 109/L at T1, 0.2731 × 109/L at T2, 0.3514 × 109/L at T3 (P = .020), 0.3968 × 109/L at T4 (P = .037), and, 0.3020 × 109/L at T5. No change in the CD4/CD45RA+ lymphocyte population was observed during treatment.
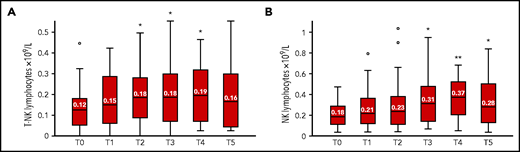
Both the T-NK (CD3+/CD56+/−CD16+/−) and NK (CD3−/CD56+/−/ CD16+/−) lymphocyte populations increased significantly during blinatumomab treatment. The median counts at T0 were, respectively, 0.1272 × 109/L and 0.1883 × 109/L vs 0.1521 × 109/L and 0.2175 × 109/L at T1, 0.1862 × 109/L (P = .013) and 0.2382 × 109/L at T2, 0.1866 × 109/L (P = .011) and 0.3148 × 109/L (P = .031) at T3, 0.1958 × 109/L (P = .048) and 0.3771 × 109/L (P = .007) at T4, and 0.1662 × 109/L and 0.2828 × 109/L (P = .039) at T5 (Figure 2).
Median number of T-NK and NK lymphocytes in 43 patients with Ph+ ALL enrolled in the GIMEMA LAL2116 D-ALBA protocol during blinatumomab administration. PB samples were tested for T-NK (A) and NK (B) lymphocytes before starting blinatumomab (T0) and after the first (T1), second (T2), third (T3), fourth (T4), and fifth (T5) cycle. Box plots represent the median (line), interquartile range (top and bottom of box), and minimum and maximum values (whiskers). °Outliers. *P < .05; **P < .01. PB, peripheral blood.
Median number of T-NK and NK lymphocytes in 43 patients with Ph+ ALL enrolled in the GIMEMA LAL2116 D-ALBA protocol during blinatumomab administration. PB samples were tested for T-NK (A) and NK (B) lymphocytes before starting blinatumomab (T0) and after the first (T1), second (T2), third (T3), fourth (T4), and fifth (T5) cycle. Box plots represent the median (line), interquartile range (top and bottom of box), and minimum and maximum values (whiskers). °Outliers. *P < .05; **P < .01. PB, peripheral blood.
Regulatory T (Treg) cells in the CD4+ population showed an overall decrease after repeated blinatumomab cycles. The percentage of CD3+/CD4+/CD25++/FOXP3+ lymphocytes (range) was 3.90% (2.2-9.6) at T0 vs 5.35% (2.88-9.78) at T1, 6.35% (3-10.10) at T2, 4.80% (1.9-7.75) at T3, 2.90% (1.70-4.15; P = .008) at T4, and 3.90% (2.28-5.23) at T5.
A correlation was determined between age and the in vivo modulation of the host immunocompetent populations after administration of blinatumomab, and no significant differences were observed in patients younger or older than 55 years of age (supplemental Table 2). Likewise, no differences were observed between molecular responders and nonresponders at the end of the dasatinib induction. So far, no correlation has been recorded between the immune modulation and the degree of molecular response after repeated cycles of blinatumomab, which was reached in ≈50% of patients.10 This rate of response holds up at a more prolonged follow-up.11 Nor could a correlation be found with the clinical outcome, because few relapses have so far been observed (4 in 43 patients).
The clinical use of blinatumomab has been aimed primarily at eliminating CD19+ leukemic cells, and the data reported so far refer mainly to its therapeutic efficacy on the neoplastic clone. The impact of blinatumomab on the host immune system has been poorly investigated. In this study of adult patients with Ph+ ALL, blinatumomab exerted a marked in vivo modulation of the T, T-NK, NK, and Treg lymphocyte populations. The increase in T-lymphocytes, particularly in CD3/CD8+ lymphocytes capable of controlling tumor cells,12 suggests that blinatumomab acts, not only against the leukemic pop…ulation but also in inducing a marked immune response. This finding is further documented by the increase in CD3/CD8/CD45RA+ cells and, in particular, in the CD3/CD8/CD45RO+ population, which is known to characterize lymphocytes that have a specific antitumor effect.13 We also observed a significant increase in T-NK and NK cells during treatment with blinatumomab. The increase in T-NK cells was probably related to the stimulus of the antibody on the CD3+ population. The NK-cell expansion may also be induced by stimulated T cells. In ALL, the T-NK-, and NK-cell populations play a role in the control of neoplasms.14-16 It should be recalled that the prolonged administration of dasatinib has a clinically significant effect on immune effector cells, resulting in a rapid lymphocyte mobilization, activation, and transmigration17 and an in vivo expansion of NK cells.18,19 Our data, obtained in patients in complete response at the initiation of blinatumomab treatment, expand on an earlier observation of an increase in CD3+ T lymphocytes reported in patients with relapsed/refractory disease with a long-term response to blinatumomab.20
Treg cells are major drivers of tumor evasion21,22 and can limit the therapeutic efficacy of blinatumomab.23 In our study, treatment with blinatumomab did not amplify Treg cells; on the contrary, we documented a significant reduction in this population at T4. Furthermore, the Treg population revealed a CD62L+/CD69− antigen profile that is associated with the absence of inhibitory effect on the proliferation of immunocompetent T lymphocytes.24 In fact, Treg cells can induce a rapid release of interleukin 10 that decreases the proliferation of CD8 lymphocytes. The consequence of this phenomenon is a reduction in the antitumor effects exerted by CD8+ T cells.23 It should also be emphasized that, in our series, even at T0, the percentage of Tregs was below the cutoff (<8.5%) reported by Duell et al23 to be associated with the therapeutic response to blinatumomab.
A recent preclinical study25 seems to contradict our results. It reported that the combination of TKIs, in particular dasatinib and ponatinib, with blinatumomab would affect the antitumor effect of the antibody by inhibiting the proliferation of T lymphocytes and the production of interferon γ. The study was performed in vitro and therefore could not mimic what occurs in vivo, considering, above all, that blinatumomab has a very short half-life. Indeed, the drug is administered in vivo by continuous infusion, to maintain its biologic activity for the longest possible time.10,26 These conditions are impossible to reproduce in vitro.
In summary, our study documented that front-line treatment of Ph+ ALL in adults with a targeted (dasatinib) and immunotherapeutic (blinatumomab) strategy, without systemic chemotherapy, in addition to reducing Treg cells, can promote a marked proliferation of immunocompetent T, T-NK, and NK cells, particularly after repeated cycles of blinatumomab. This in vivo sustained modulation of the host immune system is likely to contribute to the success of this chemotherapy-free protocol and of the high rates of molecular response that increase further with additional cycles of blinatumomab. The evidence that the modulation of immunocompetent cells also occurs in patients >55 years of age is particularly relevant, given that Ph+ ALL occurs in ∼50% of cases of B-lineage ALL in the elderly.27 Elderly patients with Ph+ ALL are in the age category most likely to benefit from chemotherapy- and transplant-free strategies.
Acknowledgment
This work was supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC), Metastases 5 × 1000 Special Program, No. 21198, Milan, Italy (R.F.).
Authorship
Contribution: M.C.P. designed the study, performed the laboratory work, analyzed the data, and contributed to writing the manuscript; G.R., N.P., M.S.d.P., and P.M. performed the laboratory work and analyzed the data; M.V., A.V., R.B., M.A., G.G., A.R., and S.C. took care of the patients and reviewed the manuscript; A.G. and R.F. designed and supervised the study, analyzed the data, and wrote the manuscript; and all authors contributed to revision the revision and approved the final submission of the manuscript.
Conflict-of-interest disclosure: Authors have participated on advisory boards or speaker’s bureau for the following companies: M.V. for Iqvia and Amgen; R.B. for Amgen, Pfizer, Jazz, Novartis, Incyte, and Servier; G.G. for Janssen, AstraZeneca, Abbvie, BeiGene, and Bayer; A.R. for Amgen, Pfizer, Sanofi, Novartis, Kite-Gilead, Celgene-BMS, Jazz, and Omeros; S.C. for Amgen, Incyte, Pfizer, and Novartis; and R.F. for Janssen, AbbVie, Amgen, Novartis, Incyte, Pfizer, and Servier. The remaining authors declare no competing financial interests.
Correspondence: Robin Foà, Hematology, Department of Translational and Precision Medicine, Sapienza University, Via Benevento 6, 00161 Rome, Italy; e-mail: rfoa@bce.uniroma1.it.
Original data are available in response to e-mail requests to the corresponding author. A very small set of data on a much smaller series of patients is reported in Foà R et al.10
The online version of this article contains a data supplement.
REFERENCES
Author notes
A.G. and R.F. contributed equally to this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal