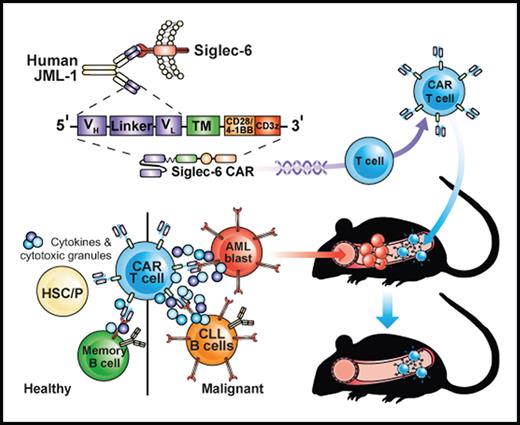
Key Points
Siglec-6 CAR T cells eliminate AML blasts (including AML stem cells) and induce complete remission from AML in a xenograft mouse model.
Normal HSPCs are Siglec-6 negative and are not affected by Siglec-6 CAR T cells in preclinical analyses.
Abstract
Acute myeloid leukemia (AML) is an attractive entity for the development of chimeric antigen receptor (CAR) T-cell immunotherapy because AML blasts are susceptible to T-cell–mediated elimination. Here, we introduce sialic acid–binding immunoglobulin-like lectin 6 (Siglec-6) as a novel target for CAR T cells in AML. We designed a Siglec-6–specific CAR with a targeting domain derived from the human monoclonal antibody JML-1. We found that Siglec-6 is commonly expressed on AML cell lines and primary AML blasts, including the subpopulation of AML stem cells. Treatment with Siglec-6 CAR T cells confers specific antileukemia reactivity that correlates with Siglec-6 expression in preclinical models, including induction of complete remission in a xenograft AML model in immunodeficient mice (NSG/U937). In addition, we confirmed Siglec-6 expression on transformed B cells in chronic lymphocytic leukemia (CLL), and specific anti-CLL reactivity of Siglec-6 CAR T cells in vitro. Of particular interest, we found that Siglec-6 is not detectable on normal hematopoietic stem and progenitor cells (HSPCs) and that treatment with Siglec-6 CAR T cells does not affect their viability and lineage differentiation in colony-formation assays. These data suggest that Siglec-6 CAR T-cell therapy may be used to effectively treat AML without the need for subsequent allogeneic hematopoietic stem cell transplantation. In mature normal hematopoietic cells, we detected Siglec-6 in a proportion of memory (and naïve) B cells and basophilic granulocytes, suggesting the potential for limited on-target/off-tumor reactivity. The lack of expression of Siglec-6 on normal HSPCs is a key to differentiating it from other Siglec family members (eg, Siglec-3 [CD33]) and other CAR target antigens (eg, CD123) that are under investigation in AML, and it warrants the clinical investigation of Siglec-6 CAR T-cell therapy.
Introduction
Sialic acid–binding immunoglobulin-like lectins (Siglecs) form a superfamily of cell surface receptors that are expressed in the hematopoietic system and are associated with inhibitory signaling in human immune cells.1 Members of the Siglec superfamily are being investigated as target antigens for chimeric antigen receptor (CAR) T-cell immunotherapy, including Siglec-2 (CD22) in B-cell acute lymphoblastic leukemia (B-ALL) and Siglec-3 (CD33) in acute myeloid leukemia (AML). CD22-specific CAR T cells have demonstrated clinical activity and re-induced remissions in patients with B-ALL who relapsed after treatment with CD19 CAR T cells.2 CD33-specific CAR T cells have been shown to be effective in preclinical models of AML and have advanced to evaluation in clinical trials because of the prevalent expression of CD33 on AML blasts.3 However, there are also challenges with targeting CD33, including the predicted ablation of normal hematopoiesis as a result of CD33 expression on normal hematopoietic stem and progenitor cells (HSPCs), and the lower expression of CD33 on AML stem cells compared with bulk AML cells.4,5 There are other candidate antigens for CAR T cells in AML, including CD123 and FLT3, that share the challenge of being expressed on normal HSPCs,6,7 as well as CLL-1, CD7, and folate receptor β that are nonuniformly expressed on AML blasts and have low-level expression on AML stem cells.5,8-10 Accordingly, there is a continuous effort to identify novel target antigens and corresponding CAR-binding domains with potential therapeutic utility in AML.
In this study, we evaluated Siglec-6 as a novel target antigen for CAR T cells in AML. Siglec-6 has been identified as the unique target antigen of a human monoclonal antibody (mAb) JML-1, which has been isolated from the post allogeneic hematopoietic stem cell transplantation (allo-HSCT) antibody repertoire in a patient with B-cell chronic lymphocytic leukemia (B-CLL).11,12 Siglec-6 consists of 3 extracellular immunoglobulin (Ig) domains and 2 intracellular immunoreceptor tyrosine-based inhibition motifs (ITIMs), closely resembling the molecular structure of Siglec-3 (CD33).1,13,14 Because of the ITIMs, Siglec-6 is thought to serve as a regulator of activating pathways in immune cells, similar to other CD33-related Siglecs.1,13,14 In normal cells and tissues, Siglec-6 expression has been reported in B cells,11,13 mast cells,15,16 and placenta.13,17 However, unlike other Siglec family members, Siglec-6 is thought to be absent on T cells, natural killer (NK) cells, neutrophils, macrophages, and monocytes.13 In malignant cells and tissues, Siglec-6 expression has been reported in CLL, mucosa-associated lymphoid tissue lymphoma,18 clonal mast cell diseases,19 and thyroid cancer.20 Here, we analyzed expression of Siglec-6 in AML cell lines, primary AML blasts, and normal developing and mature hematopoietic cells. The data show that Siglec-6 is commonly expressed in AML and is absent on normal HSPCs. We also show that T cells that were gene engineered to express a Siglec-6 CAR that consists of the variable light (VL) and variable heavy (VH) chains of the JML-1 mAb confer specific and potent anti-leukemia functions against AML and CLL in preclinical models in vitro and in vivo.
Materials and methods
Siglec-6 expression analysis by flow cytometry
Siglec-6 (CD327) expression was analyzed using allophycocyanin-conjugated mouse anti-human Siglec-6 mAb (clone 767329, R&D Systems, Minneapolis, MN) (primary AML) or REAfinity anti-human Siglec-6 (clone REA852, Miltenyi-Biotec, Bergisch-Gladbach, Germany) (all other cell types), and corresponding isotype controls (mouse IgG1 or REA control antibody [S], human IgG1). In brief, 1 × 106 cells were washed, resuspended in 100 μL phosphate-buffered saline and 0.5% fetal calf serum, and stained with anti-Siglec-6 mAb or isotype control for 30 minutes at 4°C. Before staining with clone 767329, Fc-blocking with human IgG (Jackson ImmunoResearch, West Grove, PA) was performed at 4°C for 20 minutes. 7-Amino-actinomycin D (7-AAD) was used to discriminate live and dead cells.
In vivo experiments with U937 xenograft model
The Institutional Animal Care and Use Committee (University of Würzburg) approved all in vivo experiments. NOD.Cg-PrkdcscidIl2rgtm1Wj/SzJ (NSG) mice (females, 6 to 8 weeks old) were purchased from Charles River (Sulzfeld, Germany) and inoculated with 2 × 106 firefly luciferase (FLUC)-green fluorescent protein (GFP+) U937 cells or, alternatively, 1 × 106 MOLM-13 cells. Mice were randomly allocated to treatment groups and received 5 × 106 T cells (ie, 2.5 × 106 CD4+ and 2.5 × 106 CD8+ in 200 μL of phosphate-buffered saline and 0.5% fetal calf serum) at the indicated time points. Bioluminescence imaging (BLI) was performed once per week after intraperitoneal administration of D-luciferin substrate (0.3 mg per g of body weight) (Biosynth, Staad, Switzerland) using an IVIS Lumina imaging system (PerkinElmer, Waltham, MA). Bioluminescence images were analyzed by using Living Image software (PerkinElmer).
Statistical analyses
Statistical analyses were performed using Prism software v6.07 (GraphPad, La Jolla, CA). Student t test (paired or unpaired) was used to analyze data obtained in both in vitro and in vivo experiments. We used linear regression analysis to assess correlation between Siglec-6 expression on target cells and Siglec-6 CAR T-cell–mediated cytotoxicity. The differences in survival observed in the in vivo experiments were analyzed by using a Mantel-Cox log rank test. Values of P < .05 were considered statistically significant.
Results
Siglec-6 CAR T cells recognize and eliminate AML cell lines
We generated a single-chain variable fragment (scFv) that contained the VH and VL chains of the anti-Siglec-6 mAb JML-1 and derived 2 analogous Siglec-6 CARs with CD28 vs 4-1BB costimulation (supplemental Figure 1A). The growth kinetics and yield of T cells expressing the Siglec-6 CAR at the end of manufacturing were similar to those of untransduced (UTD) T cells (supplemental Figure 1B-E). We assessed Siglec-6 expression in a set of AML cell lines and found a varying degree of Siglec-6 expression by flow cytometry (normalized mean fluorescence intensity [NMFI] range, 8.22-0.76): high on U937 and TF-1 AML cells, intermediate/low on MV4;11 and MOLM-13 AML cells, and very low/undetectable on K562 and Kasumi-1 cells. The hierarchy in Siglec-6 expression between these AML cell lines was confirmed by quantitative polymerase chain reaction (qPCR) and by super-resolution microscopy (direct stochastic optical reconstruction microscopy [dSTORM]) (Figure 1A-D; supplemental Figure 2A). To provide a matched positive control cell line, we engineered K562 cells to stably express Siglec-6 (K562/Siglec-6; supplemental Figure 2A).
Siglec-6 is expressed by AML cell lines and Siglec-6 CAR T cells recognize and eliminate AML cells in vitro. (A) Flow cytometric analysis of Siglec-6 expression on AML cell lines (U937, MV4;11, MOLM13, and Kasumi-1). Histograms show staining with anti-Siglec-6 mAb (red) and isotype control antibody (blue). Inset numbers indicate the NMFI calculated by dividing MFI obtained after staining with anti-Siglec-6 mAb by MFI of isotype control. (B) Detection of Siglec-6 expression on AML cell lines using dSTORM super-resolution microscopy. Each representative image depicts Siglec-6 molecules on the basal membrane of a cell. Scale bars represent 5 µm. (C) Real-time qPCR was performed to assess Siglec-6 messenger RNA (mRNA) transcript levels in AML cell lines. Data are normalized to MOLM-13 Siglec-6 mRNA transcript values. (D) Quantification of Siglec-6 expression on AML cell lines by dSTORM super-resolution microscopy. Each point represents a cell. (C-D) Data are representative of 3 independent experiments. (E) Specific cytolytic activity of CD8+ Siglec-6_28z CAR, Siglec-6_BBz CAR, CD19_BBz CAR, and UTD T cells against AML cell lines in a luminescence-based assay (4 hours of coculture). The assay was performed in triplicate wells with 5000 target cells per well. Data are presented as mean ± standard deviation (SD). (F) Enzyme-linked immunosorbent assay (ELISA) was performed to detect interferon-γ (IFN-γ) and interleukin-2 (IL-2) in supernatant obtained after 24-hour coculture of CD8+ Siglec-6_28z CAR, Siglec-6_BBz CAR, or UTD T cells with target cells. T cells and target cells were seeded at a 2:1 E:T ratio in triplicate wells. Data are represented as mean concentration ± SD. (G) Proliferation of CD8+ Siglec-6_28z CAR and Siglec-6_BBz CAR T cells examined by carboxyfluorescein diacetate succinimidyl ester (CFSE) dye dilution after 72 hours of coculture with target cells. The assay was performed in triplicate wells at a (2:1) E:T ratio. Histograms show proliferation of live (7-amino-actinomycin D [7-AAD–]) T cells. No exogenous cytokines were added. Data shown in panels E-G are representative of results obtained with CAR and control T-cell lines prepared from at least 5 healthy donors. (H) Correlation between specific lysis by CD8+ Siglec-6_BBz CAR T cells (after 4-hour coculture; 2.5:1 E:T ratio) and Siglec-6 normalized expression on AML cell lines. Simple linear correlation was calculated (R2 = 0.91; P = .0009). test **P < .01; ***P < .001, Student t test. ns, not significant.
Siglec-6 is expressed by AML cell lines and Siglec-6 CAR T cells recognize and eliminate AML cells in vitro. (A) Flow cytometric analysis of Siglec-6 expression on AML cell lines (U937, MV4;11, MOLM13, and Kasumi-1). Histograms show staining with anti-Siglec-6 mAb (red) and isotype control antibody (blue). Inset numbers indicate the NMFI calculated by dividing MFI obtained after staining with anti-Siglec-6 mAb by MFI of isotype control. (B) Detection of Siglec-6 expression on AML cell lines using dSTORM super-resolution microscopy. Each representative image depicts Siglec-6 molecules on the basal membrane of a cell. Scale bars represent 5 µm. (C) Real-time qPCR was performed to assess Siglec-6 messenger RNA (mRNA) transcript levels in AML cell lines. Data are normalized to MOLM-13 Siglec-6 mRNA transcript values. (D) Quantification of Siglec-6 expression on AML cell lines by dSTORM super-resolution microscopy. Each point represents a cell. (C-D) Data are representative of 3 independent experiments. (E) Specific cytolytic activity of CD8+ Siglec-6_28z CAR, Siglec-6_BBz CAR, CD19_BBz CAR, and UTD T cells against AML cell lines in a luminescence-based assay (4 hours of coculture). The assay was performed in triplicate wells with 5000 target cells per well. Data are presented as mean ± standard deviation (SD). (F) Enzyme-linked immunosorbent assay (ELISA) was performed to detect interferon-γ (IFN-γ) and interleukin-2 (IL-2) in supernatant obtained after 24-hour coculture of CD8+ Siglec-6_28z CAR, Siglec-6_BBz CAR, or UTD T cells with target cells. T cells and target cells were seeded at a 2:1 E:T ratio in triplicate wells. Data are represented as mean concentration ± SD. (G) Proliferation of CD8+ Siglec-6_28z CAR and Siglec-6_BBz CAR T cells examined by carboxyfluorescein diacetate succinimidyl ester (CFSE) dye dilution after 72 hours of coculture with target cells. The assay was performed in triplicate wells at a (2:1) E:T ratio. Histograms show proliferation of live (7-amino-actinomycin D [7-AAD–]) T cells. No exogenous cytokines were added. Data shown in panels E-G are representative of results obtained with CAR and control T-cell lines prepared from at least 5 healthy donors. (H) Correlation between specific lysis by CD8+ Siglec-6_BBz CAR T cells (after 4-hour coculture; 2.5:1 E:T ratio) and Siglec-6 normalized expression on AML cell lines. Simple linear correlation was calculated (R2 = 0.91; P = .0009). test **P < .01; ***P < .001, Student t test. ns, not significant.
Next, we sought to determine the specificity of Siglec-6 CAR T cells for AML target cells. We confirmed that CD8+ Siglec-6 CAR T cells conferred specific cytolytic activity against U937, MV4;11, and MOLM-13 AML cell lines and K562/Siglec-6 cells with a dose-response relationship from high to low effector-to-target (E:T) ratios (range, 10:1 to 1:4). We used UTD T cells as a reference and CD19 CAR T cells as comparators for specific target cell lysis (Figure 1E; supplemental Figures 2B and 3A-B). We observed productive cytokine secretion and proliferation with CD8+ and CD4+ Siglec-6 CAR T cells after stimulation with the U937, MV4;11, and MOLM-13 AML cell lines and K562/Siglec-6 cells (Figure 1F-G; supplemental Figures 2C-E and 3C-D). We observed no specific reactivity of Siglec-6 CAR T cells against K562 and Kasumi-1 cells, consistent with no observed Siglec-6 expression by qPCR and dSTORM (Figure 1B-G; supplemental Figure 2B-E). To add additional confidence to the expression of Siglec-6 on the surface of AML cell lines and the specificity of the Siglec-6 CAR, we generated isogenic U937, MV4;11, and MOLM-13 Siglec-6 knockout cell lines by CRISPR/Cas9 gene editing and confirmed that recognition by Siglec-6 CAR T cells was abolished (supplemental Figure 4A-C). Linear regression analysis showed that the rapidity and extent of cytolysis within the 4-hour assay period correlated with Siglec-6 expression on AML cell lines (R2 = 0.91; P = .0009) (Figure 1H). Overall, the cytolytic activity of CD8+ T cells expressing the Siglec-6 CAR constructs with CD28 vs 4-1BB co-stimulatory domains was similarly potent. Taken together, the data show that Siglec-6 is a prevalent target on AML cell lines and confers specific recognition by T cells expressing a Siglec-6 CAR.
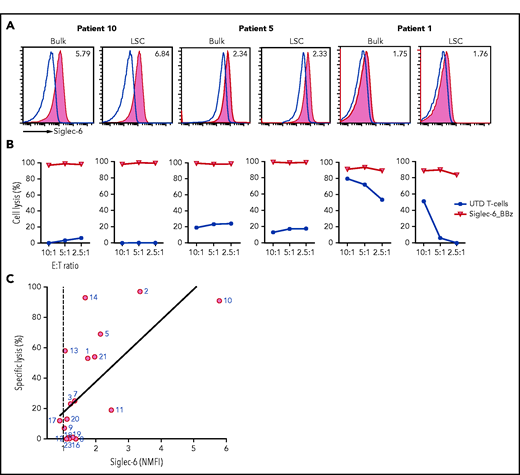
Siglec-6 is expressed on primary AML blasts, including AML stem cells
We evaluated Siglec-6 expression on primary AML blasts from a series of 20 consecutive adult patients with AML. This series comprised patients with primary and secondary AML and newly diagnosed and previously treated (relapsed/refractory) AML with distinct molecular and genetic subtypes (Table 1). To assess the potential applicability of Siglec-6 CAR T cells in this cohort, we performed a 2-criteria assessment using flow cytometric analysis (read-out: NMFI of Siglec-6 expression threshold, ≥1.1) and recognition by Siglec-6 CAR T cells (read-out: cytolysis of AML blasts threshold, ≥30%). By using flow cytometry, we detected varying degrees of Siglec-6 expression on AML blasts and ranked patients according to the NMFI (range, 5.79-0.89) (Table 1; supplemental Figure 5A-D). In the cytolysis assay, we detected rapid and potent elimination of Siglec-6+ AML blasts (Figure 2A-B). Of interest, we also observed cytolysis in AML samples that were below the expression threshold by NMFI. These data suggest that detection by flow cytometry with the directly conjugated mAb in our standard protocol is not sensitive enough to detect very-low-level Siglec-6 expression that might be sufficient to trigger the Siglec-6 CAR. Indeed, using a biotinylated anti–Siglec-6 mAb and amplification with streptavidin-phycoerythrin, enabled better detection of low-level Siglec-6 expression (supplemental Figure 6). In each of the 20 patients, at least 1 of the 2 criteria were fulfilled; in 15 patients, both criteria were fulfilled (Table 1). Similar to the observation with AML cell lines, linear regression analysis showed a correlation between Siglec-6 expression and cytolysis of AML blasts by Siglec-6 CAR T cells (R2 = 0.45; P = .0018) (Figure 2C). We also considered the subpopulation of AML stem cells that has been said to possess enhanced leukemogenic potential and resistance to therapy and is contained within the CD45dimCD34+CD38– fraction of AML blasts.5,21 By using flow cytometry, we found that AML stem cells expressed levels of Siglec-6 similar to or even higher than those of bulk AML blasts and were equally susceptible to cytolysis by Siglec-6 CAR T cells (Figure 2A-B; supplemental Figure 5B).
Expression of Siglec-6 in primary AML blasts and anti-AML reactivity of Siglec-6_BBz CAR T cells
| Patient rank . | NMFI . | Cell lysis (%) . | Disease status . | Age (y) . | Secondary AML . | Molecular biology . | Cytogenetics . | Blasts (%) . | Patient ID no. . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.89 | 30 | Relapse | 86 | No | NPM1, FLT3 (ITD) | Normal | 18.9 | 17 |
| 2 | 1.03 | 71 | Diagnosis | 74 | No | FLT3 (TKD) | –Y, del(21)(q21q22), +13 | 89.5 | 9 |
| 3 | 1.05 | 58 | Diagnosis | 30 | Yes | — | PDGFRa+ | 85.2 | 13 |
| 4 | 1.10 | 0 | Relapse | 73 | Yes | — | Normal | 75.4 | 12 |
| 5 | 1.11 | 99 | Diagnosis | 78 | No | NPM1 | Normal | 93.2 | 20 |
| 6 | 1.14 | 49 | Diagnosis | 68 | Yes | NA | NA | 43.7 | 23 |
| 7 | 1.16 | 55 | Relapse | 77 | Yes | — | Normal | 14.8 | 18 |
| 8 | 1.18 | 0 | Diagnosis | 81 | No | CEBPα biallelic | Normal | 92.5 | 19 |
| 9 | 1.22 | 41 | NA | 36 | NA | NA | NA | 48.5 | 3 |
| 10 | 1.29 | 70* | Diagnosis | 22 | No | — | MLL(11q23) translocation | 59.0 | 16 |
| 11 | 1.35 | 96* | Diagnosis | 59 | NA | NA | NA | 67.5 | 7 |
| 12 | 1.36 | 62 | Diagnosis | 59 | NA | NA | NA | 89 | 8 |
| 13 | 1.42 | NA | Diagnosis | 39 | No | CBFB_ MYH11/inv16 | Normal | 93.0 | 22 |
| 14 | 1.67 | 86* | Diagnosis | 36 | No | NPM1, FLT3 (ITD) | Normal | 85.9 | 14 |
| 15 | 1.75 | 90 | Diagnosis | 71 | No | DNMT3A, FLT3 (ITD), RUNX1 | Normal | 79.4 | 1 |
| 16 | 1.96 | 98 | Relapse | 71 | No | NPM1, FLT3 (ITD) | Extra material of unknown origin in 12p | 78.4 | 21 |
| 17 | 2.34 | 99 | Relapse | 54 | No | RUNX1 | t(12;17)(p13;q11) .ish, t(12;17)(NF1–; NF1–) | 46 | 5 |
| 18 | 2.47 | 86 | Relapse | 76 | Yes | DNMT3A, TET2, TP53 | Normal | 53.8 | 11 |
| 19 | 3.35 | 97 | Diagnosis | 81 | Yes | NA | NA | 95.1 | 2 |
| 20 | 5.79 | 98 | Diagnosis | 52 | No | — | t(4;13)(q12;q13) | 46 | 10 |
| Patient rank . | NMFI . | Cell lysis (%) . | Disease status . | Age (y) . | Secondary AML . | Molecular biology . | Cytogenetics . | Blasts (%) . | Patient ID no. . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.89 | 30 | Relapse | 86 | No | NPM1, FLT3 (ITD) | Normal | 18.9 | 17 |
| 2 | 1.03 | 71 | Diagnosis | 74 | No | FLT3 (TKD) | –Y, del(21)(q21q22), +13 | 89.5 | 9 |
| 3 | 1.05 | 58 | Diagnosis | 30 | Yes | — | PDGFRa+ | 85.2 | 13 |
| 4 | 1.10 | 0 | Relapse | 73 | Yes | — | Normal | 75.4 | 12 |
| 5 | 1.11 | 99 | Diagnosis | 78 | No | NPM1 | Normal | 93.2 | 20 |
| 6 | 1.14 | 49 | Diagnosis | 68 | Yes | NA | NA | 43.7 | 23 |
| 7 | 1.16 | 55 | Relapse | 77 | Yes | — | Normal | 14.8 | 18 |
| 8 | 1.18 | 0 | Diagnosis | 81 | No | CEBPα biallelic | Normal | 92.5 | 19 |
| 9 | 1.22 | 41 | NA | 36 | NA | NA | NA | 48.5 | 3 |
| 10 | 1.29 | 70* | Diagnosis | 22 | No | — | MLL(11q23) translocation | 59.0 | 16 |
| 11 | 1.35 | 96* | Diagnosis | 59 | NA | NA | NA | 67.5 | 7 |
| 12 | 1.36 | 62 | Diagnosis | 59 | NA | NA | NA | 89 | 8 |
| 13 | 1.42 | NA | Diagnosis | 39 | No | CBFB_ MYH11/inv16 | Normal | 93.0 | 22 |
| 14 | 1.67 | 86* | Diagnosis | 36 | No | NPM1, FLT3 (ITD) | Normal | 85.9 | 14 |
| 15 | 1.75 | 90 | Diagnosis | 71 | No | DNMT3A, FLT3 (ITD), RUNX1 | Normal | 79.4 | 1 |
| 16 | 1.96 | 98 | Relapse | 71 | No | NPM1, FLT3 (ITD) | Extra material of unknown origin in 12p | 78.4 | 21 |
| 17 | 2.34 | 99 | Relapse | 54 | No | RUNX1 | t(12;17)(p13;q11) .ish, t(12;17)(NF1–; NF1–) | 46 | 5 |
| 18 | 2.47 | 86 | Relapse | 76 | Yes | DNMT3A, TET2, TP53 | Normal | 53.8 | 11 |
| 19 | 3.35 | 97 | Diagnosis | 81 | Yes | NA | NA | 95.1 | 2 |
| 20 | 5.79 | 98 | Diagnosis | 52 | No | — | t(4;13)(q12;q13) | 46 | 10 |
The NMFI is calculated by dividing the MFI obtained after staining with anti-Siglec-6 mAb by the MFI obtained by staining with an isotype control. Cytolytic activity against primary AML blasts was analyzed in a flow cytometry-based assay after 24-hour coculture. Data in the table correspond to 2.5:1 E:T ratio. Patients with AML who had ≤5% bone marrow AML blasts were excluded from analysis.
CEBP α, CCAAT/enhancer-binding protein α; DNMT3A, DNA (cytosine-5)–methyltransferase 3A; FLT3, FMS-like tyrosine kinase 3; ITD, internal tandem duplication; NPM1, nucleophosmin 1; RUNX1; runt-related transcription factor 1; TKD, tyrosine kinase domain; TP53, tumor protein p53; MLL, mixed lineage leukemia; PDGFRa, platelet-derived growth factor receptor A.
Indicates AML blasts lysis by autologous Siglec-6 CAR T cells.
Siglec-6 is commonly expressed on primary AML blasts and recognized by Siglec-6 CAR T cells. (A) Flow cytometric analysis of Siglec-6 expression on bulk AML blasts and AML stem cells (LSCs) in 3 samples from patients with AML that had high, moderate, and low Siglec-6 expression (Table 1). Histograms show staining with anti–Siglec-6 mAb (red) and isotype control antibody (blue). Inset numbers indicate the NMFI obtained by staining with anti–Siglec-6 mAb and isotype control. (B) Cytolytic activity of CD8+ Siglec-6_BBz CAR and UTD T cells against bulk AML blasts and AML stem cells in a flow cytometry-based 24-hour assay. The experiment was performed in triplicate wells with 10 000 target cells per well. Counting beads were used to quantitate the number of residual live target cells at the end of coculture. (C) Correlation between cytolytic activity by CD8+ Siglec-6_BBz CAR T cells (after 24-hour coculture; 2.5:1 E:T ratio) and Siglec-6 normalized expression on primary AML blasts. Simple linear correlation was calculated (R2 = 0.45; P = .0018).
Siglec-6 is commonly expressed on primary AML blasts and recognized by Siglec-6 CAR T cells. (A) Flow cytometric analysis of Siglec-6 expression on bulk AML blasts and AML stem cells (LSCs) in 3 samples from patients with AML that had high, moderate, and low Siglec-6 expression (Table 1). Histograms show staining with anti–Siglec-6 mAb (red) and isotype control antibody (blue). Inset numbers indicate the NMFI obtained by staining with anti–Siglec-6 mAb and isotype control. (B) Cytolytic activity of CD8+ Siglec-6_BBz CAR and UTD T cells against bulk AML blasts and AML stem cells in a flow cytometry-based 24-hour assay. The experiment was performed in triplicate wells with 10 000 target cells per well. Counting beads were used to quantitate the number of residual live target cells at the end of coculture. (C) Correlation between cytolytic activity by CD8+ Siglec-6_BBz CAR T cells (after 24-hour coculture; 2.5:1 E:T ratio) and Siglec-6 normalized expression on primary AML blasts. Simple linear correlation was calculated (R2 = 0.45; P = .0018).
To confirm our data, we generated Siglec-6 CAR T cells from 3 patients with AML (ie, patients 7, 14, and 16; Table 1). Siglec-6 CAR T cells could be readily generated with yield and purity similar to what we had observed in cells from healthy donors (supplemental Figure 7A-B). In each of the patients, Siglec-6 CAR T cells conferred potent antileukemia reactivity with high-level cytolysis, cytokine secretion, and proliferation after stimulation with autologous AML blasts and AML cell lines (supplemental Figure 7C-H). In summary, these data show that Siglec-6 is a relevant target for CAR T cells in AML and demonstrate that Siglec-6 CAR T cells recognize and eliminate primary AML blasts in vitro.
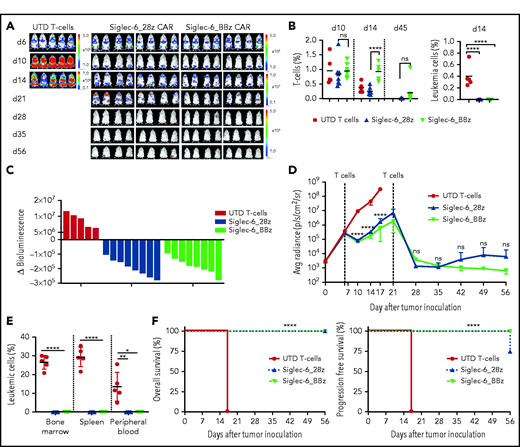
Siglec-6 CAR T cells are effective in a xenograft model of AML in vivo
We inoculated NSG mice with FLUC+GFP+ U937 AML cells and observed rapid development of systemic leukemia with circulating AML cells in peripheral blood as assessed by flow cytometry and medullar and extramedullar AML manifestations as assessed by BLI within 6 days (Figure 3A). On day 6, we treated mice with Siglec-6_28z or Siglec-6_BBz CAR T cells or UTD T cells. By day 14, we observed clearance of leukemia cells from peripheral blood (Figure 3B; supplemental Figure 8A) and a reduction of systemic leukemia burden by BLI (Figure 3A). Both Siglec-6 CAR T-cell products (ie, with CD28 vs 4-1BB co-stimulation) induced a 100% response rate (8 of 8 mice) (Figure 3C-D). Siglec-6 CAR T cells were detectable in peripheral blood on days 10 and 14, and there was superior engraftment and in vivo expansion with Siglec-6_BBz vs Siglec-6_28z CAR T cells (on day 14: 0.99% vs 0.25%, P = .0002) (Figure 3B). On day 21, there was continuous remission of AML in peripheral blood; however, in all of the mice, we observed a residual (or even increasing) BLI signal projecting to medullar and extramedullar lesions, particularly evident in mice that had received Siglec-6_28z CAR T cells. We therefore administered a second dose of Siglec-6 CAR T cells to all treatment groups on day 21 and were able to further reduce leukemia burden as assessed by BLI signal. By day 35, all of the mice that had received Siglec-6 CAR T cells had a BLI signal equal to that at baseline (ie, before leukemia engraftment). With additional follow-up, we observed sustained complete remission in 8 (100%) of 8 mice that had received the Siglec-6_BBz CAR T cells and in 6 (75%) of 8 mice that had received the Siglec-6_28z CAR T cells. In the remaining 2 (25%) of 8 mice in the Siglec-6_28z CAR group, we observed an increasing BLI signal on day 56, projecting to extramedullar loci (Figure 3A; supplemental Figure 8B). At the end of the observation period on day 56, all of the mice that had been treated with Siglec-6 CAR T cells were alive, and there were no AML cells detectable in peripheral blood, bone marrow, or spleen (Figure 3E-F; supplemental Figure 8C). All of the mice that had been treated with UTD T cells showed deleterious leukemia progression and had to be euthanized before day 21. We performed additional experiments in NSG mice that had been inoculated with MOLM-13 cells (low Siglec-6 expression). Treatment with Siglec-6 CAR T cells conferred an anti-leukemia effect and led to a significant survival benefit but was substantially less effective compared with the NSG/U937 model (high Siglec-6 expression) (supplemental Figure 9).
Siglec-6 CAR T cells confer potent antileukemia activity in a xenograft model of AML in vivo. Female NSG mice were inoculated with 2 × 106 U937 AML cells (FLUC+GFP+), and on days 6 and 21, they were treated with 5 × 106 CAR-modified or UTD T cells. T cells were formulated in a 1:1 CD4+:CD8+ ratio. (A) Serial BLI to assess leukemia progression and/or regression. Note the scale indicating upper and lower BLI thresholds at each analysis time point (right). (B) Flow cytometric analysis of peripheral blood on days 10, 14, and 45 to detect T cells and leukemia cells. Human T cells in mouse peripheral blood were defined as 7-AAD–CD45+CD3+ cells. Leukemia cells were defined as 7-AAD–CD45+GFP+ cells. (C) Waterfall plot showing change in absolute BLI values between days 6 and 10 after tumor inoculation. (D) BLI values from each treatment group showing tumor progression and regression. BLI values in panels C and D were obtained as photons per second per cm2per sr (p/s/cm2/sr) in regions of interest encompassing the entire body of each mouse. (E) Percentage of leukemic cells detected in bone marrow, spleen, and peripheral blood by flow cytometry at the end of the experiment. NB: Leukemia cells (%) values show data obtained at different time points. Mice from UTD T-cell treatment group were analyzed on day 17, and mice from the Siglec-6 CAR T-cell treatment group were analyzed on day 56. (F) Kaplan-Meier survival analysis for overall survival (left) and progression free survival (right) from different treatment groups. Data shown are representative of results obtained in independent experiments with Siglec-6 CAR T-cell from 2 donors. Mantel-Cox log-rank test ****P < .0001. *P < .05; **P < .01; ****P < .0001, Student t test (B,D-E). Avg, average; d6, day 6.
Siglec-6 CAR T cells confer potent antileukemia activity in a xenograft model of AML in vivo. Female NSG mice were inoculated with 2 × 106 U937 AML cells (FLUC+GFP+), and on days 6 and 21, they were treated with 5 × 106 CAR-modified or UTD T cells. T cells were formulated in a 1:1 CD4+:CD8+ ratio. (A) Serial BLI to assess leukemia progression and/or regression. Note the scale indicating upper and lower BLI thresholds at each analysis time point (right). (B) Flow cytometric analysis of peripheral blood on days 10, 14, and 45 to detect T cells and leukemia cells. Human T cells in mouse peripheral blood were defined as 7-AAD–CD45+CD3+ cells. Leukemia cells were defined as 7-AAD–CD45+GFP+ cells. (C) Waterfall plot showing change in absolute BLI values between days 6 and 10 after tumor inoculation. (D) BLI values from each treatment group showing tumor progression and regression. BLI values in panels C and D were obtained as photons per second per cm2per sr (p/s/cm2/sr) in regions of interest encompassing the entire body of each mouse. (E) Percentage of leukemic cells detected in bone marrow, spleen, and peripheral blood by flow cytometry at the end of the experiment. NB: Leukemia cells (%) values show data obtained at different time points. Mice from UTD T-cell treatment group were analyzed on day 17, and mice from the Siglec-6 CAR T-cell treatment group were analyzed on day 56. (F) Kaplan-Meier survival analysis for overall survival (left) and progression free survival (right) from different treatment groups. Data shown are representative of results obtained in independent experiments with Siglec-6 CAR T-cell from 2 donors. Mantel-Cox log-rank test ****P < .0001. *P < .05; **P < .01; ****P < .0001, Student t test (B,D-E). Avg, average; d6, day 6.
We were interested in elucidating potential mechanisms behind the superior in vivo performance of Siglec-6_BBz compared with Siglec-6_28z CAR T cells. In previous reports, 4-1BB co-stimulation has been shown to induce a distinct metabolic program in CAR T cells that favors in vivo persistence.22 Therefore, we performed metabolic analyses that showed an increased basal oxygen consumption rate (indicative of oxidative phosphorylation) and an increased extracellular acidification rate (indicative of aerobic glycolysis) in Siglec-6_BBz compared with Siglec-6_28z CAR T cells, indicating superior bioenergetic competence (supplemental Figure 10A-B). Taken together, the data show that Siglec-6 CAR T cells confer a potent anti-leukemia effect in vivo and are capable of inducing long-term remission of aggressive systemic AML. The data also suggest that Siglec-6_BBz rather than Siglec-6_28z CAR T cells are the preferred product for clinical translation.
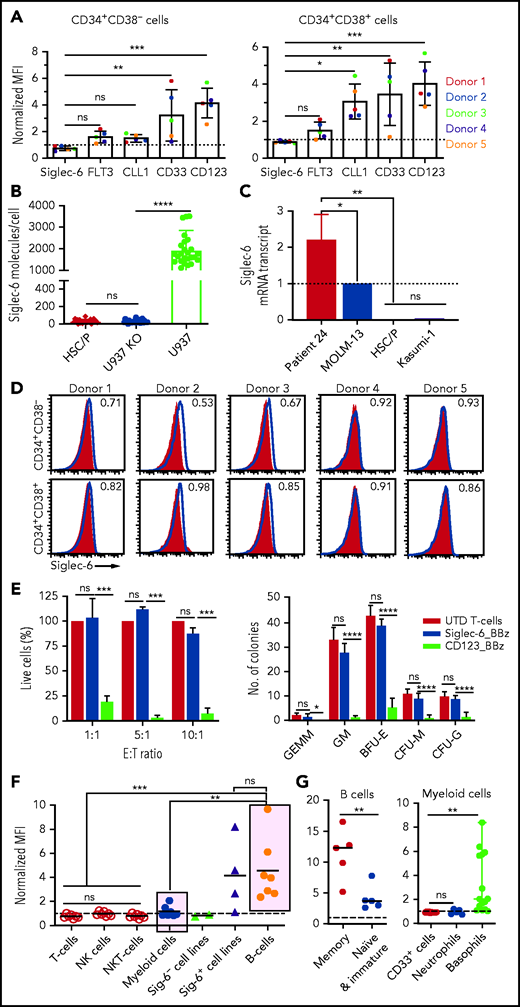
Siglec-6 is absent on normal HSPCs
We sought to evaluate potential on-target/off-tumor reactivity of Siglec-6 CAR T cells and focused on developing and mature normal hematopoietic cells. First, we obtained granulocyte colony-stimulating factor–mobilized CD34+CD38– hematopoietic stem cells and CD34+CD38+ hematopoietic progenitor cells from peripheral blood of 5 healthy donors. We performed flow cytometric analyses and detected CD123, CD33, CLL-1, and FLT3 on normal HSPCs in all 5 healthy donors, consistent with previous reports.4-7 It was encouraging to note that Siglec-6 was the only antigen that was not detectable on HSPCs in all 5 donors (Figure 4A,D; supplemental Figure 11A-B). We confirmed the absence of Siglec-6 on the surface of HSPCs by dSTORM super-resolution microscopy, and at the messenger RNA transcript level by qPCR (Figure 4B-C). To further assess the potential for on-target recognition of HSPCs, we applied the criteria for flow cytometric analysis and recognition by Siglec-6 CAR T cells that we had also applied in our evaluation of primary AML blasts. We did not detect Siglec-6 by flow cytometry (NMFI <1) (Figure 4A,D), and there was no reactivity of Siglec-6 CAR T cells against HSPCs in co-culture assays, even at high E:T ratios over a 24-hour period (Figure 4E). We used CD123 CAR T cells as a positive control and, as expected, found rapid deletion of HSPCs (Figure 4E). We plated the 24-hour co-culture of CAR T cells and HSPCs to assess their ability to initiate lineage development and found a comparable pattern of colony formation after treatment with Siglec-6 CAR T cells and UTD T cells (Figure 4E).
Normal HSPCs are not recognized by Siglec-6 CAR T cells. (A) Flow cytometric analysis of cell surface expression of different CAR target antigens on CD34+CD38– hematopoietic stem cells (HSCs) (left) and CD34+CD38+ hematopoietic progenitor cells (HPCs) (right) from 5 healthy donors. Bar diagrams show NMFI ± standard deviation (SD). Two-way analysis of variance (ANOVA) *P < .05; **P < .01;***P < .001. (B) Quantification of Siglec-6 expression on HSPCs by dSTORM super-resolution microscopy. U937 cells and Siglec-6–negative U937 knockout (KO) cells were used for comparison. Each data point represents a cell. (C) Real-time qPCR was used to assess Siglec-6 mRNA transcripts in CD34+CD38– HSCs and CD34+CD38+ HPCs. Data are normalized to MOLM-13 Siglec-6 mRNA transcripts. AML blasts from patient 24 were included in the analysis as a positive control (supplemental Figure 6). (D) Flow cytometric analysis of Siglec-6 expression on granulocyte colony-stimulating factor–mobilized CD34+CD38– HSCs and CD34+CD38+ HPCs from peripheral blood of 5 healthy donors. Inset numbers indicate the NMFI. (E) Left: percentage of live (7-AAD negative) HSCs after 24-hour coincubation with CD8+ Siglec-6_BBz CAR, CD123_BBz CAR, or UTD T cells. The assay was performed in triplicate wells with 5000 target cells per well. Counting beads were used to quantify the number of residual live HSPCs at the end of co-culture. Data are from 3 independent experiments. Right: colony formation assay was performed with residual live HSPCs after 24 hours of co-incubation with CD8+ Siglec-6_BBz CAR, CD123_BBz CAR, or UTD T cells. Graphs show the absolute number of colonies (mean ± SD) per 55-mm plate as determined by microscopy on day 14 from 3 independent experiments. (F) Flow cytometric analysis of Siglec-6 expression on healthy donor peripheral blood mononuclear cells. Siglec-6 expression by B cells (CD45+CD19+), myeloid cells (CD45+CD33+), T cells (CD45+CD3+CD56–), NK cells (CD45+CD56+CD3–), and NK T cells (CD45+CD3+CD56+) in 7 healthy donors. Siglec-6 expression by Siglec-6–positive (U937, TF-1, MV4;11, and MOLM-13) and Siglec-6–negative (K562, Kasumi-1) cell lines are plotted for reference. (G) Flow cytometric analysis of Siglec-6 expression on healthy B cells, CD33+ myeloid cells, neutrophils (CD33+CD15+CD16+), and basophils (CD33+CD123+HLA-DR–). *P < .05; **P < .01; ***P < .001; ****P < .0001, Student t test. BFU-E, burst-forming unit erythroid; CFU-G, colony-forming unit granulocyte; CFU-M, colony-forming unit macrophage; GEMM, granulocyte, erythrocyte, monocyte, megakaryocyte; GM, granulocyte-macrophage.
Normal HSPCs are not recognized by Siglec-6 CAR T cells. (A) Flow cytometric analysis of cell surface expression of different CAR target antigens on CD34+CD38– hematopoietic stem cells (HSCs) (left) and CD34+CD38+ hematopoietic progenitor cells (HPCs) (right) from 5 healthy donors. Bar diagrams show NMFI ± standard deviation (SD). Two-way analysis of variance (ANOVA) *P < .05; **P < .01;***P < .001. (B) Quantification of Siglec-6 expression on HSPCs by dSTORM super-resolution microscopy. U937 cells and Siglec-6–negative U937 knockout (KO) cells were used for comparison. Each data point represents a cell. (C) Real-time qPCR was used to assess Siglec-6 mRNA transcripts in CD34+CD38– HSCs and CD34+CD38+ HPCs. Data are normalized to MOLM-13 Siglec-6 mRNA transcripts. AML blasts from patient 24 were included in the analysis as a positive control (supplemental Figure 6). (D) Flow cytometric analysis of Siglec-6 expression on granulocyte colony-stimulating factor–mobilized CD34+CD38– HSCs and CD34+CD38+ HPCs from peripheral blood of 5 healthy donors. Inset numbers indicate the NMFI. (E) Left: percentage of live (7-AAD negative) HSCs after 24-hour coincubation with CD8+ Siglec-6_BBz CAR, CD123_BBz CAR, or UTD T cells. The assay was performed in triplicate wells with 5000 target cells per well. Counting beads were used to quantify the number of residual live HSPCs at the end of co-culture. Data are from 3 independent experiments. Right: colony formation assay was performed with residual live HSPCs after 24 hours of co-incubation with CD8+ Siglec-6_BBz CAR, CD123_BBz CAR, or UTD T cells. Graphs show the absolute number of colonies (mean ± SD) per 55-mm plate as determined by microscopy on day 14 from 3 independent experiments. (F) Flow cytometric analysis of Siglec-6 expression on healthy donor peripheral blood mononuclear cells. Siglec-6 expression by B cells (CD45+CD19+), myeloid cells (CD45+CD33+), T cells (CD45+CD3+CD56–), NK cells (CD45+CD56+CD3–), and NK T cells (CD45+CD3+CD56+) in 7 healthy donors. Siglec-6 expression by Siglec-6–positive (U937, TF-1, MV4;11, and MOLM-13) and Siglec-6–negative (K562, Kasumi-1) cell lines are plotted for reference. (G) Flow cytometric analysis of Siglec-6 expression on healthy B cells, CD33+ myeloid cells, neutrophils (CD33+CD15+CD16+), and basophils (CD33+CD123+HLA-DR–). *P < .05; **P < .01; ***P < .001; ****P < .0001, Student t test. BFU-E, burst-forming unit erythroid; CFU-G, colony-forming unit granulocyte; CFU-M, colony-forming unit macrophage; GEMM, granulocyte, erythrocyte, monocyte, megakaryocyte; GM, granulocyte-macrophage.
Second, we analyzed mature normal hematopoietic cells in peripheral blood of healthy donors (n ≥ 7) and detected high-level Siglec-6 expression on a fraction of normal B cells, weak expression on a fraction of myeloid cells, and no expression on T cells, NK cells, or NK T cells (Figure 4F). Within the normal B-cell population, Siglec-6 was detectable at high levels on memory B cells and at very low levels on naïve and immature B cells (Figure 4G; supplemental Figure 12A-B). Detailed subset analyses in myeloid cells revealed that basophilic granulocytes express Siglec-6, whereas neutrophilic granulocytes and monocytes are Siglec-6 negative. Myeloid-derived dendritic cells that we generated from monocytes in vitro were also Siglec-6 negative (Figure 4G; supplemental Figure 13A). In line with the expression analysis, we found partial deletion of B cells and basophilic granulocytes in co-culture with Siglec-6 CAR T cells, whereas neutrophilic granulocytes and monocytes were not affected (supplemental Figure 13B-C). We analyzed Siglec-6 expression in essential adult human tissues using placenta (as a reference), which is known to express Siglec-6, and spleen (as a comparator), which is expected to produce a signal as a result of infiltration from B cells and basophilic granulocytes. There was no signal in brain, breast, or liver tissue and a very low signal on occasional cells in ileum, colon, lung, kidney, heart, ovary and prostate tissues, consistent with infiltrating immune cells (supplemental Figure 14). Taken together, these data show that Siglec-6 is not expressed on normal HSPCs and that targeting Siglec-6 does not interfere with hematopoietic lineage development in vitro. Siglec-6 is expressed on memory B cells and basophilic granulocytes, suggesting the potential for limited on-target/off-tumor reactivity in patients.
Siglec-6 CAR T cells are effective against malignant B cells in CLL
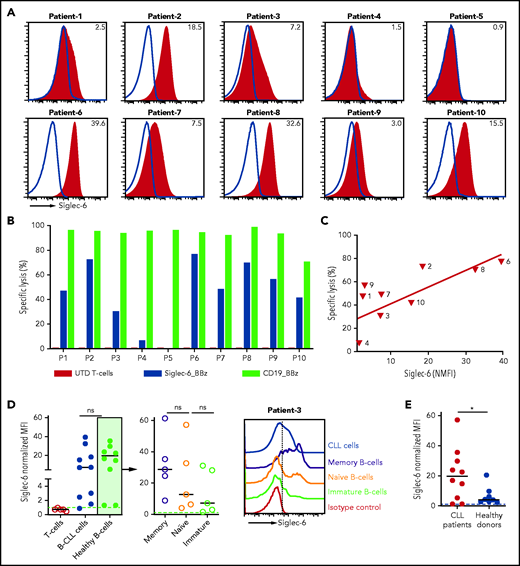
The JML-1 mAb had been discovered in a patient with CLL after allo-HSCT; accordingly, we evaluated Siglec-6 expression in a series of CLL samples from 10 patients who were treatment naïve (Table 2). We found Siglec-6 to be expressed at variable levels on B-CLL cells in 9 of 10 patients (NMFI range, 39.6-1.5) (Figure 5A; supplemental Figure 12C). We observed specific cytolytic activity of Siglec-6 CAR T cells against B-CLL cells in each of the 9 patients, and similar to our observation with AML target cells, there was a linear correlation between Siglec-6 expression and cytolysis (Figure 5B-C). For comparison, we used a CD19 CAR, and in patients with high-level Siglec-6 expression (ie, patients 2, 6, and 8), the elimination of B-CLL cells was similarly potent with Siglec-6 CAR T cells and CD19 CAR T cells (Figure 5B). We performed additional flow cytometric analyses and found that in patients with CLL, there was a higher level of Siglec-6 expression on normal B cells compared with cells from healthy donors, including Siglec-6 expression on naïve and immature normal B cells (Figure 5D-E; supplemental Figure 12D). Accordingly, we also observed partial deletion of normal B cells in coculture with Siglec-6 CAR T cells (supplemental Figure 15).
Characteristics of patients with CLL
| Patient rank . | NMFI . | Cell lysis (%) . | Age (y) . | IGHV mutation . | Cytogenetics . | CLL cells (%) . | Patient no. . |
|---|---|---|---|---|---|---|---|
| 1 | 0.9 | 0 | 66 | No | +12 | 89 | 5 |
| 2 | 1.5 | 7 | 91 | Yes | — | 36 | 4 |
| 3 | 2.5 | 47 | 54 | Yes | del13q | 24 | 1 |
| 4 | 3.0 | 57 | 53 | Yes | — | 84 | 9 |
| 5 | 7.2 | 31 | 90 | Yes | del13q | 89 | 3 |
| 6 | 7.5 | 49 | 48 | No | del11q | 96 | 7 |
| 7 | 15.5 | 41 | 61 | Yes | — | 60 | 10 |
| 8 | 18.5 | 73 | 58 | Yes | — | 79 | 2 |
| 9 | 32.6 | 70 | 60 | Yes | — | 69 | 8 |
| 10 | 39.6 | 77 | 66 | Yes | +12 | 96 | 6 |
| Patient rank . | NMFI . | Cell lysis (%) . | Age (y) . | IGHV mutation . | Cytogenetics . | CLL cells (%) . | Patient no. . |
|---|---|---|---|---|---|---|---|
| 1 | 0.9 | 0 | 66 | No | +12 | 89 | 5 |
| 2 | 1.5 | 7 | 91 | Yes | — | 36 | 4 |
| 3 | 2.5 | 47 | 54 | Yes | del13q | 24 | 1 |
| 4 | 3.0 | 57 | 53 | Yes | — | 84 | 9 |
| 5 | 7.2 | 31 | 90 | Yes | del13q | 89 | 3 |
| 6 | 7.5 | 49 | 48 | No | del11q | 96 | 7 |
| 7 | 15.5 | 41 | 61 | Yes | — | 60 | 10 |
| 8 | 18.5 | 73 | 58 | Yes | — | 79 | 2 |
| 9 | 32.6 | 70 | 60 | Yes | — | 69 | 8 |
| 10 | 39.6 | 77 | 66 | Yes | +12 | 96 | 6 |
Siglec-6 expression was analyzed in 10 treatment-naïve patients with CLL. Cytolytic activity against primary B-CLL cells was analyzed in a flow cytometry-based assay after 4 hours of coculture. CLL cells (%) values refer to the frequency in peripheral blood.
Siglec-6 CAR T cells recognize malignant B cells in B-CLL. (A) Flow cytometric analysis of Siglec-6 expression on B-CLL cells from 10 patients. Histograms show staining with anti-Siglec-6 mAb (red) and isotype control antibody (blue). Inset numbers indicate the NMFI. (B) Cytolytic activity of CD8+ Siglec-6_BBz CAR, CD19_BBz CAR, and UTD T cells against B-CLL cells in a flow cytometry-based assay. Target cells were seeded in triplicate wells (10 000 cells per well) and were cocultured with effector cells at a 5:1 E:T ratio. Counting beads were used to quantify the number of residual live target cells after 4 hours of coculture (P1, patient 1). (C) Correlation between B-CLL–specific cell lysis by CD8+ Siglec-6_BBz CAR T cells (after 4 hours of coculture, 5:1 E:T ratio) and Siglec-6 expression on primary B-CLL cells. Simple linear correlation was calculated (R2 = 0.54; P = .01). (D) Flow cytometric analysis of Siglec-6 expression on healthy B cells (CD45+CD19+CD5–CD20high) from patients with CLL. Left: pooled data on Siglec-6 expression on B-CLL cells from 10 patients and on healthy B-cell subsets from 5 of 10 patients with CLL. The remaining 5 patients did not have enough healthy B cells in the peripheral blood for subset analysis. Right: a representative histogram from patient 3, which shows Siglec-6 expression on healthy immature (CD45+CD19+CD5–CD20highCD10+), naïve (CD45+CD19+CD5–CD20highCD10–CD27–), and memory (CD45+CD19+CD5–CD20highCD10–CD27+) B cells compared with B-CLL cells. (E) Siglec-6 expression on healthy B cells from patients with CLL and healthy donors. *P < .05, Student t test.
Siglec-6 CAR T cells recognize malignant B cells in B-CLL. (A) Flow cytometric analysis of Siglec-6 expression on B-CLL cells from 10 patients. Histograms show staining with anti-Siglec-6 mAb (red) and isotype control antibody (blue). Inset numbers indicate the NMFI. (B) Cytolytic activity of CD8+ Siglec-6_BBz CAR, CD19_BBz CAR, and UTD T cells against B-CLL cells in a flow cytometry-based assay. Target cells were seeded in triplicate wells (10 000 cells per well) and were cocultured with effector cells at a 5:1 E:T ratio. Counting beads were used to quantify the number of residual live target cells after 4 hours of coculture (P1, patient 1). (C) Correlation between B-CLL–specific cell lysis by CD8+ Siglec-6_BBz CAR T cells (after 4 hours of coculture, 5:1 E:T ratio) and Siglec-6 expression on primary B-CLL cells. Simple linear correlation was calculated (R2 = 0.54; P = .01). (D) Flow cytometric analysis of Siglec-6 expression on healthy B cells (CD45+CD19+CD5–CD20high) from patients with CLL. Left: pooled data on Siglec-6 expression on B-CLL cells from 10 patients and on healthy B-cell subsets from 5 of 10 patients with CLL. The remaining 5 patients did not have enough healthy B cells in the peripheral blood for subset analysis. Right: a representative histogram from patient 3, which shows Siglec-6 expression on healthy immature (CD45+CD19+CD5–CD20highCD10+), naïve (CD45+CD19+CD5–CD20highCD10–CD27–), and memory (CD45+CD19+CD5–CD20highCD10–CD27+) B cells compared with B-CLL cells. (E) Siglec-6 expression on healthy B cells from patients with CLL and healthy donors. *P < .05, Student t test.
Collectively, these data show that Siglec-6 is a novel target antigen for CAR T-cell therapy in AML and CLL. Siglec-6 has a favorable expression profile with absence on normal HSPCs and restricted expression on a fraction of normal B cells and basophilic granulocytes. The data suggest that targeting Siglec-6 will be effective for treating AML and CLL in patients with high-level Siglec-6 expression on leukemic cells.
Discussion
The development of adoptive immunotherapy with CAR T cells in AML has been substantially more challenging compared with that for ALL, in which CD19 CAR T-cell therapy is now an approved treatment. In ALL, the clinical translation and development of CD19 CAR T cells has been facilitated by the identification of CD19 as a “poster child” antigen that is highly and uniformly expressed on leukemic cells, absent on all essential normal tissues, and associated with predictable on-target/off-tumor recognition that leads to deletion of normal B cells.23-25 In addition, the target patient population of CD19 CAR T cells in ALL are children and young adults that are medically fit and able to tolerate the acute (and chronic) adverse effects of CAR T-cell therapy with acceptable morbidity and mortality.26 In AML, the situation is more challenging because the lead target antigens CD33 and CD123, even though they are commonly expressed on AML blasts,27 are also present on normal HSPCs. A predicted outcome of effectively targeting CD33 and CD123 on AML blasts is deletion of normal HSPCs, which would potentially require subsequent allo-HSCT to reconstitute normal hematopoiesis.4-6 In AML, the target patient population is substantially older than that in ALL, has a higher incidence of comorbidities, and is anticipated to be less able to tolerate CAR T-cell–induced toxicity.28,29
Here, we introduce Siglec-6 as a novel antigen for CAR T cells in AML. Our data show that Siglec-6 is prevalently expressed on primary AML blasts and AML cell lines and is absent on normal HSPCs. We also show that Siglec-6 CAR T cells rapidly eliminate AML cells in both in vitro and in vivo models. These data suggest that targeting Siglec-6 may enable AML to be treated effectively without inducing myeloablation. Accordingly, Siglec-6 CAR T-cell therapy may help patients with AML who are medically unfit and are not considered candidates for an allo-HSCT and patients who relapse after allo-HSCT.
The experience with CD19 CAR T cells in ALL and B-cell maturation antigen CAR T cells in multiple myeloma has illustrated several additional requirements for effective and potentially curative CAR T-cell therapy in hematology indications.25,26,30 One requirement is uniform and stable expression of the target antigen on malignant cells, because otherwise, outgrowth of malignant cells that have downregulated or lost the target antigen will occur.2,30-32 We have performed a 2-criteria assessment to evaluate the susceptibility of primary AML blasts to Siglec-6 CAR T cells. Flow cytometric assessment showed uniform expression of Siglec-6 on primary AML blasts in a subset of patients. In some patients, the NMFI after staining with an anti-Siglec-6 antibody and an isotype control was below the 1.1 threshold; we still observed specific cytolytic activity of Siglec-6 CAR T cells. These data suggest that on some primary AML blasts and AML cell lines, Siglec-6 is expressed at a density that is at or below the detection limit of flow cytometry (in the range of 1000 molecules per cell), which we confirmed to be the case by quantitative dSTORM super-resolution microscopy. However, low-level antigen expression on target cells can also be sufficient for recognition and elimination by CAR T cells.33-35 The induction of cytokine secretion and proliferation in CAR T cells requires higher antigen density on target cells compared with cytolytic activity.34,36 In the NSG/U937 xenograft model, a relatively high dose of Siglec-6 CAR T cells was required for inducing remission, and the anti-leukemia effect was reinvigorated after administering a second dose of Siglec-6 CAR T cells, suggesting that continued refinement of Siglec-6 CAR design to optimize low-level antigen recognition and signaling is warranted.
At present, there is no clinical experience of targeting Siglec-6 with any immunotherapeutic modality and therefore, it is unknown whether Siglec-6 might be downregulated or internalized under therapeutic pressure. Dynamic expression with upregulation of Siglec-6 on mast cells in colorectal cancer has been reported.16 The Siglec family member Siglec-2 (CD22) is internalized upon antigen-binding, which is exploited to increase the uptake of anti-CD22 antibody drug conjugates.37 It is unknown whether novel mutations or alternative splicing might occur at the Siglec-6 locus, because these phenomena have been described as mechanisms of ALL relapse and escape after CD19 CAR T-cell therapy.31,38,39 AML is a heterogeneous disease, and no previously reported CAR target antigen is highly and uniformly expressed on leukemic cells in every patient with AML.5,27 It is likely that the optimal single CAR target antigen or combination of them will have to be selected in a personalized approach for treating AML. Our data suggest that patients with AML who have high-level Siglec-6 expression are the ones that most likely benefit from Siglec-6 CAR T-cell therapy. In patients with AML who have low-level Siglec-6 expression, multiantigen targeting (eg, in combination with CD70 or TIM3, which are absent or low on normal hematopoietic stem cells) might be a preferable option.5,40
An important attribute of the Siglec-6 CAR presented in this study is that the scFv was derived from a human IgG1 antibody. The development of humoral and cellular immune responses to scFv’s with non-human VL and VH chains has been described as a mechanism that limits in vivo persistence after the first administration of CAR T cells and leads to rapid rejection after a second administration.23 This risk is greatly mitigated with the human scFv in our Siglec-6 CAR, even though immunogenic epitopes may still originate from the fusion sites of VL and VH in the scFv and the fusion sites between scFv, spacer domain, and signaling module in the CAR construct.
Because the JML-1 antibody from which we derived the scFv for our Siglec-6 CAR originates from a human patient with B-CLL, we expect a low potential for off-tumor recognition and a favorable safety profile in humans. In silico analyses in normal adult tissues showed that Siglec-6 expression is restricted to placenta,20 which we confirmed in a human tissue array by immunohistochemistry. Siglec-6 is present on mast cells that may release histamine upon destruction by Siglec-6 CAR T cells, which mandates careful prophylaxis and monitoring. We show that Siglec-6 is expressed on memory B cells and basophilic granulocytes but not on neutrophilic granulocytes, monocytes, or myeloid dendritic cells. Siglec-6 is present on malignant B cells in CLL, even though less consistently and at a lower density compared with the alternative CLL CAR target antigens CD19, CD20, and ROR1.41-43 A predicted advantage of targeting Siglec-6 rather than CD19 in CLL is that it is less toxic to normal B cells. While this article was being revised, a study by Kovalovsky et al44 demonstrated efficacy of Siglec-6 CAR T cells against CLL cells in vitro and MEC-1 CLL cells overexpressing the Siglec-6 target antigen in vivo.
In summary, we present Siglec-6 as a novel target for CAR T cells in AML. The data suggest that Siglec-6 CAR T-cell therapy will be effective in patients with high Siglec-6 expression on leukemic cells and will be associated with an acceptable toxicity profile.
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft (project number 324392634, TRR 221) (M.H., H.J., H.E.), the European Union’s Horizon 2020 Research and Innovation Programme (733297 EURE-CART [M.H. and H.E.] and 754658 CARAMBA [M.H., H.E.]), and the German Excellence Initiative fellowship awarded to the Graduate School of Life Sciences (H.J.) at the University of Würzburg, by the patient advocacy group “Hilfe im Kampf gegen den Krebs eV,” (Würzburg, Germany), “Forschung hilft”-Stiftung zur Förderung der Krebsforschung an der Universität Würzburg. A.N.-B. was supported by the Fundación Española de Hematología y Hemoterapia (Beca Estancias en el Extranjero Convocatoria 2018).
Authorship
Contribution: H.J., A.N.-B., and M.M. designed and performed experiments, analyzed data, and wrote the manuscript; S.F., C.V., A.Y., M.B.V., M.G., J.R.S., and R.M. designed and performed experiments and analyzed data; S.K., S.T., and H.B. provided biological material and analyzed data; D.M., K.M., and M.L. designed experiments and analyzed data; H.E. and M.S. revised the manuscript and cosupervised the project; and M.H. designed experiments, analyzed data, wrote the manuscript, and supervised the project.
Conflict-of-interest disclosure: H.J. and M.H. are co-inventors on a patent application related to the Siglec-6 CAR filed by the University of Würzburg and licensed by T-CURX, Würzburg, Germany. H.J. and M.H. are co-inventors on patent applications and granted patents related to other CAR T-cell technologies filed by the University of Würzburg. M.H. is a co-founder and equity owner of T-CURX. S.T. has received research funding from Celgene and Gilead; has received speaker and consulting honorarium from Abbvie, Celgene/BMS, EUSA Pharma, Gilead, Janssen, Medigene, Novartis, and Pfizer; has received financial support of educational activities and conference attendance from Celgene, Gilead, and Medigene. The remaining authors declare no competing financial interests.
Correspondence: Michael Hudecek, Universitätsklinikum Würzburg, Medizinische Klinik und Poliklinik II, Oberdürrbacher Straße 6, 97080 Würzburg, Germany; e-mail: hudecek_m@ukw.de.
There is a Blood Commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
REFERENCES
Author notes
*H.J., A.N.-B., and M.M. contributed equally to this work.

![Siglec-6 is expressed by AML cell lines and Siglec-6 CAR T cells recognize and eliminate AML cells in vitro. (A) Flow cytometric analysis of Siglec-6 expression on AML cell lines (U937, MV4;11, MOLM13, and Kasumi-1). Histograms show staining with anti-Siglec-6 mAb (red) and isotype control antibody (blue). Inset numbers indicate the NMFI calculated by dividing MFI obtained after staining with anti-Siglec-6 mAb by MFI of isotype control. (B) Detection of Siglec-6 expression on AML cell lines using dSTORM super-resolution microscopy. Each representative image depicts Siglec-6 molecules on the basal membrane of a cell. Scale bars represent 5 µm. (C) Real-time qPCR was performed to assess Siglec-6 messenger RNA (mRNA) transcript levels in AML cell lines. Data are normalized to MOLM-13 Siglec-6 mRNA transcript values. (D) Quantification of Siglec-6 expression on AML cell lines by dSTORM super-resolution microscopy. Each point represents a cell. (C-D) Data are representative of 3 independent experiments. (E) Specific cytolytic activity of CD8+ Siglec-6_28z CAR, Siglec-6_BBz CAR, CD19_BBz CAR, and UTD T cells against AML cell lines in a luminescence-based assay (4 hours of coculture). The assay was performed in triplicate wells with 5000 target cells per well. Data are presented as mean ± standard deviation (SD). (F) Enzyme-linked immunosorbent assay (ELISA) was performed to detect interferon-γ (IFN-γ) and interleukin-2 (IL-2) in supernatant obtained after 24-hour coculture of CD8+ Siglec-6_28z CAR, Siglec-6_BBz CAR, or UTD T cells with target cells. T cells and target cells were seeded at a 2:1 E:T ratio in triplicate wells. Data are represented as mean concentration ± SD. (G) Proliferation of CD8+ Siglec-6_28z CAR and Siglec-6_BBz CAR T cells examined by carboxyfluorescein diacetate succinimidyl ester (CFSE) dye dilution after 72 hours of coculture with target cells. The assay was performed in triplicate wells at a (2:1) E:T ratio. Histograms show proliferation of live (7-amino-actinomycin D [7-AAD–]) T cells. No exogenous cytokines were added. Data shown in panels E-G are representative of results obtained with CAR and control T-cell lines prepared from at least 5 healthy donors. (H) Correlation between specific lysis by CD8+ Siglec-6_BBz CAR T cells (after 4-hour coculture; 2.5:1 E:T ratio) and Siglec-6 normalized expression on AML cell lines. Simple linear correlation was calculated (R2 = 0.91; P = .0009). test **P < .01; ***P < .001, Student t test. ns, not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/138/19/10.1182_blood.2020009192/4/m_bloodbld2020009192f1.png?Expires=1770230167&Signature=5IfbvRklZWp3GJL6~uFqLBseLqNzr5g3Y3ARfyjtLdOXjReBw0JiLUIHEL~CVK26ucmRqsXY1iHBlLso2Tr7ymYrEjDci14lNLrqu5Fxd0qADQi1F7xLPQYHE-OARIPJyVobu-ZNDl5zfI800H-JnZHTprvl2JRC0n307xR367XUChKQk2QYAAy69l1kthhE6J0Bd85N1wfDeqz0vtAjziDjmPQ-eonNIzaqVR4X9t9AMkakNNUK0bQFj4eSZAR4qwTsS4CA~J0QQWjfefKTlrZkrLbDS94kbmPV9Q56WjtDhQTWflLgi35CSyNW6A462K1DvOzZ0EN0KLp2-itmCA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal