In this issue of Blood, Jetani et al1 explore models of CAR T-cell targeting of Sialic acid-binding Ig-like lectin-6 (Siglec-6) in acute myeloid leukemia (AML). Whereas CAR T cells are a useful new treatment for patients with refractory B-cell acute lymphoblastic leukemia (B-ALL), developing CAR T-cell therapies for AML has been hampered by a lack of suitable targets. Most obvious CAR targets for AML are also expressed on hematopoietic stem cells (HSCs) or myeloid cells. Given the propensity for CAR T cells to persist, prolonged myeloid aplasia would be expected if myeloid cells or HSCs were recognized. Importantly, Siglec-6 has no significant expression on HSCs or myeloid cells; consequently, Siglec-6 CAR T cells should spare normal hematopoiesis.
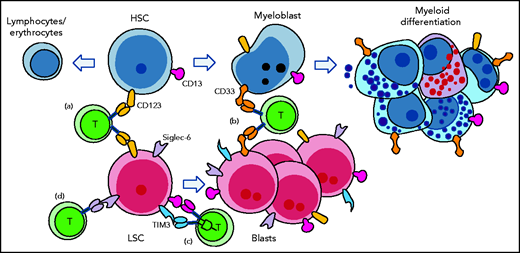
Targeting AML with CAR T cells poses additional challenges. AML is a heterogenous disorder arising from dysregulation at diverse points in myeloid differentiation. CAR T-cell targets should be broadly expressed on all AML subtypes and should target leukemia stem cells (LSCs), a cell population held responsible for relapse. However, LSCs may be phenotypically distinct from the bulk population and may differ among patients. Different LSC subpopulations may even be present in a single patient (see figure). Finally, potential AML targets should not be expressed on other critical nonmyeloid or nonhematopoietic cells.
The best explored AML target antigens are CD332 and CD123.3 These targets are not ideal, as they are expressed by normal myeloid cells and HSCs, respectively. With targets that do not spare normal hematopoiesis, 1 pragmatic strategy is to use CAR T cells to act as a “bridge” to allogeneic HSC transplant (allo-HSCT). In that case, conditioning chemotherapy should eradicate the CAR T cells and rescue the patient from aplasia. However, this strategy involves a lengthy period of myelosuppression and may be poorly tolerated. There are limited, early clinical data on CAR T-cell therapy in AML using such targets, but responses have been reported (reviewed in Mardiana and Gill 4).
CAR T-cell therapy for AML ideally targets LSCs and AML blasts, but spares HSCs and myelopoiesis. Early approaches such as targeting of CD123 (a) and CD33 (b) did not spare HSCs or myelopoiesis, resulting in aplasia. (c) More complex targeting approaches where CAR T activation is triggered only by the presence of 2 antigens can allow increased specificity. In this example, CD13 is expressed by LSC and blasts, but it is also expressed by HSCs and myeloid cells. Although TIM3 is expressed outside the hematopoietic system, it is expressed on AML cells but not on normal HSCs. (d) Finally, some antigens such as Siglec-6, which are expressed on AML cells but not on normal hematopoietic cells may allow simple and selective targeting of AML.
CAR T-cell therapy for AML ideally targets LSCs and AML blasts, but spares HSCs and myelopoiesis. Early approaches such as targeting of CD123 (a) and CD33 (b) did not spare HSCs or myelopoiesis, resulting in aplasia. (c) More complex targeting approaches where CAR T activation is triggered only by the presence of 2 antigens can allow increased specificity. In this example, CD13 is expressed by LSC and blasts, but it is also expressed by HSCs and myeloid cells. Although TIM3 is expressed outside the hematopoietic system, it is expressed on AML cells but not on normal HSCs. (d) Finally, some antigens such as Siglec-6, which are expressed on AML cells but not on normal hematopoietic cells may allow simple and selective targeting of AML.
More complex strategies have been developed. “Logic gating” of CAR signaling can restrict recognition to a pattern of antigen expression, thus improving specificity.5 For example, He et al6 described CAR T cells that mediated cytolysis only after joint recognition of CD13 and TIM-3. Given that AML blasts express both targets, but HSCs lack TIM-3, HSCs were relatively spared. Because of the difficulties in sparing normal hematopoiesis, a radical solution of concomitant administration of donor HSCs, genetically edited so as not to express cognate target, has been proposed.7 More recently, targeting CD708 and TIM39 has been suggested. These antigens are expressed by blasts and LSCs, but not on HSCs, although expression is found on NK cells and monocytes.
How does Siglec-6 fare against other targets and approaches? Jetani et al show that Siglec-6 is expressed in ∼60% of AML cases, expression is retained on LSCs, but there is little expression on HSCs. Further, expression within the hematopoietic system was restricted to memory B cell and basophil populations. Outside the hematopoietic system, no expression was identified other than the placenta. Furthermore, Jetani et al also demonstrate that Siglec-6 may be a useful target in B-Cell chronic lymphoblastic lymphoma (B-CLL). Siglecs are a large family of proteins that are thought to promote cell-cell interactions and regulate innate and adaptive immune systems through glycan recognition. It is worth noting that cancer immunotherapies targeting Siglecs are not new: CD22 (Siglec-2) and CD33 (Siglec-3) have been targeted with well-investigated immunoconjugates and CAR T cell therapies for ALL and AML, respectively.
The investigators next generated CARs based on a human monoclonal antibody (mAb) and demonstrated function in vitro, as well as in a range of AML models. Siglec-6 expression level is low but appears sufficient to direct CAR-mediated lysis against LSCs among other cells. HSCs were relatively spared after in vitro coincubation with Siglec-6 CAR T cells, although in vivo HSC engraftment studies were not described. One possible limitation of Siglec-6 is that proliferation and cytokine release was observed only when Siglec-6 CAR T cells were co-cultured with target cells expressing higher levels of target antigen.
In summary, Jetani et al expand the possibilities of CAR T-cell targeting in AML. Siglec-6 CAR T cell therapy could be useful for ∼60% of patients with AML and unlike many other targets, should spare normal hematopoiesis. It is hoped that clinical exploration of this and other strategies will bring CAR T cell therapy to patients with refractory AML.
Conflict-of-interest disclosure: M.P. receives a salary contribution from and owns stock in Autolus Therapeutics. S.G. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal