TO THE EDITOR:
The ChAdOx1 nCoV-19 is a recombinant chimpanzee adenoviral vector vaccine encoding the spike glycoprotein of severe acute respiratory syndrome coronavirus 2, which has a good efficacy rate and safety profile.1 Over the past 2 months, concern has been raised over reported thrombotic events associated with thrombocytopenia after ChAdOx1 nCoV-19 vaccination, a complication called vaccine-induced immune thrombotic thrombocytopenia (VITT).2-6 The pathophysiology of VITT is still unclear but seems to be similar to spontaneous autoimmune heparin-induced thrombocytopenia (aHIT).2,7 In fact, as in aHIT, VITT patients develop platelet factor-4 (PF4) antibodies without any recent exposure to heparin. These antibodies are able to activate platelets and induce procoagulant platelet phenotype via crosslinking the Fc γ receptor IIA on platelet surface. IV immunoglobulin (IVIG) has been successfully used in the treatment of spontaneous aHIT.8,9 We and others have recently shown that IVIG inhibits the in vitro induction of procoagulant platelet phenotype by sera from VITT patients.2,7 Herein, we report our clinical experience on the use of IVIG in the management of VITT and present novel laboratory analysis of the effect of IVIG therapy on anti-PF4 antibody level and platelet activation in VITT patients.
The study cohort consisted of patients who were admitted to our hospitals between February 1 and May 5, 2021 with suspected VITT due to neurological or hematological symptoms after first immunization with ChAdOx1 nCoV-19 (Vaxzevria; AstraZeneca, London, United Kingdom). The diagnosis of VITT was serologically confirmed according to the recommendations of the International Society on Thrombosis and Haemostasis Scientific and Standardization Subcommittee on Platelet Immunology,10 using an immunoglobulin G (IgG)-enzyme immune assay (EIA) to detect IgG antibodies against PF4 (Hyphen Biomed, Neuville-sur-Oise, France). The ability of the sera to activate platelets was tested using a modified heparin-induced platelet aggregation assay as previously described.7 Sera-induced procoagulant platelets were analyzed using flow cytometer as previously described.11 For more details, see the supplemental material, available on the Blood Web site. The study was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the Institutional Review Board of the University of Tuebingen (236/2021BO1). We used GraphPad Prism, version 7.0 (GraphPad, La Jolla, CA) for statistical analysis. A value of P < .05 was accepted as statistically significant.
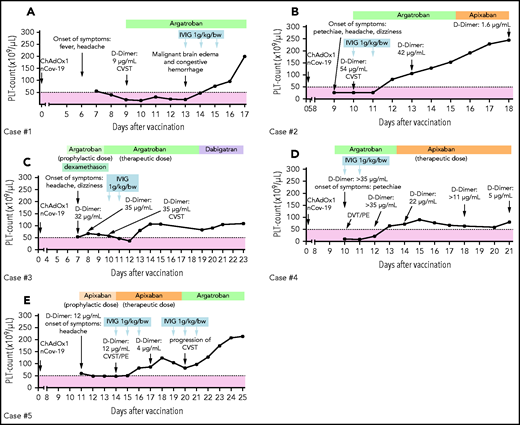
Five patients (3 females) with a median age of 47 years (range, 20-57) were included in this study. The duration between vaccination and hospital admission was 7 to 9 days. All patients had severe thrombocytopenia (41.2 ± 9.7 × 109/L; range, 10-60; Figure 1A-E) and increased D-dimer (9 µg/mL or higher; range, 9-54). At admission, several thrombotic events, including cerebral venous sinus thrombosis (CVST; 4 patients, cases 1-3 and 5), pulmonary embolism (2 patients, cases 4 and 5), and deep vein thrombosis (case 4), were detected. Detailed case descriptions and patient characteristics are given in the supplemental methods and in supplemental Table 1A. VITT diagnosis was confirmed by detecting anti-PF4 IgG antibodies in EIA (optical density [OD] 2.98 ± 0.23; range, 2.07-3.36), platelet activation in the modified heparin-induced platelet aggregation assay (median time to platelet aggregation, 5 minutes; range, 5-5 minutes ), and formation of procoagulant platelets (CD62p/PS+ platelets mean: 45 ± 7; range, 23-66). Laboratory investigations at admission and after IVIG therapy are presented in supplemental Table 1B.
Individual course of the platelet counts and therapies. Five cases (A-E) of VITT after severe acute respiratory syndrome coronavirus 2 vaccination were identified. Patients were treated with nonheparin anticoagulation (argatroban, green blocks; danaparoid, lavender blocks; direct oral anticoagulants, orange blocks) combined with IVIG. Patients receiving therapeutic anticoagulation with platelet counts below 50 × 109/L (dashed line) were considered to be at enhanced risk for major hemorrhage. CSVT, cerebral sinus vein thrombosis; DVT, deep vein thrombosis; PE, pulmonary embolism; PLT, platelet.
Individual course of the platelet counts and therapies. Five cases (A-E) of VITT after severe acute respiratory syndrome coronavirus 2 vaccination were identified. Patients were treated with nonheparin anticoagulation (argatroban, green blocks; danaparoid, lavender blocks; direct oral anticoagulants, orange blocks) combined with IVIG. Patients receiving therapeutic anticoagulation with platelet counts below 50 × 109/L (dashed line) were considered to be at enhanced risk for major hemorrhage. CSVT, cerebral sinus vein thrombosis; DVT, deep vein thrombosis; PE, pulmonary embolism; PLT, platelet.
All patients received parenteral anticoagulation with argatroban (n = 4) or danaparoid (n = 1), and 1 patient (case 5) initially received apixaban. Two patients (cases 3 and 5 on a prophylactic dose of argatroban and apixapan, respectively) developed a new thromboembolic complication at day 4 and 3 of hospitalization (before IVIG administration), respectively. Anticoagulation was continued in these patients with argatroban in a therapeutic dosage (Figure 1C,E).
IVIG was administered at a dose of 1 g/kg body weight for 2 to 5 days. Median of total IVIG dose was 140 g (range, 95 to 600 g). Absolute platelet increment was 32.6 ± 17.1 × 109/L within 48 hours (P vs baseline, .12) and 94.2 ± 23.3 × 109/L within 72 hours after IVIG (P vs baseline, .01; Figure 2A). A complete platelet response (platelet count ≥100 × 109/L) was achieved in 4 patients within 96 hours. One patient had a platelet response (platelet count ≥30 × 109/L and at least twofold increase the baseline count) within 72 hours after IVIG therapy. From a clinical perspective, increasing the platelet count is important in thrombocytopenic patients requiring therapeutic anticoagulation. This is most critical when thrombosis occurs at unusual sites, such as CVST, because of the increased mortality risk due to hemorrhagic transformation after an arterial stroke or CVST. In our cohort, 1 patient (case 1) suffering from VITT-associated CVST had postthrombotic hemorrhage during the thrombocytopenic period, prior to receiving IVIG.
Effect of IVIG therapy on PLT count and procoagulant platelets. Platelet count increment (A) and procoagulant platelets after IVIG therapy (B-C). Procoagulant platelets (CD62P/Phosphatidylserine [PS]+) were analyzed in patients before and after IVIG therapy via Annexin V-FITC and CD62p-APC antibody staining. Where indicated, PLTs were pretreated with PF4 (C). Data are presented as fold increase compared with healthy control. ns, not significant. *P < .05. The number of sera tested is reported in each graphic.
Effect of IVIG therapy on PLT count and procoagulant platelets. Platelet count increment (A) and procoagulant platelets after IVIG therapy (B-C). Procoagulant platelets (CD62P/Phosphatidylserine [PS]+) were analyzed in patients before and after IVIG therapy via Annexin V-FITC and CD62p-APC antibody staining. Where indicated, PLTs were pretreated with PF4 (C). Data are presented as fold increase compared with healthy control. ns, not significant. *P < .05. The number of sera tested is reported in each graphic.
Successful use of IVIG in the treatment of aHIT has been reported.8,9,12 Mainly because of similarities between aHIT and VITT, recent societal guidelines recommend the use of IVIG in VITT with the assumption that IVIG could mitigate the platelet activation induced by anti-PF4 antibodies and thus reduce the platelet consumption and the development of new thrombosis.10,13,14 However, concerns of increased new thrombotic events limit its use.15 Serial D-dimer levels were available from 3 cases, and all of them showed a decrease within 72 hours after IVIG therapy (Figure 1). We observed progression of CVST in 1 patient (case 5). Other patients receiving nonheparin anticoagulation at therapeutic doses combined by IVIG did not develop new thrombosis, indicating sufficient antithrombotic efficacy. Similarly, Thaler et al successfully used IVIG (1 g/kg for 2 consecutive days) and argatroban in a patient with VITT.16 Tiede et al reported a positive platelet response in 3 VITT patients after IVIG therapy (1 g/kg for 2 consecutive days).5 However, 2 patients developed new thromboembolic events (extensive splanchnic vein thrombosis and popliteal artery occlusion).5 New thromboembolic events are common in patients with VITT.5-7 Therefore, clinicians should pay attention to dynamic changes in clinical as well as laboratory parameters and exercise extra vigilance in VITT patients in order to detect new thromboembolic events in a timely manner.
To assess the mechanism by which high-dose IVIG downregulates hypercoagulability in VITT, we analyzed the ability of VITT patients’ sera to generate procoagulant platelets before and after IVIG therapy. The reactivity in PF4 EIA did not change significantly after IVIG administration (n = 4, 3.21 ± 0.06 OD vs 3.18 ± 0.08 OD; P: .798; supplemental Table 1B). On the other hand, the ability of the sera from VITT patients to induce procoagulant platelets reduced after IVIG therapy in 3 cases in the absence and in 2 out of 4 cases in the presence of PF4 (Figure 2B-C; supplemental Table 1B). Noteworthy, diluted sera showed specific platelet activation only in the presence of PF4 (supplemental Figure 1A-D). These data suggest that IVIG interferes with the pathogenic anti-PF4 antibodies by competing with them to bind to Fc γ receptor IIA receptors, which might be in vivo associated with reductions in platelet activation and disseminated intravascular coagulation. The later ones are confirmed in our study by the rapid response in platelet count and decrease in D-dimer levels.7,9 However, the effect of IVIG on other cells cannot be ruled out as another explanation for the observed therapeutic benefit.
In summary, we showed that high-dose IVIG inhibits antibody-mediated procoagulant platelet generation, rapidly increases the platelet count, and finally, deescalates the hypercoagulable state in VITT. Adjunct use of IVIG can be recommended as a therapeutic option to prevent disease progression.
Acknowledgments
This work was supported by grants from the German Research Foundation and from the Herzstiftung BA5158/4 and TSG-Study (T.B.), by special funds from the state of Baden-Württemberg for autopsy-based COVID-19 research, and the DEFEAT PANDEMIcs network funded by the BMBF (Federal Ministry of Education and Research) (P.M. and F.F.).
Authorship
Contribution: G.U., K.A., and T.B. designed the study; P.M., U.Z., A.M., P.R., M.G., G.C.P., J.M., M.B., K.F., H.H., N.H., M.M., C.L., and S.N. were responsible for the treatment of the patients and collected and analyzed the clinical data; G.U., K.A., U.J.S., F.F., and T.B. reviewed medical reports; G.U., K.A., and A.S. performed the experiments; G.U., K.A., A.S., and T.B. analyzed the data, interpreted the results, and wrote the manuscript; and all authors read and approved the manuscript.
Conflict-of-interest disclosure: T.B. has received research funding from CoaChrom Diagnostica GmbH, DFG, Robert Bosch GmbH, Stiftung Transfusionsmedizin und Immunhämatologie eV, Ergomed, Surrey, DRK Blutspendedienst, Deutsche Herzstiftung, Ministerium fuer Wissenschaft, Forschung und Kunst Baden-Wuerttembergm, has received lecture honoraria from Aspen Germany GmbH, Bayer Vital GmbH, Bristol Myers Squibb GmbH & Co, Doctrina Med AG, Meet the Experts Academy UG, Schoechl Medical Education GmbH, Mattsee, Stago GmbH, Mitsubishi Tanabe Pharma GmbH, Novo Nordisk Pharma GmbH, has provided consulting services to Terumo, has provided expert witness testimony relating to heparin-induced thrombocytopenia and non–heparin-induced thrombocytopenia thrombocytopenic and coagulopathic disorders. All of these are outside the current work. The remaining authors declare no competing financial interests.
Correspondence: Tamam Bakchoul, Institute for Clinical and Experimental Transfusion Medicine, Medical Faculty of Tuebingen, Otfried-Müller Str. 4/1, 72072 Tuebingen, Germany; e-mail: tamam.bakchoul@med.uni-tuebingen.de.
Data may be requested for academic collaboration from the corresponding author.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.

![Effect of IVIG therapy on PLT count and procoagulant platelets. Platelet count increment (A) and procoagulant platelets after IVIG therapy (B-C). Procoagulant platelets (CD62P/Phosphatidylserine [PS]+) were analyzed in patients before and after IVIG therapy via Annexin V-FITC and CD62p-APC antibody staining. Where indicated, PLTs were pretreated with PF4 (C). Data are presented as fold increase compared with healthy control. ns, not significant. *P < .05. The number of sera tested is reported in each graphic.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/138/11/10.1182_blood.2021012479/3/m_bloodbld2021012479f2.png?Expires=1769129762&Signature=yIAFlRXsi6nOkKhLC7k7Ok5GvcfGnyfBTkfS-vDHw5SE7gSxPupiqp-QP1G9h6wuMd4UEr7BJSg6wAMFFhfU91YDbjvur3SBkQGgFWA-Eg78Soq24DV72vCKV1tQrSubP3Pesizkn5sZ-s2cfHQoDCnZ4xl6HMepTjGZEssZxHIAN4JNnFQy0VZ-Ic90kSbpVhJyvPhGHvwNmBeSgFRIb6bvfhcVIEuNVId2JSmREroOYsUn7CMMEnMAP0vsSqVeo7KKl-SbyWd0OLdCLiOCOAzvtJX3snR8XSDQslJyWX428u9b-0rg~C90t8wZd0oZQRSzisw~TRRUXO-3ry-tVQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal