Abstract
In the last decade, enormous progress has been made in the development of gene therapy for hemophilia A and B. After the first encouraging results of intravenously administered adeno-associated virus (AAV)-based liver-directed gene therapy in patients with severe hemophilia B were reported in 2011, many gene therapy studies have been initiated. Most of these studies, using AAV vectors with various gene constructs, showed sufficient factor VIII and IX expression in patients to significantly reduce the number of bleeds and the need for prophylaxis in most patients with severe hemophilia. This resulted in great clinical benefit for nearly all patients. In this review, we will summarize the most recent findings of reported and ongoing gene therapy trials. We will highlight the successful outcome of trials with focus on the results of recently reported phase 1 trials and preliminary results of phase 2b/3 trials for hemophilia A and B. These new reports also reveal the impact of side effects and drawbacks associated with gene therapy. We will therefore also discuss the limitations and remaining issues of the current gene therapy approaches. These issues must be resolved before gene therapy will be widely available for the hemophilia patient population.
Current and emerging treatments for hemophilia A and B
Hemophilia is an X-linked hereditary bleeding disorder caused by defects in the F8 or F9 gene, which results in a deficiency of coagulation factor VIII (FVIII) or FIX. Patients with severe hemophilia receive intravenously administered factor concentrate several times per week, thereby reducing joint bleeds and long-term arthropathy.1 Despite this intensive prophylactic regimen, bleedings may still occur, and eventually arthropathy develops over time in many patients.2,3 In the last decade, long-acting FVIII and FIX products have been developed requiring less intravenous injections, resulting in higher trough factor levels and a low bleeding rate.4,5 Other developments are the nonfactor treatments, of which subcutaneously administered emicizumab, a monoclonal antibody mimicking the action of FVIII, is now registered for use in patients with hemophilia A.6,7 Despite these great improvements, patients still need regular treatment. Also, the risk of bleeding is not completely abolished, and additional treatment is still needed before and after surgical interventions. Gene therapy for hemophilia is another developed treatment modality, and recent studies show long-term factor expression and great clinical benefit for patients. However, with the introduction of extended half-life products and nonfactor treatment, the benefit/risk ratio of gene therapy vs other treatments has to be taken into account and considered for each individual patient.8-12
Gene therapy: rationale and historical studies
Gene therapy is a suitable treatment of hemophilia for various reasons. Hemophilia is caused by a single gene defect, a minimal expression of FVIII or FIX already leads to major improvement of the bleeding phenotype, and gene expression can be evaluated easily by measuring factor levels in plasma. The first studies using an ex vivo approach of gene therapy by gene transfer in autologous fibroblasts or hematopoietic stem cells, resulted in short-lasting low expression of FVIII.13 Adeno-associated virus (AAV)-based gene therapy was introduced 20 years ago by intramuscular injection of recombinant AAV (rAAV)-FIX in patients with hemophilia B. The procedure was safe, and expression in muscle biopsies lasted for more than 3 years; however, it resulted in a minimal increase of plasma FIX (most patients <1%).14-16
In 2006, the first AAV2-based liver-directed gene therapy clinical study in patients with hemophilia B showed temporarily expression of FIX. The vector was administered in the hepatic artery, and FIX expression lasted for 2 months.17 Long-term follow-up revealed no adverse events, no liver toxicity, and no development of hepatocellular carcinoma (HCC) even after a period of 12 to 15 years after vector administration.18 In 2011, the first study in patients with hemophilia B treated with intravenously administered liver-directed AAV8-based therapy showed expression levels of FIX between 1 and 6 IU/dL, which resulted in a reduction of bleedings without the need for prophylaxis.19 After 3 years, 6 patients treated with the highest vector dose had median FIX levels of 5 IU/dL, showing long-term stable expression, now even lasting for >8 years.9,20 These promising results were the basis for further development of gene therapy by several research groups and companies. Currently 47 trials on gene therapy for hemophilia have been carried out or are ongoing (clinical trials.gov; January 2021). For tables summarizing all hemophilia A and B gene therapy trials, we refer to recent reviews.20,21
Results of recent AAV-based gene therapy trials for hemophilia A and B
Hemophilia A
The first study on intravenously administered AAV-based liver-directed gene therapy for hemophilia A patients was reported in 2017.10 Because of the size of the full-length FVIII gene, which is too large to be packed in an AAV viral vector, a construct encoding for B-domain deleted FVIII was used. Initial findings of this FVIII gene therapy trial using AAV5 (valoctocogene roxaparvovec) were very encouraging, showing median levels of 77 IU/dL (range, 19-164 IU/dL) at 1 year using a vector dose of 6 × 1013 vector genomes (vg)/kg. The reported FVIII levels were obtained with a 1-stage clotting assay (OSA), which gives 1.65-fold higher levels than chromogenic assays (CSAs). Long-term data after 3-year follow-up showed a marked reduction of the expression compared with the FVIII levels at 1 year (median CSA, 20 vs 60 IU/dL).22 According to the recently reported 4-year data from the 6 × 1013 vg/kg cohort, the decline in FVIII activity continues (median CSA, 16.4 IU/dL).23 Recently, the 1-year data of the phase 3 study in which 134 patients received 6 × 1013 vg/kg of valoctocogene roxaparvovec were reported.24 Median FVIII activity using the CSA was 23.9 IU/dL after 1 year (n = 132; mean, 42.9; standard deviation [SD], 45.5), and the median annualized bleeding rate was 0.0 (mean, 0.8; SD, 3.0). Further details of the study results are awaited, but the large sample size of this study will hopefully allow investigation of several factors that may affect the level and duration of expression of FVIII. Another study on AAV gene therapy in 12 patients with hemophilia A by Spark (SPK8011) showed FVIII levels between 12 and 30 IU/dL (assay type not specified), sufficient to prevent bleeding without the need of prophylaxis. In this study, lower vector doses were infused 0.5 to 2 × 1012 vg/kg compared with the Biomarin study.25 Recently, data of a subset of 5 patients who achieved a steady-state FVIII expression after 8 to 12 weeks revealed no decline of FVIII over a period of 2 years (FVIII:C between 5.2% and 19.8%). However, 2 patients of the initial cohort lost expression of FVIII and restarted prophylaxis.26 Recently, the first data of a phase 1/2 dose-finding study on the use of AAVhu37 capsid vector technology (BAY 2599023, Bayer GET8 study) were presented. In total, 6 patients in 3 dose cohorts showed variable FVIII expression, which was sustained over time up to 16 months. The 2 patients in the highest dose cohort (2 × 1013 gene copies [gc]/kg) had FVIII levels (CSA) of 12 and 72 IU/dL, but the durability of FVIII response cannot be assessed because of the short follow-up.27 The study using AAV6-B-domain deleted FVIII (giroctocogene fitelparvovec [SB-525; ALTA study Sangamo/Pfizer]) showed highly variable FVIII expression levels measured with CSA.28 Five patients in the highest dose cohort (3 × 1013 vg/kg) achieved FVIII levels within the normal range (mean at 8 weeks, 61.5 IU/dL; SD, 26.1 IU/dL) with no bleeding events and no requirements of FVIII administration at 24 weeks. After a follow-up of 1 year, the median steady-state FVIII activity (CSA) was 50.2 IU/dL (mean, 80.1; SD, 93.3); however, the individual data of most patients show a decline over time29 (Table 1).
Data of reported results of intravenously administered AAV-based gene therapy phase 1 to 3 trials for hemophilia A and B
| Sponsor . | Generic/product name . | NC no. . | Phase . | N . | Dose of vector (/kg BW) . | Expression level short term (1-6 mo)* . | 1 y (IU/dL or %) . | >2 y (IU/dL or %) . | Duration and stability . | Reference . |
|---|---|---|---|---|---|---|---|---|---|---|
| Hemophilia A | ||||||||||
| Biomarin Pharmaceuticals | Valoctocogene roxaparvovec (AAV5 BMN 270) | NCT02576795 | 1/2 | 7 | 6 × 1013 | Gradual increase up to 24 wk | 60 (median) 64 (mean) CSA | 36 (median) 26 (mean) | Expression declining after 1 y to 33 (mean) and 20 (median) after 3 y | 10,22 |
| BioMarin Pharmaceutical | Valoctocogene roxaparvovec (AAV5 BMN 270) | NCT03370913 | 3 | 134 | 6 × 1013 | n.a. | 23.9 (median) 42.9 (SD 45.5) CSA | n.a. | No long-term data available | 24 |
| Spark Therapeutics | SPK-8011, AAV LK03-co-BDD-F8 | NCT03003533 | 1/2 | 7 | 2 × 1012 | 16-49 (n = 5), response < 5 in 2 patients | n.a | 5.2-19.8 (n = 5) | Two patients lost expression | 25,26 |
| Sangamo Therapeutics/ Pfizer | Giroctocogene fitelparvovec SB-525, AAV6-co-BDD-F8 (ALTA-Study) | NCT03061201 | 1/2 | 5 | 3 × 1013 | Increase to normal range within 5 wk | 50.2 (median steady state) 80.1 mean (SD 93.3) | n.a. | 28,29 | |
| Bayer | BAY2599023 (DTX 201) AAVhu37- GET-8 study | NCT03588299 | 1/2 | 2 | 2 × 1013 | 12 and 72 | n.a | n.a | Follow-up too short for evaluation of durability | 27 |
| Hemophilia B | ||||||||||
| UCL/St JudesCRH | AAV8 FIX-WT | NCT00979238 | 1 | 6 | 2 × 1012 | 1.4-7.2 (range) (OSA) | 5.1 mean (SD 1.7) | Stable expression over 8 y | 9,20 | |
| Ultragenics Pharm | DTX101, AAVrh10FIX | NCT02618915 | 1/2 | 3 | 5 × 1012 | 12-20 (range) reached within 3-8 wk | Gradual decrease to baseline | Transient, short-term expression | 56 | |
| Baxalta/Takeda | AAV8-coF9-Padua Bax 335 | NCT01687608 | 1/2 | 2 | 3 × 1012 | 45.3 (mean peak levels) (range 32-59) (OSA) | n.a. | 20 (one patient) | Transient, short-term expression in 7; loss of expression in 6 out of 7 | 54,55 |
| UniQure BioPharma | AAV5-hFIX (AMT-060) | NCT02396342 | 1/2 | 5 | 2 × 1013 | 6.9 (mean) (95% CI, 2.6-11.3) (OSA) | n.a. | 7.4 (95% CI 4.2-10.6) | Stable expression over 5 y | 12,30 |
| Spark Therapeutics/ Pfizer | Fidanacogene elaparvovec SPK-9001, mutant AAV8-coF9-Padua Factor IX-long study | NCT03307980 | 1/2 | 10 | 5 × 1011 | 33.7 mean (SD 18.5) Range 14-81 (OSA) | n.a. | n.a. | 11 | |
| UniQure Biopharma | Etranacogene dezaparvovec AAV5-hFIXco-Padua (AMT-061) | NCT03489291 | 2b | 3 | 2 × 1013 | 47 (mean) (range 33.2-57.0) (OSA) | n.a. | n.a. | No long-term data available | 31 |
| Freeline Therapeutics | FLT180a (B-AMAZE) | NCT03369444 | 1 | 2 | 1.5 × 1012 | 160 (67-253) Mean (range at 26 wk) | n.a | n.a. | Studies continued with lower dose | 33 |
| UniQure Biopharma | AAV5-hFIXco-Padua (AMT-061)HOPE-B | NCT03569891 | 3 | 54 | 2 × 1013 | 37.2 (mean) 19.6 SD Range 1-97 (OSA) | n.a. | n.a | No long-term data available | 32 |
| Sponsor . | Generic/product name . | NC no. . | Phase . | N . | Dose of vector (/kg BW) . | Expression level short term (1-6 mo)* . | 1 y (IU/dL or %) . | >2 y (IU/dL or %) . | Duration and stability . | Reference . |
|---|---|---|---|---|---|---|---|---|---|---|
| Hemophilia A | ||||||||||
| Biomarin Pharmaceuticals | Valoctocogene roxaparvovec (AAV5 BMN 270) | NCT02576795 | 1/2 | 7 | 6 × 1013 | Gradual increase up to 24 wk | 60 (median) 64 (mean) CSA | 36 (median) 26 (mean) | Expression declining after 1 y to 33 (mean) and 20 (median) after 3 y | 10,22 |
| BioMarin Pharmaceutical | Valoctocogene roxaparvovec (AAV5 BMN 270) | NCT03370913 | 3 | 134 | 6 × 1013 | n.a. | 23.9 (median) 42.9 (SD 45.5) CSA | n.a. | No long-term data available | 24 |
| Spark Therapeutics | SPK-8011, AAV LK03-co-BDD-F8 | NCT03003533 | 1/2 | 7 | 2 × 1012 | 16-49 (n = 5), response < 5 in 2 patients | n.a | 5.2-19.8 (n = 5) | Two patients lost expression | 25,26 |
| Sangamo Therapeutics/ Pfizer | Giroctocogene fitelparvovec SB-525, AAV6-co-BDD-F8 (ALTA-Study) | NCT03061201 | 1/2 | 5 | 3 × 1013 | Increase to normal range within 5 wk | 50.2 (median steady state) 80.1 mean (SD 93.3) | n.a. | 28,29 | |
| Bayer | BAY2599023 (DTX 201) AAVhu37- GET-8 study | NCT03588299 | 1/2 | 2 | 2 × 1013 | 12 and 72 | n.a | n.a | Follow-up too short for evaluation of durability | 27 |
| Hemophilia B | ||||||||||
| UCL/St JudesCRH | AAV8 FIX-WT | NCT00979238 | 1 | 6 | 2 × 1012 | 1.4-7.2 (range) (OSA) | 5.1 mean (SD 1.7) | Stable expression over 8 y | 9,20 | |
| Ultragenics Pharm | DTX101, AAVrh10FIX | NCT02618915 | 1/2 | 3 | 5 × 1012 | 12-20 (range) reached within 3-8 wk | Gradual decrease to baseline | Transient, short-term expression | 56 | |
| Baxalta/Takeda | AAV8-coF9-Padua Bax 335 | NCT01687608 | 1/2 | 2 | 3 × 1012 | 45.3 (mean peak levels) (range 32-59) (OSA) | n.a. | 20 (one patient) | Transient, short-term expression in 7; loss of expression in 6 out of 7 | 54,55 |
| UniQure BioPharma | AAV5-hFIX (AMT-060) | NCT02396342 | 1/2 | 5 | 2 × 1013 | 6.9 (mean) (95% CI, 2.6-11.3) (OSA) | n.a. | 7.4 (95% CI 4.2-10.6) | Stable expression over 5 y | 12,30 |
| Spark Therapeutics/ Pfizer | Fidanacogene elaparvovec SPK-9001, mutant AAV8-coF9-Padua Factor IX-long study | NCT03307980 | 1/2 | 10 | 5 × 1011 | 33.7 mean (SD 18.5) Range 14-81 (OSA) | n.a. | n.a. | 11 | |
| UniQure Biopharma | Etranacogene dezaparvovec AAV5-hFIXco-Padua (AMT-061) | NCT03489291 | 2b | 3 | 2 × 1013 | 47 (mean) (range 33.2-57.0) (OSA) | n.a. | n.a. | No long-term data available | 31 |
| Freeline Therapeutics | FLT180a (B-AMAZE) | NCT03369444 | 1 | 2 | 1.5 × 1012 | 160 (67-253) Mean (range at 26 wk) | n.a | n.a. | Studies continued with lower dose | 33 |
| UniQure Biopharma | AAV5-hFIXco-Padua (AMT-061)HOPE-B | NCT03569891 | 3 | 54 | 2 × 1013 | 37.2 (mean) 19.6 SD Range 1-97 (OSA) | n.a. | n.a | No long-term data available | 32 |
Only data for the highest dose of vector administered in the phase 1/2 studies are given. N = number of patients included. n.a., not available.
FVIII or FIX activity levels were obtained by OSAs or CSAs. In case no assay type is specified in the table, this is unknown.
Unfortunately, data of the various phase 1 studies is difficult to compare because of a limited number of observations. There is high variability in FVIII expression not only between the various studies but also within dose cohorts in the various studies. In addition, the reported outcomes in the studies are not uniform regarding measurements of FVIII levels (CSA or OSA). An important observation in 3 of the abovementioned studies, including the preliminary results of the phase 3 study with valoctacogene roxaparvovec, is the decline of FVIII expression over time.
Hemophilia B
Most AAV-based gene therapy studies to date have been performed in patients with hemophilia B (Table 1). As mentioned above, the first study showing long-term expression of FIX using AAV8 liver-directed gene therapy was performed by Nathwani et al.9,20 Another phase 1 study with AMT-060 (uniQure), a rAAV5 capsid containing the WT-FIX gene showed vector dose-dependent expression of FIX in 2 dose cohorts, with expression levels around 7 IU/dL in the highest dose cohort.12 Recent data after 5 years of follow-up showed stable and durable expression, with a mean FIX level (OSA) of 5.2 IU/dL (95% confidence interval [CI]: 2.0-8.4) and 7.4 IU/dL (4.2-10.6) in the low dose and high dose cohort, respectively. This was associated with a sustained reduction of factor IX use and ongoing reduction of the number of bleeds.30 To obtain higher FIX activity levels, fidanacogene elaparvovec (SPK9001; Spark) was developed, an AAV-FIX-Padua (FIX R338L) construct, which contains a FIX variant with a five- to eightfold higher specific activity. The phase 1 study with this Padua variant showed a mean FIX activity of 33.7 ± 18.5 IU/dL.11 This FIX-Padua variant is now used in all ongoing FIX gene therapy trials. A phase 2b study in 3 patients and a phase 3 study in 54 patients with etranacogene dezaparvovec (AMT-061, uniQure), using the same vector as AMT-060, showed a mean FIX activity (OSA) of 47 and 37.2 IU/dL, respectively, 6 months after gene transfer.31 In the phase 3 study, this enabled discontinuation of prophylaxis in 96% of patients, and 72% of patients did not report any bleeding during follow-up.32 This is an important study because patients with preexisting neutralizing antibodies to AAV5 were also included. One patient with the highest titer (3212) did not respond to treatment, but the other patients with titers up to 678 had FIX expression similar to patients without neutralizing antibodies.33 The phase 1 B-AMAZE study using FLT180a, a AAVS3 capsid carrying a F9 variant with a gain-of-function mutation, included 10 patients, who were treated in 4 cohorts (maximum dose, 1.5 × 1012 vg/kg). High expression levels of FIX were shown shortly after gene therapy of 24 to 168 IU/dL at 3 weeks, which were stable during follow-up of 1 year. One of the patients with a high expression of FIX (>200 IU/dL) developed a thrombotic occlusion of arteriovenous fistula for which anticoagulant treatment was started.34 It is still unclear if the obtained FIX levels after gene therapy are sufficient to prevent bleeding during surgery or trauma. Recently, George et al35 presented data on 2 surgeries in patients with hemophilia B previously treated with fidanacogene elaparvovec. Surgery because of appendicitis and a lumbar discectomy were uneventful without the need for exogenous FIX concentrate in patients with FIX levels of 26.3 and 11.8 IU/dL.
Currently, several phase 3 studies are ongoing for hemophilia A and B. After a lead-in phase of 6 months to record bleeding rates and factor concentrate use on current (prophylactic) treatment, these patients underwent gene therapy and are followed for a period of 1 year and then for an additional 5 years in a separate study, as recommended by the US Food and Drug Administration (FDA).36 Recently, an updated guide entitled “Human Gene Therapy for Hemophilia; Guidance for Industry” was published by the FDA and contains recommendations for the development of human gene therapy products for hemophilia.37 The guidance includes recommendations for preclinical studies, the design of clinical trials, and the related development of coagulation FVIII and FIX activity assays, as well as on (surrogate) end points, such as factor levels and bleeding rates. The most advanced hemophilia A gene therapy program, which was expected to be approved in 2020, was rejected by the FDA. BioMarin provided 3-year data from the phase 1/2 trial and requested accelerated approval based on preliminary data in a limited number of patients of the phase 3 trial. The FDA concluded that, because of the differences between the phase 1/2 and phase 3 study, they could not rely on the data of the phase 1/2 to support durability of effect. The FDA recommended completion of the phase 3 study and submission of 2-year follow-up safety and efficacy data on all study participants. Gene therapy will drive a significant development in hemophilia care, and hemophilia centers should be adequately prepared to ensure that patients have an equal chance of being offered gene therapy as a therapeutic option. The recently proposed “hub-and-spoke” model by the European Association for Haemophilia and Allied Disorders–European Haemophilia Consortium will help to differentiate between centers with different experiences and ensure that there is monitoring and transfer of experience and knowledge.38
Outcome of gene therapy?
Reduction of bleeding and factor use
As mentioned above, most trials in both hemophilia A and B lead to meaningful expression of FVIII and FIX and result in a strong decrease of both the number of bleedings and the need for prophylaxis.9,12,22 This leads to a tremendous clinical benefit and changes the lives of patients with severe hemophilia.
Achieved levels of FVIII and FIX
For both patients and doctors, the expectations of the achievable factor levels have changed over time. Initially, the aim was to achieve levels of >5 IU/dL to prevent spontaneous joint bleedings. Based on the findings of the phase 1/2 studies, the current desired FVIII and FIX levels after gene therapy are >12 IU/dL and ideally should be above the hemostatic value (>40 IU/dL).39 Several hemophilia experts have advocated that factor levels should be the main outcome measurement of gene therapy trials.40 It is important, however, to notice that the reported levels after gene therapy are strongly dependent on the type of assay used. FVIII levels obtained with OSAs provide higher levels than the CSAs.23 A kinetic bias between OSA and CSA readout times has been suggested, leading to higher OSA levels caused by accelerated early FXa and thrombin formation.41 In gene therapy for hemophilia B using the full-size FIX gene with the Padua variant OSA yielded ≈1.6 times higher results than CSA.42 It is still debated whether the levels obtained with OSA or CSA correlate better with the hemostatic potential and bleeding phenotype. Therefore, it is recommended that both tests should be used to quantify FVIII and FIX activity after gene therapy and that the possibly more accurate lower values measured by the CSA test be relied on.41
Changes in health-related quality of life and loss of identity
Recently, core outcomes have been identified such as the frequency of bleeds, factor activity level, duration of expression, chronic pain, health care resource use, and mental health to evaluate the comparative value of gene therapy.43 The potential impact of gene therapy on patient reported outcomes (PROs) and quality of life has not yet been determined. Significant improvements were reported for Haemophilia Quality of Life Questionnaire for Adults domains Future, Treatment, View, Sport, Feeling, and Total Score at 1 year after treatment.44 Further research will be important to understand the appropriateness of already validated hemophilia-specific PRO instruments to assess health-related quality of life after gene therapy, including ensuring that these validated instruments allow the collection of data to compare gene therapy with traditional treatments. It could be expected that these domains would also show improvements in the long term. However, it is still unclear how the need for frequent visits during the first 2 years and the possible treatment with immunosuppressive drugs, as well as the unknown question of the durability of factor expression and long-term safety will influence PRO. In addition, a sense of loss of identity as a person with hemophilia and member of the hemophilia community may have an emotionally negative impact on gene therapy outcomes and counteract the potential cumulative benefits of gene therapy. Three patients with hemophilia B from the AMT-060 study were interviewed about their experiences with conventional and gene therapy and the effects of the therapy on everyday life. All 3 participants have become more active and participate more in sports. However, they expressed the fear that they did not know how long the effect would last and felt that psychologic support would be necessary if the FIX level was to drop again. No patient expressed concern about possible negative long-term consequences of gene therapy.45
Limitations of gene therapy
Immunologic response, liver function abnormalities, and liver toxicity
Thus far, limited side effects after intravenous infusion of AAV-based gene therapy have been reported; however, mild infusion-related complaints and, in some studies, even more severe reactions with hypotension and fever were encountered.12,29 Administration of AAV vectors will induce innate immune response, followed by adaptive immune responses leading to the development of neutralizing antibodies in all individuals, that will be present long term.46,47 In addition cytotoxic T-cell responses against the vector capsid may occur in 30% to 60% of patients.46 This immune response may be associated with liver function abnormalities (alanine transaminase [ALT] elevations). This risk of developing this response seems to be vector dose dependent.19 It may also be associated with loss of factor expression if not treated immediately with corticosteroids.17,19,48 Remarkably, in several studies, liver function abnormalities occurred without a measurable immunologic cytotoxic T-cell response in the peripheral blood as determined by enzyme-linked immunospot assays; however, this assay is not well standardized and has several limitations. Detailed studies on clinical immunogenicity of AAV5 gene therapy using various humoral and cellular immune response assays showed no clear association between these responses and safety or efficacy measures.49 In recent studies in patients treated for hemophilia A, liver function abnormalities persist over a longer time span, and it is still unknown what the cause of these persistent transaminitis is, but it may be of different origin than the immune response–related liver function abnormalities observed mainly early after gene therapy. It seems to occur more often after gene therapy in patients with hemophilia A. It has been suggested that this may, besides immunologic responses, be caused by cellular stress because hepatocytes physiologically do not synthesize FVIII; cell death caused by high amounts of viral capsids has also been considered.50,51 In the highest dose cohort of the SPK8011 study, 5 of 7 patients required corticosteroid treatment for ALT elevations, immune response, or FVIII decline.25 In the ALTA study, 4 patients receiving giroctocogene fitelparvovec had ALT elevations, responding to corticosteroid treatment, of which 3 had recurrent ALT elevations.29 Also 2 patients treated with high-dose (2 × 1013 gc/kg) BAY2599023 experienced recurrent ALT elevations, requiring long-term corticosteroids for >6 months.52 Because of these observations in many currently initiated trials, corticosteroids with or without additional immunosuppressive drugs are given immediately after vector infusion to prevent liver function abnormalities. The importance of obtaining more insights into the cause of liver function abnormalities is emphasized by the recent reports of 3 children with X-linked myotubular myopathy who experienced severe hepatotoxicity after high-dose (3 × 1014 gc/kg) AAV6 gene therapy. Eventually this resulted in death caused by sepsis and a bleeding event.53,54 The precise mechanism of the hepatotoxicity in these cases is not yet known.
Failure of gene therapy
Some gene therapy approaches resulted in only temporary expression of FVIII and FIX and were considered unsuccesful.8 This was mainly because of immunologic responses and initial low levels of expression measured in the circulation.55-57 In a dose-ascending AAV8-based study using the FIX Padua variant, 7 of 8 participants with severe and moderate hemophilia B achieved transient, dose-dependent measurable FIX activity (peak FIX, 32-58.5 IU/dL) in the highest dose cohort. However, FIX activity was maintained in only 1 of 7 patients55 (Table 1). The cause of the loss of transgene expression in the other 6 patients is not known; however, this might have been caused by immune responses by CpG oligodeoxynucleotides introduced in BAX335 by codon optimization. In another study using AAVrh10, only short-term expression of FIX was observed, with peaks between 12 and 20 IU/dL and subsequent loss of expression in 5 of 6 individuals. This may be related to AAVrh10 capsid-induced immune responses, because these patients developed ALT elevations, and despite treatment with corticosteroids, FIX expression was not preserved.57 In studies that are considered successful, several individual patients did not have expression or very low expression of factor, which should also be considered a failure of this therapy. The mechanism behind these failures and the large variability of expression in general should be further investigated.12,23,26,29
Integration and oncogenesis after AAV gene therapy?
It is assumed that rAAV remain mainly episomal after hepatocyte transduction. However, integration occurs in the host genome, and the possible implications for oncogenesis have been widely discussed.58-60 Given the fact that very high AAV vector loads (>1015) are being delivered, even a low percentage of integration may still be important. In mice, intravenous injection of genome-containing particles of an AAV vector resulted in the development of HCC in 33% of the animals, which was linked to AAV vector integration resulting in clonal expansion of transformed cells.61 Long-term results of gene transfer studies in dogs revealed integration at a low frequency, mainly in intergenic regions of the dog genome, without development of malignancy.47,60 In another study, which confirmed stable and sustained FVIII expression for up to 10 years, 2 of 9 dogs experienced a gradual increase in FVIII activity that was 4 times the steady-state level, and clonal expansions with integration near genes previously associated with growth control and cancer, however, without any evidence of tumorigenesis.62,63 In liver biopsies of clinical study participants, a dose-dependent increase of vector DNA corresponding with FVIII expression levels has been detected.64 None of the 4 patients treated 12 to 15 years ago with AAV2-based intravascular gene therapy developed HCC.18 Despite these reassuring (pre)clinical data in dogs and humans, recently, HCC developed in a hemophilia B patient 1 year after treatment with etranacogene dezaparvovec gene therapy.65 Investigations of tumor biopsies have shown no evidence of clonal expansion or any dominant integration event. Additionally, whole genome sequencing confirmed that the HCC had genetic mutations that are independent of vector integration.65 This event also shows the importance of collecting long-term data on safety and efficacy in a global registry for patients with gene therapy.66
Limitations in the possibility of gene therapy
Preexisting neutralizing AAV antibodies
Preexisting neutralizing antibodies against AAV are widely distributed in the general population.67 In patients with hemophilia A from the United Kingdom, 21% and 23% had antibodies to AAV 5 and AAV8, respectively, with a high degree of cross-reactivity. Seroprevalence increased with age and exposure to plasma products.68 A major problem with regard to the importance of detection of these antibodies in circulation is the fact that various assays are in use, and these are not standardized. Preclinical studies demonstrated the capacity of humoral immunity to prevent successful transduction of AAV into hepatocytes.69,70 Therefore, preexisting neutralizing antibodies are still an exclusion criteria in studies, and it is expected that these patients will also be ineligible in the future. However, in the uniQure AMT-060 studies, 3 patients were included that, on hindsight, turned out to have AAV5 neutralizing antibodies, using a more sensitive luciferase-based assay. These 3 patients had expression and benefit of treatment, and FIX levels were not related to the titer of the neutralizing antibodies.71 Therefore, in the phase 3 study, individuals with preexistent AAV5 neutralizing antibodies were also included, and preliminary data show that neutralizing antibodies titers up to 678 do not seem to affect FIX expression.33 It has been demonstrated in nonhuman primates with preexisting high-titer neutralizing antibodies against AAV5 that immune adsorption is effective to reduce the titer of neutralizing antibodies before gene therapy, which resulted in therapeutic levels of FIX expression.72 Preclinical studies have demonstrated that the immune response induced by AAV antibodies could be inhibited by an endopeptidase (imlifidase) able to degrade circulating immunoglobulin G, which would provide the prospect of treatment of a larger patient population.73 It is important to note that, after treatment with AAV-based gene therapy, high-titer neutralizing antibodies circulate, which thus far makes it unlikely that patients can be retreated with AAV, although imlifidase may overcome this problem by degrading the anti-AAV immunoglobulin G.73
Conclusions
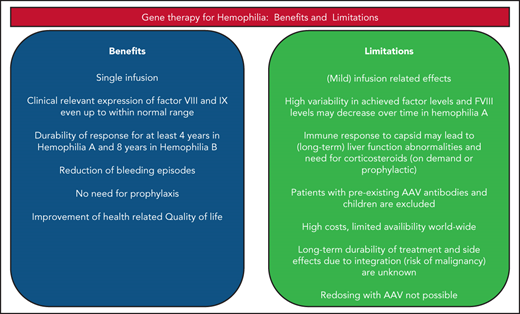
Great clinical benefit has been achieved in patients with hemophilia A and B with intravenously administered AAV-based liver-directed gene therapy. The expectations to be able to transform patients with severe hemophilia into patients with mild hemophilia using gene therapy have been met, and FVIII and FIX levels even in the normal range have been achieved. In most patients, prophylaxis could be discontinued, and limited or no bleedings occurred. Unfortunately, several open questions and issues still remain, which are addressed in Table 2: the variability in expression levels, occurrence of immunologic responses, and the need for immunosuppressive therapy because of the occurrence of liver function abnormalities, of which the nature is yet unresolved. Also, safety issues regarding integration and potential development of malignancy need further research. A major issue has emerged given the observed decline of FVIII expression levels within 3 to 4 years after gene therapy. Therefore, long-term results of ongoing phase 3 studies are needed before gene therapy may become widely available.
Main findings on clinical benefit and remaining issues in hemophilia gene therapy
| Various outcomes and aspects of gene therapy . | Findings in favor of gene therapy . | Remaining issues of gene therapy . |
|---|---|---|
| Clinical benefit | Great clinical benefit in most patients: Reduction of bleeding and cessation of prophylaxis for patients after a single IV infusion of AAV-based gene therapy | Uncertainty about the long-term efficacy |
| Eligibility of patients | Treatment of both hemophilia A and B patients | Not available for patients with AAV antibodies, with liver disease, children, and with severe comorbidity |
| Expression levels | Factor levels sufficient to reduce bleeding and stopping prophylaxis in a large majority of patients | Variability in expression levels of FVIII and FIX, from 0 to >200 IU/dL; reason for variability with similar gene constructs is yet unknown |
| Durability | Stable expression in FIX based gene therapy for over 8 y; despite reduction of FVIII levels over time, duration of clinical response at least 4 y | Limited durability of expression in hemophilia A in the only study with long-term data after 4 y; some patients lose expression early because of immune response; unknown why expression levels of FVIII decrease over time |
| Antibodies against factor VIII and FIX | None reported thus far | Patients with a history of inhibitors have been excluded from phase 1-3 trials |
| Toxicity | Limited infusion-related toxicity | Some patients experience infusion-related reactions, including fever and hypotension; unexplained liver function abnormalities in around 30% of patients, not only related to short-term immune response but also >3 mo after infusion, requiring corticosteroid treatment; in AAV based trials in other genetic disorders severe hepatic toxicity with very high vector doses |
| Immune response | In a minority of patients, depending upon vector dose and construct, leading to ALT elevation, but in most cases well responding to corticosteroids preventing decline of factor levels | If not treated appropriately and timely this may lead to reduction of FVIII and FIX levels and even failure of therapy; some gene therapy programs use prophylactic corticosteroids and other immunosuppressive therapy to prevent liver function abnormalities |
| Availability | Studies are performed in various countries in Europe, United States, and Asia | Thus far available only in centers with gene therapy study expertise; gene therapy will not be available for many patients in the world because of logistic reasons and high costs |
| Costs | May be cost-effective by reducing the need for prophylaxis with expensive long-term factor concentrate | Expected high costs for one single treatment, although exact costs are yet unknown |
| Safety | Limited integration of AAV, mainly episomal located | Integration does occur in the host genome. Potential of developing malignancy and association with malignancy should be investigated |
| Redosing | May be possible in the future with non–AAV-based gene therapy | Not possible with similar AAV-based technology because of high and persistent AAV antibodies after gene therapy |
| Various outcomes and aspects of gene therapy . | Findings in favor of gene therapy . | Remaining issues of gene therapy . |
|---|---|---|
| Clinical benefit | Great clinical benefit in most patients: Reduction of bleeding and cessation of prophylaxis for patients after a single IV infusion of AAV-based gene therapy | Uncertainty about the long-term efficacy |
| Eligibility of patients | Treatment of both hemophilia A and B patients | Not available for patients with AAV antibodies, with liver disease, children, and with severe comorbidity |
| Expression levels | Factor levels sufficient to reduce bleeding and stopping prophylaxis in a large majority of patients | Variability in expression levels of FVIII and FIX, from 0 to >200 IU/dL; reason for variability with similar gene constructs is yet unknown |
| Durability | Stable expression in FIX based gene therapy for over 8 y; despite reduction of FVIII levels over time, duration of clinical response at least 4 y | Limited durability of expression in hemophilia A in the only study with long-term data after 4 y; some patients lose expression early because of immune response; unknown why expression levels of FVIII decrease over time |
| Antibodies against factor VIII and FIX | None reported thus far | Patients with a history of inhibitors have been excluded from phase 1-3 trials |
| Toxicity | Limited infusion-related toxicity | Some patients experience infusion-related reactions, including fever and hypotension; unexplained liver function abnormalities in around 30% of patients, not only related to short-term immune response but also >3 mo after infusion, requiring corticosteroid treatment; in AAV based trials in other genetic disorders severe hepatic toxicity with very high vector doses |
| Immune response | In a minority of patients, depending upon vector dose and construct, leading to ALT elevation, but in most cases well responding to corticosteroids preventing decline of factor levels | If not treated appropriately and timely this may lead to reduction of FVIII and FIX levels and even failure of therapy; some gene therapy programs use prophylactic corticosteroids and other immunosuppressive therapy to prevent liver function abnormalities |
| Availability | Studies are performed in various countries in Europe, United States, and Asia | Thus far available only in centers with gene therapy study expertise; gene therapy will not be available for many patients in the world because of logistic reasons and high costs |
| Costs | May be cost-effective by reducing the need for prophylaxis with expensive long-term factor concentrate | Expected high costs for one single treatment, although exact costs are yet unknown |
| Safety | Limited integration of AAV, mainly episomal located | Integration does occur in the host genome. Potential of developing malignancy and association with malignancy should be investigated |
| Redosing | May be possible in the future with non–AAV-based gene therapy | Not possible with similar AAV-based technology because of high and persistent AAV antibodies after gene therapy |
Authorship
Contribution: F.W.G.L. and W.M. wrote the manuscript and approved the final manuscript.
Conflict-of-interest disclosure: F.W.G.L. received unrestricted research grants from CSL Behring, Shire/Takeda, and uniQure; is a consultant for CSL Behring, Shire/Takeda, Biomarin, and uniQure, of which the fees go to the University; has received travel support from SOBI; and is a Data Safety Monitoring Board member of a study sponsored by Roche. W.M. received unrestricted research grants from Amgen, Bayer, Biotest, CSL, LFB, Novo Nordisk, Octapharma, Pfizer, Shire, and Sobi; is a consultant for Ablynx, Amgen, Aventis, Bayer, Biomarin, Freeline, Leo, LFB, Octapharma, Sobi, Novartis, Novo Nordisk, Octapharma, Pfizer, Roche, Sanofi, Sobi, and UniQure; and is a DSMB member of a study sponsored by Octapharma.
Correspondence: Frank W. G. Leebeek, Erasmus MC, University Medical Center Rotterdam, Department of Hematology, Dr Molewaterplein 40, 3015 GD Rotterdam, The Netherlands; e-mail: f.leebeek@erasmusmc.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal