Mucosa-associated lymphoid tissue (MALT)1, a key regulator of normal and pathological B-cell receptor (BCR) signaling, is a promising target for lymphoma therapy. In this issue of Blood, Fontan et al report that mammalian target of rapamycin (mTOR) inhibitors can sensitize diffuse large B-cell lymphoma (DLBCL) to pharmacological MALT1 targeting and synergize with MALT1 inhibitors to kill DLBCL cells.1
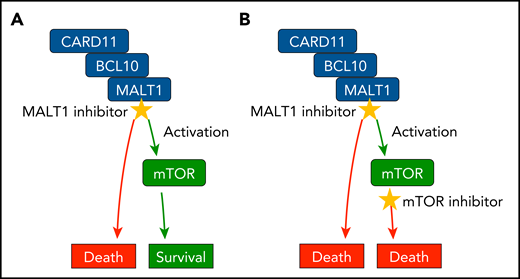
Rationale for MALT1 and mTOR coinhibition. (A) MALT1 inhibitor treatment is toxic for ABC DLBCL cells, but also activates mTOR signaling in the tumor cell, which induces secondary survival programs that mediate resistance. (B) Coinhibition of MALT1 and mTOR disrupts these mechanisms and triggers synergistic ABC DLBCL cell killing.
Rationale for MALT1 and mTOR coinhibition. (A) MALT1 inhibitor treatment is toxic for ABC DLBCL cells, but also activates mTOR signaling in the tumor cell, which induces secondary survival programs that mediate resistance. (B) Coinhibition of MALT1 and mTOR disrupts these mechanisms and triggers synergistic ABC DLBCL cell killing.
The BCR is critical for the function of normal B cells, but aberrant BCR signaling can also sustain malignant B-cell growth in aggressive and indolent lymphomas.2 Furthermore, a variety of gain-of-function mutations within BCR signaling molecules are recurrently detected in B-cell malignancies and are causally connected to the diseases. Although pharmacological targeting of BCR signaling pathways can be effective for B-cell lymphoma treatment, its full therapeutic potential has not yet been realized.2
The signals from the BCR are physiologically initiated by proximal tyrosine kinases like SYK and BTK and transduced to downstream effector cascades via lipid and serine/threonine kinases like phosphatidylinositol 3-kinase (PI3K), AKT, and PKC. The pivotal link that channels BCR proximal signaling to the essential NF-κB survival pathway is the CARD11-BCL10-MALT1 (CBM) signalosome,3 which positions ubiquitin regulators for canonical NF-κB and MAPK activation. The MALT1 paracaspase subunit also possesses a unique proteolytic domain, which cleaves and inactivates negative NF-κB regulators like A20 and other factors to further amplify the immune receptor signals.3 Since the initial demonstration that pharmacological MALT1 protease inhibition is lethal for activated B-cell–type (ABC) DLBCL cells,4,5 several groups have developed small-molecule inhibitors of MALT1, and the first-in-human study involving MALT1 inhibitors has now been initiated in lymphoma patients (registered at www.clinicaltrials.gov as #NCT03900598).
Because lymphoma cells frequently compensate for the targeted inhibition of oncogenic signaling by rewiring biochemical survival networks, limiting therapeutic responses, Fontan et al searched for cellular factors that could mitigate or enhance the effects of MALT1 inhibitors. To this end, they first performed an unbiased short hairpin RNA knockdown screen in an ABC DLBCL cell line in the presence of MALT1 inhibitors and used bioinformatics to identify the pathways associated with resistance or sensitivity to MALT1 inhibition. Interestingly, loss of activating proteins in the BCR, PI3K-AKT, or Toll-like receptor (TLR) pathway sensitized the lymphoma cells to MALT1 inhibition, whereas depletion of negative regulators of BCR and NF-κB signaling or several metabolic programs mediated resistance. On the basis of these results, the authors designed a secondary drug screen with combinations of MALT1 inhibitors and clinically available compounds that target key signaling kinases in the BCR, PI3K-AKT, and TLR pathways. Although combinations of MALT1 inhibitors with SYK, PKC, or BTK inhibitors were largely additive, PI3K inhibitors were mostly synergistic with MALT1 inhibitors in ABC DLBCL cell killing.
The combination of PI3Kδ inhibition and MALT1 targeting was the most synergistic and effective. The PI3Kδ inhibitor CAL-101 is US Food and Drug Administration approved for lymphoid neoplasms, and this specific combination was studied in further detail and in xenograft models in vivo. Although each compound by itself had only a modest effect on the expansion of an ABC DLBCL line in immune-deficient NOD-SCID mice, the combination of the MALT1 and PI3Kδ inhibitors significantly impaired tumor growth. However, after prolonged MALT1 and PI3Kδ inhibition, the tumors resumed expansion, indicating the development of resistance. Detailed analysis of the escaping tumors demonstrated increased NF-κB signaling and increased mTOR pathway activity, suggesting feedback mechanisms that attenuate the MALT1 inhibitor response. Because even short-term inhibition of MALT1 in ABC DLBCL cells resulted in a rapid activation of mTOR signaling, the authors finally combined MALT1 inhibitors with rapamycin or temsirolimus, which inhibits the mTOR complex 1 (mTORC1), and tested the combined effects on ABC DLBCL cell lines and primary patients’ samples in 3-dimensional organoid culture. In all cases, the MALT1/mTORC1 inhibitory combinations induced apoptosis and inhibited proliferation in a highly synergistic manner. Moreover, in the abovementioned xenograft model, the combination of the MALT1 and mTORC1 inhibitors resulted in marked regression of lymphoma compared with the individual drugs alone and led to a significant survival benefit for the animals.
Together, this study demonstrates that the pharmacological blockage of MALT1 in ABC DLBCL induces tumor cell–intrinsic activation of mTOR activity, which provides a secondary survival advantage and resistance in the lymphoma cells. mTOR inhibitors can disrupt this rescue pathway and thereby work synergistically with MALT1 inhibitors, resulting in ABC DLBCL cell killing (see figure). These results provide an attractive clinical angle to enhance the effectiveness of therapeutic MALT1 blockage with mTOR inhibitors. The findings are also consistent with the fact that MALT1 colocalizes within the CBM complex with BCR, MyD88, TLR9, and mTOR on endolysosomes in DLBCL cells.6 Thus, it is possible that MALT1 could directly control mTOR activity by cleaving regulatory proteins in DLBCL cells, which should be investigated. Moreover, MALT1 has also been validated as a therapeutic target for other B-cell malignancies with aberrant BCR signaling, such as chronic lymphocytic leukemia and mantle cell lymphoma.7,8 It will be of interest to investigate whether treatment of these lymphomas would also benefit from combined MALT1 and mTOR inhibition. However, MALT1 activity as well as mTOR activity is important in malignant B cells and critical in immune cells in the tumor microenvironment, including conventional and regulatory T cells and innate immune cells.2,3,9,10 Because systemic MALT1 inhibition may have pleiotropic effects and could result in autoimmunity or the reprogramming of regulatory T cells, enhancing antitumor immune responses, it will be important to define the in vivo consequences of combined MALT1 and mTOR inhibition with regard to B-cell lymphomas that grow in the presence of the complex immune system.
Conflict-of-interest disclosure: The author declares no competing financial interests.