Key Points
Dose/schedule-adjusted Rd-R prolonged EFS in elderly intermediate-fit patients with MM.
Rd-R induced progression-free and overall survival similar to standard continuous Rd.
Abstract
Lenalidomide-dexamethasone (Rd) is standard treatment for elderly patients with multiple myeloma (MM). In this randomized phase 3 study, we investigated efficacy and feasibility of dose/schedule-adjusted Rd followed by maintenance at 10 mg per day without dexamethasone (Rd-R) vs continuous Rd in elderly, intermediate-fit newly diagnosed patients with MM. Primary end point was event-free survival (EFS), defined as progression/death from any cause, lenalidomide discontinuation, or hematologic grade 4 or nonhematologic grade 3 to 4 adverse event (AE). Of 199 evaluable patients, 101 received Rd-R and 98 continuous Rd. Median follow-up was 37 months. EFS was 10.4 vs 6.9 months (hazard ratio [HR], 0.70; 95% confidence interval [CI], 0.51-0.95; P = .02); median progression-free survival, 20.2 vs 18.3 months (HR, 0.78; 95% CI, 0.55-1.10; P = .16); and 3-year overall survival, 74% vs 63% (HR, 0.62; 95% CI, 0.37-1.03; P = .06) with Rd-R vs Rd, respectively. Rate of ≥1 nonhematologic grade ≥3 AE was 33% vs 43% (P = .14) in Rd-R vs Rd groups, with neutropenia (21% vs 18%), infections (10% vs 12%), and skin disorders (7% vs 3%) the most frequent; constitutional and central nervous system AEs mainly related to dexamethasone were more frequent with Rd. Lenalidomide was discontinued for AEs in 24% vs 30% and reduced in 45% vs 62% of patients receiving Rd-R vs Rd, respectively. In intermediate-fit patients, switching to reduced-dose lenalidomide maintenance without dexamethasone after 9 Rd cycles was feasible, with similar outcomes to standard continuous Rd. This trial was registered at www.clinicaltrials.gov as #NCT02215980.
Introduction
The International Myeloma Working Group (IMWG) frailty score identifies fit, intermediate-fit, and frail patients with multiple myeloma (MM) that have different survival predictions and risks of toxicity from treatment in newly diagnosed MM (NDMM). According to the score, intermediate-fit patients are those age 76 to 80 years or younger with impairments in functional abilities (Activities of Daily Living [ADL] ≤4 or Instrumental Activities of Daily Living [IADL] ≤5) or comorbidities (Charlson Comorbidity Index [CCI] ≥2).1
Combination therapies, including lenalidomide-dexamethasone (Rd), bortezomib-melphalan-prednisone (VMP), and bortezomib-Rd,2-4 are considered standard treatment options for elderly patients not eligible for autologous stem cell transplantation (ASCT).2-4 Monoclonal antibody–based treatments, including daratumumab plus either Rd or VMP,5,6 have been approved by the European Medicines Agency and US Food and Drug Administration and have recently become new standards of care in this setting.
Rd is effective and safe in elderly patients with NDMM of all ages. However, the outcomes of patients age >75 years in the FIRST trial were suboptimal compared with those of younger patients (progression-free survival [PFS], 20 vs 28 months; overall survival [OS], 52 vs 60.9 months).2,7 The impact of age on efficacy was less evident in the MAIA trial, in which the outcomes of patients age <75 and ≥75 years receiving Rd were comparable (median PFS, 32 vs 34 months).8 Although life expectancy should be considered, there is large variability within the same age groups (eg, from 4.9 to 14.2 years in the population of those age 75 years), reflecting health status variability.9
Clinical trials usually have stringent eligibility criteria, and patients with myeloma age ≥75 years or with comorbidities or functional impairments are an understudied population. In the FIRST and MAIA trials,2,6 which led to the approval of Rd and daratumumab-Rd, median age was 73 years, with 35% and 43.5% of patients age >75 years, respectively. Nevertheless, patients with Eastern Cooperative Oncology Group performance status >2 or impaired organ function (eg, significant cytopenia or hepatic impairment) were excluded. Furthermore, older patients are more susceptible to adverse events (AEs) that may negatively affect the duration of treatment or outcome because of increased comorbidities, altered pharmacodynamics, or functional impairments.10 Standard treatments may induce a high rate of grade 3 to 4 AEs (75% to 91%), leading to discontinuation because of AEs in 30% of patients.2,3,11-13
Continuous therapy, such as Rd or daratumumab-Rd, until progressive disease (PD) and daratumumab-VMP followed by daratumumab maintenance until PD have proven to be more effective compared with fixed-duration therapy.5,7 Currently, there are no clear data on the advantage of continuous steroid treatment compared with fixed-duration treatment in NDMM. Nevertheless, steroids are scarcely tolerated in the long term, even in younger patients,14 and whether sparing dexamethasone is as effective as prolonged steroid exposure remains an open issue.
In the FIRST trial, among patients age >75 years treated with continuous Rd, one-third discontinued lenalidomide and 44% required dose reduction. Moreover, at 18 months, only 30% of patients age ≥75 years were receiving the full planned dose of lenalidomide.7 In the EMN01 study, in which the lenalidomide dose was reduced to 10 mg during maintenance after full-dose induction, median PFS in intermediate-fit patients was 16.6 months, 16% of patients discontinued treatment because of AEs, and 16% reduced lenalidomide.15,16 Therefore, reducing the dose of lenalidomide after induction seems a valuable strategy to improve the feasibility of treatment in elderly intermediate-fit patients.
We designed a trial for elderly intermediate-fit patients with NDMM and compared Rd induction followed by lenalidomide maintenance at 10 mg per day without steroids (Rd-R) vs standard continuous Rd. We aimed to evaluate whether the Rd schedule could be further optimized and whether avoiding continuous steroids would be a more appropriate strategy in this group of patients.
Methods
Study patients
Patients with NDMM age >65 and ≤80 years who were ineligible for ASCT and defined as intermediate-fit according to the IMWG frailty score (score, 1) were eligible for enrollment.1 Fit (IMWG frailty score, 0) and frail (score, ≥2) patients were excluded from the trial. The definition of intermediate-fit patients, based on age and CCI, ADL, and IADL scores, is summarized in Table 1.
IMWG frailty score to define patient frailty status
| Parameter . | Score . |
|---|---|
| Age, y | |
| ≤75 | 0 |
| 76-80 | 1 |
| >80 | 2 |
| ADL | |
| >4 | 0 |
| ≤4 | 1 |
| IADL | |
| >5 | 0 |
| ≤5 | 1 |
| CCI | |
| ≤1 | 0 |
| ≥2 | 1 |
| Patient status | |
| Fit | 0 |
| Intermediate-fit | 1 |
| Frail | ≥2 |
| Parameter . | Score . |
|---|---|
| Age, y | |
| ≤75 | 0 |
| 76-80 | 1 |
| >80 | 2 |
| ADL | |
| >4 | 0 |
| ≤4 | 1 |
| IADL | |
| >5 | 0 |
| ≤5 | 1 |
| CCI | |
| ≤1 | 0 |
| ≥2 | 1 |
| Patient status | |
| Fit | 0 |
| Intermediate-fit | 1 |
| Frail | ≥2 |
Inclusion criteria were measurable and symptomatic disease. Patients could be enrolled regardless of abnormal baseline laboratory values (eg, absolute neutrophil count, <1 × 109/L; platelet count, <80 × 109/L; hemoglobin, <8 g/dL; creatinine clearance, <30 mL/min), so those who are generally excluded from clinical trials were included. Exclusion criteria were the presence of another malignancy, uncontrolled active infection, and active hepatitis B or C or HIV infection. Patients agreed to use contraception throughout the study. The study was approved by the institutional review board at each of the participating centers. All patients provided written informed consent before entering the study, which was performed in accordance with the Declaration of Helsinki.
Study design and intervention
This was a multicenter randomized (1:1) phase 3 clinical trial involving 33 Italian centers. The primary end point was event-free survival (EFS); secondary end points included PFS, OS, response rate, and incidence of dose reduction and drug discontinuation.
Patients randomly assigned to Rd-R received 9 28-day induction cycles of lenalidomide (25 mg per day for 21 days) and dexamethasone (20 mg on days 1, 8, 15, and 22) followed by lenalidomide maintenance (10 mg per day for 21 days) until PD or intolerance. Patients allocated to continuous Rd received 28-day cycles of lenalidomide (25 mg per day for 21 days) and dexamethasone (20 mg on days 1, 8, 15, and 22) until PD or intolerance, as adopted in patients age >75 years in the FIRST trial.7 Antithrombotic prophylaxis was mandatory during lenalidomide therapy; aspirin or low molecular weight heparin were chosen according to the thrombotic risk of the patient.17 Granulocyte colony-stimulating factor, blood, and platelet transfusions were allowed during the study. In case of neutropenia or recurrent infections, antibacterial prophylaxis was recommended but not mandatory. Treatment was discontinued in case of withdrawal of consent, PD, or AEs that, in the investigator’s opinion, could cause severe or permanent harm to the patient. Less serious toxicities were managed through dose reductions of the study drugs.
Assessments of end points
The primary end point of the study was EFS, which was defined as the occurrence of grade 4 hematologic AEs, grade 3 to 4 nonhematologic AEs (including second primary malignancy [SPM]), discontinuation of lenalidomide, PD, or death. EFS was calculated from the time of enrollment until the occurrence of any of the defining events mentioned above, whichever came first. PFS was calculated from the time of enrollment until the date of PD or death resulting from any cause, whichever came first. OS was calculated from the time of enrollment until the date of death resulting from any cause. Patients who did not experience any event were censored at the date of last follow-up. Evaluation of response to treatment was performed according to the International Response Criteria for Multiple Myeloma.18 AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0). Efficacy assessment was performed every 4 weeks during study treatment. Safety assessment was performed every 2 weeks for the first 9 cycles and every 4 weeks thereafter. The lead/corresponding author analyzed the data, and all authors had access to primary clinical trial data and approved the manuscript.
Statistical analysis
The study was designed to compare EFS between patients in the experimental Rd-R arm vs standard continuous Rd arm. With a 1-sided α error of 0.05, a sample size of 210 patients was determined to provide 80% power to detect a hazard ratio (HR) of 0.62 in favor of Rd-R, assuming 2 years of accrual, study duration of 5 years, and 15% dropout rate. To yield the necessary number of events (n = 139) for the primary analysis, calculated with the Schoenfeld formula, 210 patients were needed. Patients were analyzed on an intention-to-treat basis for all time-to-event end points. Interim safety analyses were planned when the first 50 patients completed the first cycle and the first year of treatment. Response rates and safety were analyzed in patients receiving at least 1 dose of study drug. Kaplan-Meier curves were generated for all time-to-event end points, and the long-rank test was used to compare treatment arms. A Cox proportional hazards model, adjusted for the main prognostic factors (chromosomal abnormalities, lactate dehydrogenase, and International Staging System [ISS]) and age (>75 vs ≤75 years), was used to estimate HR values and 95% confidence intervals (CIs) for the intention-to-treat population. Response rates and AEs rates were compared using the Fisher’s test. Times of observation were censored on 4 March 2020. Data were analyzed using R software (version 3.6.2).
Results
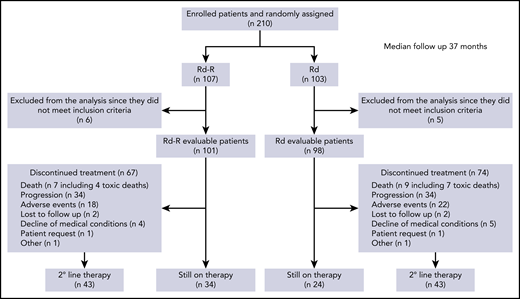
A total of 210 patients were enrolled between 28 October 2014 and 3 October 2017. Eleven patients did not meet the inclusion criteria and were excluded from the analysis. Therefore, 199 patients were randomly assigned to Rd-R (n = 101) or continuous Rd (n = 98) and could be evaluated (Figure 1).
Patient and disease characteristics were well balanced between the 2 arms (Table 2). Median age was 75 years in the Rd-R arm and 76 years in the Rd arm (P = .06); 21% and 26% of patients were ISS stage 3 (P = .5), and 19% vs 23% had high-risk chromosomal abnormalities (P = .68), respectively; 52% of patients in the Rd-R group and 43% in the Rd group were defined as intermediate-fit, not because of age but because of geriatric impairments (CCI or ADL or IADL) only.
Baseline patient and disease characteristics
| . | Rd-R (n = 101) . | Rd (n = 98) . | All patients (n = 199) . |
|---|---|---|---|
| Age, y | |||
| Median | 75 | 76 | 76 |
| IQR | 73-77 | 74-79 | 73-78 |
| 76-80 | 48 (48) | 56 (57) | 104 (52) |
| Sex | |||
| Male | 53 (52) | 49 (50) | 102 (51) |
| Female | 48 (48) | 49 (50) | 97 (49) |
| ECOG PS | |||
| 0 | 35 (37) | 36 (37) | 71 (37) |
| 1 | 50 (52) | 51 (53) | 101 (52) |
| 2 | 11 (11) | 10 (10) | 21 (11) |
| Missing | 5 | 1 | 6 |
| Creatinine clearance, mL/min | |||
| ≤60 | 36 (36) | 48 (49) | 84 (42) |
| >60 | 65 (64) | 50 (51) | 115 (58) |
| ISS | |||
| 1 | 32 (32) | 37 (38) | 69 (35) |
| 2 | 48 (47) | 36 (37) | 84 (42) |
| 3 | 21 (21) | 25 (25) | 46 (23) |
| Chromosomal abnormalities | |||
| Standard risk | 55 (81) | 56 (77) | 111 (79) |
| High risk* | 13 (19) | 17 (23) | 30 (21) |
| Data missing† | 33 | 25 | 58 |
| Amp1q | 25 (38) | 34 (49) | 59 (44) |
| Intermediate-fit | |||
| Age | 48 (48) | 56 (57) | 104 (52) |
| Geriatric impairment | |||
| CCI ≥2 | 23 (23) | 17 (18) | 40 (20) |
| ADL ≤4 | 9 (9) | 12 (12) | 21 (11) |
| IADL ≤5 | 21 (21) | 13 (13) | 34 (17) |
| . | Rd-R (n = 101) . | Rd (n = 98) . | All patients (n = 199) . |
|---|---|---|---|
| Age, y | |||
| Median | 75 | 76 | 76 |
| IQR | 73-77 | 74-79 | 73-78 |
| 76-80 | 48 (48) | 56 (57) | 104 (52) |
| Sex | |||
| Male | 53 (52) | 49 (50) | 102 (51) |
| Female | 48 (48) | 49 (50) | 97 (49) |
| ECOG PS | |||
| 0 | 35 (37) | 36 (37) | 71 (37) |
| 1 | 50 (52) | 51 (53) | 101 (52) |
| 2 | 11 (11) | 10 (10) | 21 (11) |
| Missing | 5 | 1 | 6 |
| Creatinine clearance, mL/min | |||
| ≤60 | 36 (36) | 48 (49) | 84 (42) |
| >60 | 65 (64) | 50 (51) | 115 (58) |
| ISS | |||
| 1 | 32 (32) | 37 (38) | 69 (35) |
| 2 | 48 (47) | 36 (37) | 84 (42) |
| 3 | 21 (21) | 25 (25) | 46 (23) |
| Chromosomal abnormalities | |||
| Standard risk | 55 (81) | 56 (77) | 111 (79) |
| High risk* | 13 (19) | 17 (23) | 30 (21) |
| Data missing† | 33 | 25 | 58 |
| Amp1q | 25 (38) | 34 (49) | 59 (44) |
| Intermediate-fit | |||
| Age | 48 (48) | 56 (57) | 104 (52) |
| Geriatric impairment | |||
| CCI ≥2 | 23 (23) | 17 (18) | 40 (20) |
| ADL ≤4 | 9 (9) | 12 (12) | 21 (11) |
| IADL ≤5 | 21 (21) | 13 (13) | 34 (17) |
Data presented as n (%) of patients with available data, unless otherwise specified.
ECOG PS, European Eastern Cooperative Group performance status.
At least 1 of the following: del17, t(14;16), or t(4;14). Positivity cutoff: del17, 10%; t(14;16) or t(4;14), 15%.
Data on del17, t(14;16), or t(4;14) missing.
Efficacy
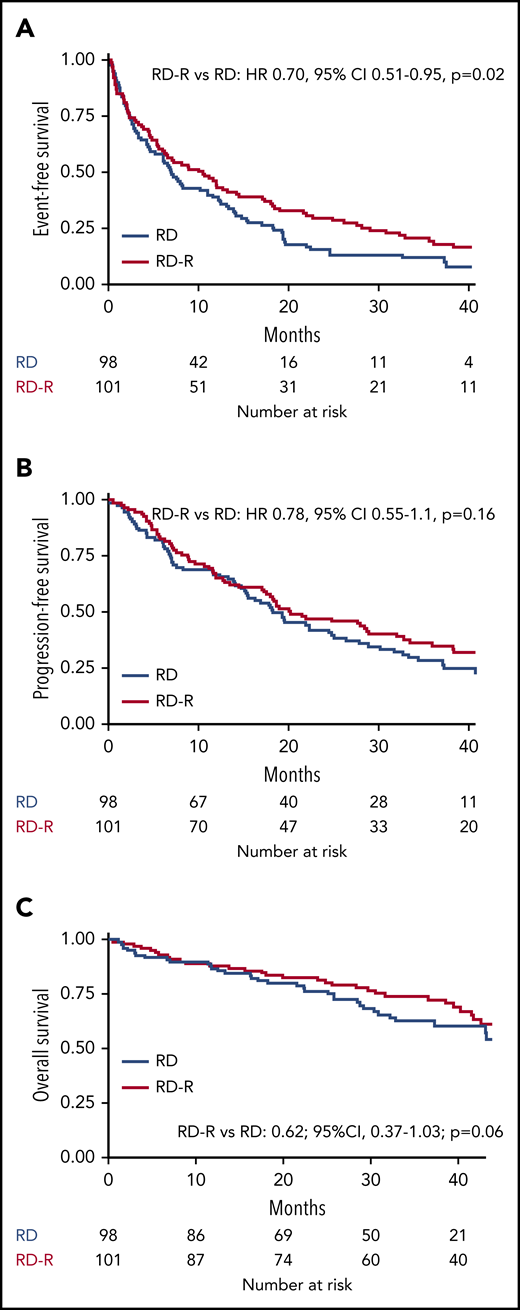
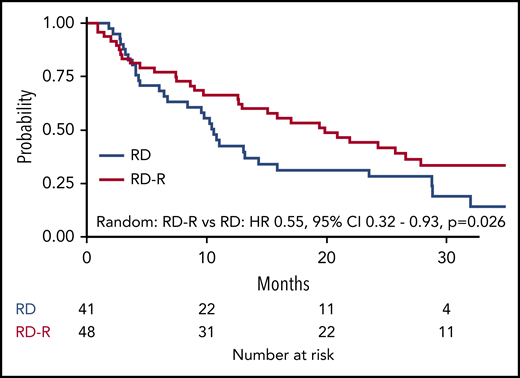
After a median follow-up of 37 months (range 27-45 months), median EFS was 10.4 months in the Rd-R arm and 6.9 months in the Rd arm (HR, 0.70; 95% CI, 0.51-0.95; P = .02; Figure 2A). The EFS advantage in the Rd-R arm was maintained beyond cycle 9 (median, 19.8 vs 10.6 months; HR, 0.55; 95% CI, 0.32-0.93; P = .03; Figure 3).
Kaplan-Meier curves for time-to-event end points. (A) EFS. (B) PFS. (C) OS.
Overall, grade 4 hematologic AEs occurred in 8%; grade 3 to 4 nonhematologic AEs, including SPM, in 37%; discontinuation of lenalidomide in 14%; and PD or death in 42% of patients. Rates of grade 4 hematologic toxicities were 11% vs 6%, grade 3 to 4 nonhematologic AEs were 31% vs 40%, and discontinuation rates of lenalidomide were 16% vs 11% in Rd-R vs continuous Rd groups, respectively. No difference in terms of EFS was observed in patients considered intermediate-fit according to age vs intermediate-fit because of comorbidities or functional impairments (median EFS, 8.1 vs 7.6 months; HR, 0.93; 95% CI, 0.68-1.28; P = .67).
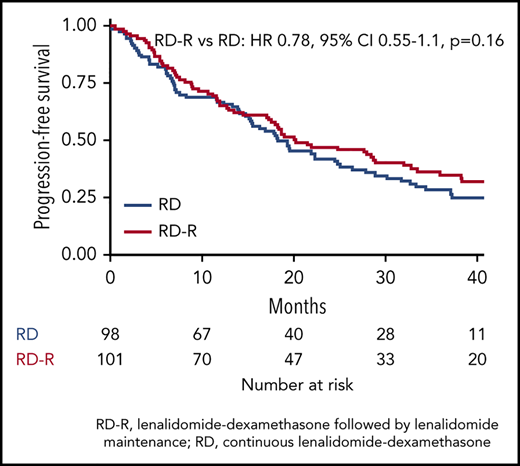
Median PFS was 20.2 months with Rd-R and 18.3 months with Rd (HR, 0.78; 95% CI, 0.55-1.1; P = .16; Figure 2B). A total of 70 patients died (Rd-R, n = 32; Rd, n = 38), mainly because of PD (n = 15 patients in each group). Median OS was not reached; the 3-year OS rate was 74% with Rd-R and 63% with continuous Rd (HR, 0.62; 95% CI, 0.37-1.03; P = .06; Figure 2C).
Among patients still receiving therapy after 9 cycles, no difference in median PFS was observed between Rd-R and Rd (24.3 vs 18.7 months; HR, 0.73; 95% CI, 0.44-1.18; P = .19).
Best response was similar in the 2 groups; the overall response rate (at least partial response during the whole treatment period) was 78% vs 68% (P = .15) and the rate of very good partial response or better was 51% vs 39% (P = .09) in Rd-R vs continuous Rd arms, respectively (Table 3). Median time to response was 2 vs 1.9 months and median time to best response was 7.5 vs 6.8 months in Rd-R and Rd arms, respectively. Among patients still receiving therapy after 9 cycles, 17% in the Rd-R arm and 25% in the Rd arm (P = .2) had in improvement in response thereafter.
Best response rates
| Best response . | Rd-R (n = 101) . | Rd (n = 98) . | All patients (n = 199) . |
|---|---|---|---|
| ORR | 79 (78) | 67 (68) | 146 (73) |
| ≥VGPR | 52 (51) | 38 (39) | 90 (45) |
| sCR | 4 (4) | — | 4 (2) |
| CR | 1 (1) | 1 (1) | 2 (1) |
| nCR* | 19 (19) | 15 (15) | 34 (17) |
| VGPR | 28 (28) | 22 (22) | 50 (25) |
| PR | 27 (27) | 29 (30) | 56 (28) |
| SD | 12 (12) | 17 (17) | 29 (15) |
| PD | — | 6 (6) | 6 (3) |
| Not evaluable | 10 (10) | 8 (8) | 18 (9) |
| Best response . | Rd-R (n = 101) . | Rd (n = 98) . | All patients (n = 199) . |
|---|---|---|---|
| ORR | 79 (78) | 67 (68) | 146 (73) |
| ≥VGPR | 52 (51) | 38 (39) | 90 (45) |
| sCR | 4 (4) | — | 4 (2) |
| CR | 1 (1) | 1 (1) | 2 (1) |
| nCR* | 19 (19) | 15 (15) | 34 (17) |
| VGPR | 28 (28) | 22 (22) | 50 (25) |
| PR | 27 (27) | 29 (30) | 56 (28) |
| SD | 12 (12) | 17 (17) | 29 (15) |
| PD | — | 6 (6) | 6 (3) |
| Not evaluable | 10 (10) | 8 (8) | 18 (9) |
Data presented as n (%).
CR, complete response; nCR, near complete response; ORR, overall response rate; PR, partial response; sCR, stringent complete response; SD, stable disease; VGPR, very good partial response.
Immunofixation negative; bone marrow examination not performed.
A trend toward a PFS benefit in favor of Rd-R was observed in standard-risk patients (HR, 0.62; 95% CI, 0.39-1.00) but not in high-risk patients (HR, 1.10; 95% CI, 0.47-2.55).
Safety
Median duration of lenalidomide treatment was 17.3 months with Rd-R (interquartile range [IQR], 7.2-34.7) and 12.8 months with continuous Rd (IQR 4.5-30.3). Median duration of dexamethasone treatment was 8.6 months with Rd-R (IQR, 7.5-9.3) vs 11.7 months with continuous Rd (IQR, 4.9-25.3). Median cumulative dose of lenalidomide delivered was 5798 mg in the Rd-R arm and 5408 mg in the Rd arm. As expected, because of the study design, median cumulative dose of dexamethasone delivered was inferior in the Rd-R arm compared with the Rd arm (720 vs 955 mg).
At least 1 grade ≥3 hematologic AE was reported in 26% of patients receiving Rd-R and 20% of patients receiving Rd (P = .40). The most frequent grade ≥3 hematologic toxicity was neutropenia, at 21% vs 18%, respectively.
The most frequent grade ≥3 toxicities were nonhematologic. At least 1 grade ≥3 nonhematologic AE was reported in 33% of patients receiving Rd-R and 43% of patients receiving Rd (P = .15). The most frequent grade ≥3 nonhematologic toxicities in Rd-R vs Rd arms were infection (10% vs 12%), constitutional (3% vs 12%), dermatologic (7% vs 3%), and central nervous toxicities (2% vs 6%), with no significant differences detected between the 2 arms. Granulocyte colony-stimulating factor was administered in 20% of patients receiving Rd-R and 17% of patients receiving Rd (P = .72). A low incidence of grade ≥3 thromboembolic events (Rd-R, 1%; Rd, 4%) was recorded. Overall, 63 patients (32%) received low molecular weight heparin; 65 (33%), aspirin; and 18 (9%), warfarin; 7 (3%) did not receive any prophylaxis; and prophylaxis information was unknown in the remaining 46 (23%) patients. Grade ≥3 peripheral neuropathy was not significant in either arm. Four SPMs were recorded, 2 (2%) with Rd-R and 2 (2%) with Rd (1 each of pancreatic neoplasm, Bowen disease, prostatic cancer, and Hodgkin lymphoma). One patient in the Rd-R group was diagnosed with gastric cancer a few days after starting cycle 1 (Table 4). Among patients still receiving therapy after cycle 9, the rate of at least 1 grade ≥3 nonhematologic AE was 17% vs 25% (P = .28), and the most frequent AEs were constitutional (fatigue, 1% vs 3%) and gastrointestinal (diarrhea, 3% vs 7%) in patients who received Rd-R vs continuous Rd, respectively.
Grade ≥3 AEs, drug discontinuation, dose reduction, and cumulative dose
| . | Rd-R (n = 101) . | Rd (n = 98) . | All patients (n = 199) . |
|---|---|---|---|
| Hematologic | |||
| At least 1 event | 26 (26) | 20 (20) | 46 (23) |
| Anemia | 9 (9) | 3 (3) | 12 (6) |
| Neutropenia | 21 (21) | 18 (18) | 39 (20) |
| Thrombocytopenia | 4 (4) | 2 (2) | 6 (3) |
| Nonhematologic | |||
| At least 1 event | 33 (33) | 42 (43) | 75 (38) |
| Cardiologic | 2 (2) | 2 (2) | 4 (2) |
| Atrial fibrillation | 1 (1) | 1 (1) | 2 (1) |
| Heart failure | 1 (1) | 1 (1) | 2 (1) |
| Infection | 10 (10) | 12 (12) | 22 (11) |
| Pneumonia | 7 (7) | 4 (4) | 11 (6) |
| Febrile neutropenia | 3 (3) | 1 (1) | 4 (2) |
| Sepsis/septic shock | 0 | 5 (5) | 5 (3) |
| Other | 1 (1) | 3 (3) | 4 (2) |
| Constitutional | 3 (3) | 12 (12) | 15 (8) |
| Fatigue | 2 (2) | 7 (7) | 9 (5) |
| Fever | 0 | 1 (1) | 1 (1) |
| Other | 1 (1) | 6 (6) | 7 (4) |
| Dermatologic | 7 (7) | 3 (3) | 10 (5) |
| Rash | 5 (5) | 2 (2) | 7 (4) |
| Other | 2 (2) | 1 (1) | 3 (2) |
| Gastrointestinal | 5 (5) | 5 (5) | 10 (5) |
| Diarrhea | 3 (3) | 4 (4) | 7 (4) |
| Other | 3 (3) | 2 (2) | 5 (3) |
| Nervous | 2 (2) | 6 (6) | 8 (4) |
| Anxiety | 0 | 2 (2) | 2 (1) |
| Confusion | 0 | 1 (1) | 1 (1) |
| Insomnia | 0 | 1 (1) | 1 (1) |
| Tremor | 0 | 2 (2) | 2 (1) |
| Stroke | 1 (1) | 0 | 1 (1) |
| Other | 1 (1) | 4 (4) | 5 (3) |
| Vascular | 2 (2) | 4 (4) | 6 (3) |
| Thromboembolism | 1 (1) | 4 (4) | 5 (3) |
| Other | 1 (1) | 1 (1) | 2 (1) |
| SPM | 2 (2) | 2 (2) | 4 (2) |
| Pancreatic | 1 (1) | 0 | 1 (1) |
| Hodgkin lymphoma | 0 | 1 (1) | 1 (1) |
| Bowen disease | 1 (1) | 0 | 1 (1) |
| Prostate | 0 | 1 (1) | 1 (1) |
| Lenalidomide | |||
| At least 1 dose reduction, %* | 45 | 62 | 53 |
| Discontinuation for AEs, % | 24 | 30 | 27 |
| Cumulative dose, mg | 5798 | 5408 | 5524 |
| Dexamethasone | |||
| Dose reduction, %† | 17 | 31 | 24 |
| Discontinuation for AEs, %† | 14 | 34 | 24 |
| Cumulative dose,† | 720 | 955 | 740 |
| . | Rd-R (n = 101) . | Rd (n = 98) . | All patients (n = 199) . |
|---|---|---|---|
| Hematologic | |||
| At least 1 event | 26 (26) | 20 (20) | 46 (23) |
| Anemia | 9 (9) | 3 (3) | 12 (6) |
| Neutropenia | 21 (21) | 18 (18) | 39 (20) |
| Thrombocytopenia | 4 (4) | 2 (2) | 6 (3) |
| Nonhematologic | |||
| At least 1 event | 33 (33) | 42 (43) | 75 (38) |
| Cardiologic | 2 (2) | 2 (2) | 4 (2) |
| Atrial fibrillation | 1 (1) | 1 (1) | 2 (1) |
| Heart failure | 1 (1) | 1 (1) | 2 (1) |
| Infection | 10 (10) | 12 (12) | 22 (11) |
| Pneumonia | 7 (7) | 4 (4) | 11 (6) |
| Febrile neutropenia | 3 (3) | 1 (1) | 4 (2) |
| Sepsis/septic shock | 0 | 5 (5) | 5 (3) |
| Other | 1 (1) | 3 (3) | 4 (2) |
| Constitutional | 3 (3) | 12 (12) | 15 (8) |
| Fatigue | 2 (2) | 7 (7) | 9 (5) |
| Fever | 0 | 1 (1) | 1 (1) |
| Other | 1 (1) | 6 (6) | 7 (4) |
| Dermatologic | 7 (7) | 3 (3) | 10 (5) |
| Rash | 5 (5) | 2 (2) | 7 (4) |
| Other | 2 (2) | 1 (1) | 3 (2) |
| Gastrointestinal | 5 (5) | 5 (5) | 10 (5) |
| Diarrhea | 3 (3) | 4 (4) | 7 (4) |
| Other | 3 (3) | 2 (2) | 5 (3) |
| Nervous | 2 (2) | 6 (6) | 8 (4) |
| Anxiety | 0 | 2 (2) | 2 (1) |
| Confusion | 0 | 1 (1) | 1 (1) |
| Insomnia | 0 | 1 (1) | 1 (1) |
| Tremor | 0 | 2 (2) | 2 (1) |
| Stroke | 1 (1) | 0 | 1 (1) |
| Other | 1 (1) | 4 (4) | 5 (3) |
| Vascular | 2 (2) | 4 (4) | 6 (3) |
| Thromboembolism | 1 (1) | 4 (4) | 5 (3) |
| Other | 1 (1) | 1 (1) | 2 (1) |
| SPM | 2 (2) | 2 (2) | 4 (2) |
| Pancreatic | 1 (1) | 0 | 1 (1) |
| Hodgkin lymphoma | 0 | 1 (1) | 1 (1) |
| Bowen disease | 1 (1) | 0 | 1 (1) |
| Prostate | 0 | 1 (1) | 1 (1) |
| Lenalidomide | |||
| At least 1 dose reduction, %* | 45 | 62 | 53 |
| Discontinuation for AEs, % | 24 | 30 | 27 |
| Cumulative dose, mg | 5798 | 5408 | 5524 |
| Dexamethasone | |||
| Dose reduction, %† | 17 | 31 | 24 |
| Discontinuation for AEs, %† | 14 | 34 | 24 |
| Cumulative dose,† | 720 | 955 | 740 |
Data presented as n (%), unless otherwise specified. All AEs in table were considered treatment related or unknown according to treating physician.
Including patients who started lenalidomide at reduced dose.
As planned in the study design, dexamethasone was stopped in Rd-R arm after 9 cycles.
After 9 cycles, cumulative incidence of grade ≥3 hematologic AEs at 2 years was 16% vs 13% in the Rd-R arm vs Rd arm; cumulative incidence of nonhematologic toxicity at 2 years was inferior in the Rd-R arm vs Rd arm (10% vs 17%; supplemental Figure 1A-B, available on the Blood Web site).
The rate of lenalidomide discontinuation because of AEs was 24% with Rd-R vs 30% with continuous Rd (P = .42); lenalidomide was reduced in 45 (45%) and 61 (62%) patients (P = .01) and dexamethasone in 17 (17%) and 30 (31%) patients, respectively (Table 4). In detail, 40 and 48 patients had at least 1 lenalidomide dose reduction in the first 9 cycles in the Rd-R vs Rd groups, respectively (including patients starting lenalidomide at a reduced dose). Fifteen and 20 patients had at least 1 lenalidomide dose reduction beyond cycle 9 in the Rd-R vs Rd groups, respectively. Regarding dexamethasone, 17 and 22 patients had at least 1 dose reduction in the first 9 cycles in the Rd-R vs Rd groups, respectively, whereas 8 patients received reduced dexamethasone beyond cycle 9 in the continuous Rd group. During the first 60 days from the start of therapy, early dropout occurred in 9% of patients; causes included toxicity (50%) and death resulting from toxicity (16%), decline of patient condition or loss to follow-up (17%), death not related to PD (11%), and PD (6%). The main cause of early discontinuation because of toxicity was infection (38%).
Overall, 40 deaths not related to PD occurred: 17 (17%) in the Rd-R arm, and 23 (23%) in the Rd arm. Of these, 13 occurred during treatment and were considered related to study drugs (Rd-R, n = 5 [pneumonia, n = 2; chronic obstructive pulmonary disease exacerbation, n = 1; Escherichia coli infection, n = 1; sepsis, n = 1]; continuous Rd, n = 8 [sepsis, n = 3; E coli infection, n = 1; postsurgical infection, n = 1; respiratory failure, n = 1; cardiac arrest, n = 1; heart failure, n = 1), 5 resulted from decline in medical condition (Rd-R, n = 2; Rd, n = 3), 3 were accidental deaths, 13 resulted from unknown causes, and 6 resulted from other diseases.
At the time of analysis, 58 (29%) patients were still receiving therapy: 34 (34%) in the Rd-R arm, and 24 (24%) in the Rd arm. Overall, 141 (71%) patients had discontinued treatment: 67 (66%) in the Rd-R arm, and 74 (75%) in the Rd arm. The main reasons for discontinuation in the Rd-R vs Rd arms, respectively, were PD (51% vs 46%) and toxicity (13% vs 28%); overall, 61% of patients received second-line therapy, mainly bortezomib based (81%).
Discussion
This is the first randomized phase 3 trial comparing an adapted Rd-R treatment schedule sparing steroids and reducing lenalidomide dose at maintenance, with standard continuous Rd, in intermediate-fit patients with NDMM ineligible for ASCT. After a median follow-up of 37 months, no difference in PFS or OS was observed between Rd-R and continuous Rd groups, whereas EFS (accounting for a combination of toxicity and efficacy) was significantly prolonged in the Rd-R arm. Furthermore, Rd-R resulted in better tolerability compared with Rd, particularly in terms of nonhematologic toxicity (grade ≥3, 33% vs 43%) and lenalidomide dose reduction (45% vs 62%).
The Rd combination is an effective treatment in transplant-ineligible patients with NDMM.2 Toxicity is a major concern in intermediate-fit and frail patients, because they are at higher risk of AEs, leading to treatment discontinuation.1 The goal of treatment in intermediate-fit patients is to achieve deep responses and reduce toxicity, while preserving quality of life.19 How to appropriately modulate treatment intensity in this group of patients has been investigated in small trials, but a definitive strategy has not yet been determined.20-22 Therefore, to account for both efficacy and safety, we chose EFS as the primary end point of our study. In our trial, EFS curves already dissociated within the first 9 months, when treatment doses and schedules were similar between the 2 arms. Despite the same initial treatment, the median age of patients at baseline was 75 and 76 years and 21% and 25% of patients were ISS stage 3 in the Rd-R and Rd arms, respectively. Although these differences were minor and not significant, they might in part explain the EFS difference during the first 9 months in this particular population. Nevertheless, beyond cycle 9, the EFS advantage favoring the Rd-R arm remained significant, supporting the feasibility of reducing treatment intensity over time. Neither the early interruption of dexamethasone in the Rd-R arm nor the lenalidomide dose reduction to 10 mg as maintenance after the first 9 cycles affected the efficacy of this regimen.
In the FIRST trial, patients age >75 years receiving continuous Rd composed 35% of participants, and in the EMN01 trial, patients age >75 years treated with Rd for 9 cycles followed by lenalidomide maintenance made up 37% of participants; furthermore, 26% of patients in the EMN01 trial were intermediate-fit. In our trial, 52% of patients were age >75 years, and all patients were intermediate-fit.7,15,16 As reported in the subanalysis of the FIRST trial including patients age >75 years, median PFS was 20 months with continuous Rd; in intermediate-fit patients enrolled in the EMN01 trial, PFS was 16.6 months. Despite the limitations of cross-trial comparisons, our results are comparable to those reported in the FIRST and EMN01 trials; our gentler approach with dose/schedule-adjusted Rd-R induced a median PFS of 20.2 months.
Although no substantial PFS difference between Rd-R vs continuous Rd was seen, a trend toward better PFS was observed in standard-risk patients treated with Rd-R vs Rd; no difference was observed in high-risk patients, but this was limited by a lower number of patients. There is no evidence that lenalidomide improves the outcomes of patients with high-risk cytogenetics2,23 ; triplets or more intensive regimens including newer drugs5,6,24 are needed to overcome their poor prognosis. However, the feasibility and tolerance of more intensive regimens should be carefully considered in high-risk intermediate-fit patients, and more data are needed. In standard-risk patients, reduced-dose Rd-R could be the appropriate strategy to balance efficacy and safety.
In our study, the safety profile of Rd was consistent with those observed in previous studies.7,16 Low incidence of venous thromboembolism and SPM was observed. The major hematologic toxicity was neutropenia, which was slightly higher with Rd-R, probably because of the absence of steroids during maintenance. However, this did not translate to a higher rate of infection.
The main grade ≥3 nonhematologic toxicity in both arms was infection, with a rate similar to that reported in the EMN01 trial (9%) and inferior to that reported in the subanalysis of the FIRST trial (29%). Central nervous system AEs and general symptoms were slightly higher with continuous Rd, likely because of the longer administration of dexamethasone. In the Rd arm, 31% of patients required dexamethasone dose reductions for AEs (as compared with 17% in the Rd-R arm), confirming that steroids are scarcely tolerated, particularly in these elderly patients, and they might hamper treatment adherence.14
The early dropout rate (ie, within the first 60 days) in our study was 9%, primarily because of early toxicities, mostly infections. Overall, 6.5% of patients died as a result of toxicity, and infection was the most frequent cause of death resulting from toxicity (10 patients died as a result of infection among 13 deaths resulting from toxicity). In a large population of patients with NDMM, the incidence of death resulting from toxicity was 5% in patients age 75 to 79 years.25
Indeed, infection is an important cause of morbidity and mortality in patients with MM. In our study, antimicrobial prophylaxis was recommended in case of neutropenia or recurrent infections but was not mandatory. As reported in a recent phase 3 trial, the addition of prophylactic levofloxacin to active myeloma treatment during the first 12 weeks of therapy significantly reduced febrile episodes and deaths compared with placebo, particularly in transplantation-ineligible patients (HR, 0.51).26 In the future, adequate antibiotic prophylaxis, at least for the first 12 weeks of therapy, is warranted, especially for intermediate-fit and frail patients.
Our analysis has some limitations. The trial was designed with EFS as the primary end point, and therefore, there was not enough power to detect a statistically significant difference in PFS. However, the outcomes of the 2 arms were comparable. No stratification at randomization was planned according to prognostic factors, or features conferring the intermediate-fit status (age or geriatric impairments). However, patient characteristics were well balanced between the 2 groups. Rd is quite an old treatment, and newer combinations are being explored for intermediate-fit patients with NDMM22 ; however, our results provide practical insight into the management of these patients and confirm that sparing steroids after 9 to 12 months, often adopted in clinical practice, is a feasible strategy. This strategy could be further evaluated in the future as a starting point for newer combinations, including novel agents (a trial comparing daratumumab-lenalidomide vs Rd in frail patients is under way; registered at www.clinicaltrials.gov as #NCT03993912).
In summary, we confirmed the efficacy and feasibility of continuous lenalidomide therapy. An optimization of this combination, sparing steroids and reducing lenalidomide dose after induction (Rd-R), can allow patients to remain on treatment longer, maintaining disease control over time. Our results suggest that, at least in intermediate-fit elderly NDMM patients, treatment intensity during continuous treatment can be deescalated without a negative impact on outcome. Ongoing and future trials including frailty-adjusted strategies to optimize treatment in the era of personalized therapy will evaluate this steroid-free approach, with newer drugs and combinations.
Requests for data and analyses performed in this study should be addressed directly to the corresponding author, who will evaluate the requests and can provide the requested information.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.L., M.B., and S.B. conceived, planned, and supervised the study; A.L. collected and analyzed the data; A.C. performed the statistical analysis; A.L. wrote the manuscript; and all authors provided patients and/or study materials, provided comments on the manuscript, and gave final approval.
Conflict-of-interest disclosure: A.L. has received honoraria from Amgen, Bristol-Myers Squibb, Celgene, Janssen, and GlaxoSmithKline and served on advisory boards for Bristol-Myers Squibb, Celgene, Janssen, and Takeda. G.G. has served on advisory boards for AbbVie, Janssen, and AstraZeneca and speakers bureaus for AbbVie and Janssen. M.D.’A. has served on an advisory board for GlaxoSmithKline. M.O. has received honoraria from and served on advisory boards for Amgen, Bristol-Myers Squibb, Celgene, Janssen, and Takeda. G.B. has received honoraria from Novartis, Celgene, Amgen, and Takeda. M.G. has received honoraria from Bristol-Myers Squibb, Celgene, Janssen, and Takeda. R.M. has received honoraria from AbbVie, Roche, Janssen, and Shire. N.G. has received research grants from Celgene and Janssen Pharmaceutical; received clinical trial sponsorship from Janssen Pharmaceutical, Millennium Pharmaceutical, and GlaxoSmithKline; served on advisory boards for Celgene, Takeda, and Janssen Pharmaceutical; and received congress fees from Janssen Pharmaceutical, Celgene, and Bristol-Myers Squibb. F.P. has served on advisory boards for Celgene, Bristol-Myers Squibb, and Janssen. T.C.d.T. has received research fund, meeting and travel expenses support from Celgene. P.C. has served as a speaker and/or on advisory boards for AbbVie, ADC Theraputics, Amgen, Celgene, Daiichi Sankyo, Gilead, Incyte, Janssen, Jazz Pharmaceuticals, Kite, KiowaKirin, Novartis, Roche, Sanofi, Servier, and Takeda. P. Tacchetti has received honoraria from Janssen, Celgene, Bristol-Myers Squibb, Amgen, Takeda, AbbVie, and Oncopeptides. M.B. has received honoraria from Sanofi, Celgene, Amgen, Janssen, Novartis, Bristol-Myers Squibb, and AbbVie; served on advisory boards for Janssen and GlaxoSmithKline; and received research funding from Sanofi, Celgene, Amgen, Janssen, Novartis, Bristol-Myers Squibb, and Mundipharma. S.B. has received honoraria from Celgene, Amgen, Janssen, and Bristol-Myers Squibb; served on advisory boards for Celgene, Amgen, Janssen, and Karyopharm; and received consultancy fees from Janssen and Takeda.
Correspondence: Alessandra Larocca, Myeloma Unit, Division of Hematology, University of Torino, Azienda Ospedaliero-Universitaria Città della Salute e della Scienza di Torino, via Genova 3, 10126, Torino, Italy; e-mail: alelarocca@hotmail.com.