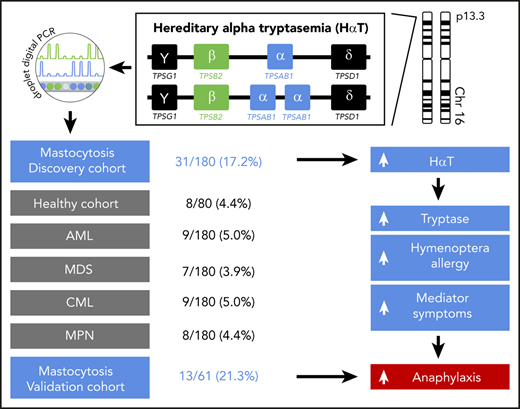
Key Points
Mastocytosis is strongly associated with hereditary α tryptasemia (HαT).
Patients with mastocytosis and HαT have a high risk for hymenoptera venom allergy and severe anaphylaxis.
Abstract
Mastocytosis is a hematopoietic neoplasm characterized by expansion of KIT D816V-mutated clonal mast cells in various organs and severe or even life-threatening anaphylactic reactions. Recently, hereditary α-tryptasemia (HαT) has been described as a common genetic trait with increased copy numbers of the α-tryptase encoding gene, TPSAB1, and associated with an increased basal serum tryptase level and a risk of mast cell activation. The purpose of our study was to elucidate the clinical relevance of HαT in patients with mastocytosis. TPSAB1 germline copy number variants were assessed by digital polymerase chain reaction in 180 mastocytosis patients, 180 sex-matched control subjects, 720 patients with other myeloid neoplasms, and 61 additional mastocytosis patients of an independent validation cohort. α-Tryptase encoding TPSAB1 copy number gains, compatible with HαT, were identified in 17.2% of mastocytosis patients and 4.4% of the control population (P < .001). Patients with HαT exhibited higher tryptase levels than patients without HαT (median tryptase in HαT+ cases: 49.6 ng/mL vs HαT− cases: 34.5 ng/mL, P = .004) independent of the mast cell burden. Hymenoptera venom hypersensitivity reactions and severe cardiovascular mediator-related symptoms/anaphylaxis were by far more frequently observed in mastocytosis patients with HαT than in those without HαT. Results were confirmed in an independent validation cohort. The high prevalence of HαT in mastocytosis hints at a potential pathogenic role of germline α-tryptase encoding TPSAB1 copy number gains in disease evolution. Together, our data suggest that HαT is a novel emerging robust biomarker in mastocytosis that is useful for determining the individual patient´s risk of developing severe anaphylaxis.
Introduction
Mastocytosis is a hematopoietic neoplasm characterized by infiltration of clonal mast cells (MC) in various organs. In patients with cutaneous mastocytosis (CM), the disease is limited to the skin, whereas in patients with systemic mastocytosis (SM), the bone marrow (BM) and other extracutaneous organs are affected.1-3 Based on clinical and laboratory variables, the World Health Organization recognizes various forms of SM, including indolent SM (ISM), smoldering SM (SSM), aggressive SM, SM with an associated hematologic neoplasm SM-AHN, and mast cell leukemia.1,4-6 The latter 3 entities are collectively referred to as advanced SM, based on their increased risk of progression and death. Most patients with SM harbor an activating somatic mutation in the receptor tyrosine kinase KIT.7,8 The presence of a codon 816 mutation in the KIT gene in an extracutaneous organ represents a diagnostic criterion for SM, and KIT D816V is also recognized as a major target of therapy.1,9 Overall, the management of patients with mastocytosis requires an interdisciplinary setting to address hematologic, allergy-related, and dermatologic aspects.10 Although a number of novel (genetic) biomarkers have recently been proposed for estimating disease progression and responses to treatment in advanced SM,11-17 the prediction of severe mediator-related symptoms and life-threatening anaphylactic reactions in patients with SM remains a clinical challenge.
Tryptases are serine proteases abundantly expressed in MC.18,19 Serum tryptase is a useful marker for the assessment of MC activation and a biomarker of the MC burden in SM.20,21 In the absence of MC activation, the basal serum tryptase level is rather stable. A persistent increase >20 ng/mL is a widely accepted minor diagnostic criterion for SM.1 Basal serum tryptase levels correlate with the MC burden in patients with SM.22 A tryptase concentration of 125 ng/mL or higher is a prognostic variable in the international prognostic scoring system for mastocytosis.1,16,22 A markedly elevated tryptase level >200 ng/mL represents a B-finding and thus criterion for SSM where the disease burden is usually high.1 During a severe anaphylactic event, MC release large amounts of tryptase, and the serum tryptase level increases substantially over the individual’s baseline.20,23 An event-related transient elevation of the serum tryptase level by at least 20% over the individual baseline plus 2 ng/mL is a criterion of systemic MC activation in patients with MC activation syndromes.24-27
The tryptase locus contains four tryptase encoding genes (TPSG1, TPSB2, TPSAB1, and TPSD1), of which only TPSB2 and TPSAB1 encode the secreted isoforms of tryptase. Although TPSB2 is considered to encode only β-tryptase isoforms, the TPSAB1 locus encodes either α or β isoforms.28,29 In addition to the canonical genotypes (0α:4β, 1α:3β, and 2α:2β) based on isoform expression of 4 tryptase genes at these 2 genetic loci, increased germline TPSAB1 copy number encoding α-tryptase has recently been described as hereditary α-tryptasemia (HαT).28,30 HαT is a relatively common autosomal dominant genetic trait in apparently healthy individuals characterized by (slightly) elevated basal serum tryptase and sometimes associated with systemic immediate hypersensitivity reactions, cutaneous flushing and pruritus, functional gastrointestinal diseases, connective tissue abnormalities, and symptoms suggestive of autonomic dysfunction.28,30-33 A gene-dose effect between the number of α-tryptase genes and basal serum tryptase levels and severity of clinical symptoms has also been described.30,34 The aim of the present study was to elucidate the clinical relevance of germline TPSAB1 copy number gains in a large cohort of mastocytosis patients.
Methods
Patients
We examined peripheral blood and BM samples from 180 mastocytosis patients (98 females, 82 males) that were diagnosed between January 1991 and June 2018 in Vienna and were included in our local registry. Blood and BM samples for assessment of all parameters included in this study were obtained at diagnosis after informed consent was given. Histopathologic examination of BM sections, quantification of BM MC infiltration, and measurement of total serum tryptase levels were performed as described.35 According to World Health Organization criteria,1,5 16 patients were diagnosed with CM, 5 with the proposed category of mastocytosis in the skin (MIS; BM involvement not confirmed),36 118 with ISM, 10 with SSM, 9 with aggressive SM, 3 with mast cell leukemia, and 19 with SM with an associated hematologic neoplasm. Allergy and mediator-related symptoms were assessed according to published recommendations as described in detail in the supplemental Methods available on the Blood Web site.36 The patients’ characteristics are shown in Table 1. In addition, the TPSAB1 copy number variation (CNV) was tested in 180 anonymized samples of sex-matched individuals from the same geographic area undergoing genetic testing for reasons not related to any hematologic neoplasm and 720 samples of patients with different myeloid malignancies: acute myeloid leukemia, n = 180; myelodysplastic syndromes (MDS) including MDS/myeloproliferative neoplasm (MDS/MPN) overlap disorders, n = 180; myeloproliferative neoplasm (MPN), n = 180; and chronic myeloid leukemia, n = 180 (supplemental Table 1). Finally, 61 ISM patients from the Medical University of Gdańsk were analyzed as an independent validation cohort (Table 1). The study was approved by the institutional review boards (Vienna: EK: 184/2014; EK:1549/2012; EK:1750/2017; EK 19-012-VK; Gdańsk: NKBBN/331/2017).
Patients’ characteristics of the discovery and validation cohort
| . | Discovery cohort . | Validation cohort . | P . | |||||
|---|---|---|---|---|---|---|---|---|
| Disease subtype . | All patients (n = 180) . | All patients (n = 61) . | ||||||
| CM (n = 16) . | MIS (n = 5) . | ISM (n = 118) . | SSM (n = 10) . | Advanced SM (n = 31) . | ||||
| Age, median (range), y | 38 (11-76) | 35 (26-48) | 46 (18-82) | 48 (19-73) | 60 (21-90) | 48 (11-91) | 49 (23-71) | .50 |
| Sex, female/male, n | 11/5 | 4/1 | 69/49 | 5/5 | 9/22 | 98/82 | 35/26 | .77 |
| Mast cell infiltration (% BM histology), median (range) | NA | NA | 10 (0-50) | 30 (10-80) | 15 (1-70) | 10 (0-80) | NA | n.t. |
| KIT D816V mutation, n (% positive) | 11/16 (68.8) | 3/5 (60) | 113/116 (97.4) | 9/10 (90.0) | 23/30 (76.7)* | 160/177 (90.4) | 40/50 (80.0) | .08 |
| KIT D816V mutant allele burden, median (range), % | 0.03 (0.004-0.54) | 0.03 (0.025-0.18) | 0.18 (0.002-46.9) | 0.11 (0.016-35.4) | 2.05 (0.005-46.7) | 0.18 (0.002-46.9) | NA | n.t. |
| Serum tryptase level, median (range), µg/L | 7.4 (3.2-23.3) | 11.3 (5.8-21.9) | 35.7 (5.3-938) | 313.3 (153-1090) | 89.3 (1.8-1617) | 36.6 (1.8-1617) | 39 (6.57-163) | .82 |
| α-Tryptase encoding TPSAB1 gain, n (% positive) | 2/16 (12.5) | 1/5 (20) | 22/118 (18.6) | 2/10 (20.0) | 4/31 (12.9) | 31/180 (17.2) | 13/61 (21.3) | .57 |
| . | Discovery cohort . | Validation cohort . | P . | |||||
|---|---|---|---|---|---|---|---|---|
| Disease subtype . | All patients (n = 180) . | All patients (n = 61) . | ||||||
| CM (n = 16) . | MIS (n = 5) . | ISM (n = 118) . | SSM (n = 10) . | Advanced SM (n = 31) . | ||||
| Age, median (range), y | 38 (11-76) | 35 (26-48) | 46 (18-82) | 48 (19-73) | 60 (21-90) | 48 (11-91) | 49 (23-71) | .50 |
| Sex, female/male, n | 11/5 | 4/1 | 69/49 | 5/5 | 9/22 | 98/82 | 35/26 | .77 |
| Mast cell infiltration (% BM histology), median (range) | NA | NA | 10 (0-50) | 30 (10-80) | 15 (1-70) | 10 (0-80) | NA | n.t. |
| KIT D816V mutation, n (% positive) | 11/16 (68.8) | 3/5 (60) | 113/116 (97.4) | 9/10 (90.0) | 23/30 (76.7)* | 160/177 (90.4) | 40/50 (80.0) | .08 |
| KIT D816V mutant allele burden, median (range), % | 0.03 (0.004-0.54) | 0.03 (0.025-0.18) | 0.18 (0.002-46.9) | 0.11 (0.016-35.4) | 2.05 (0.005-46.7) | 0.18 (0.002-46.9) | NA | n.t. |
| Serum tryptase level, median (range), µg/L | 7.4 (3.2-23.3) | 11.3 (5.8-21.9) | 35.7 (5.3-938) | 313.3 (153-1090) | 89.3 (1.8-1617) | 36.6 (1.8-1617) | 39 (6.57-163) | .82 |
| α-Tryptase encoding TPSAB1 gain, n (% positive) | 2/16 (12.5) | 1/5 (20) | 22/118 (18.6) | 2/10 (20.0) | 4/31 (12.9) | 31/180 (17.2) | 13/61 (21.3) | .57 |
NA, not available; n.t., not tested.
One additional patient was tested positive for KIT D816H.
Assessment of allergy and mediator-related symptoms
Mediator-related symptoms were evaluated in mastocytosis patients using 5 key parameters: skin-related symptoms (flushing, pruritus, blistering), cardiovascular symptoms and vascular instability (hypotension, anaphylactic shock), skeletal symptoms (bone pain), gastrointestinal tract symptoms (abdominal cramping, diarrhea, abdominal pain/cramping, ulcus duodeni, ulcus ventriculi), and neurologic symptoms (headache). The mediator-related symptoms were graded as 0 (no symptoms), 1 (mild and infrequent symptoms), 2 (moderate), 3 (severe), and 4 (severe adverse event requiring emergency therapy and hospitalization) according to published recommendations.36 Grades 3 and 4 were summarized as severe mediator-induced symptoms. A history of allergy against pollen, foods, drugs, animals, insect venom, or other triggers was recorded clinically. In addition, specific immunoglobin E (IgE) antibody testing and/or skin prick testing was performed in a subset of patients.
Genetic analysis
Genomic DNA was extracted from peripheral blood or BM cells using the QIAsymphony Sp Instrument with the QIAsymphony DNA Midi Kit (Qiagen, Hilden, Germany). KIT D816V mutation analysis was performed by droplet digital polymerase chain reaction (ddPCR) as described.35,37 The KIT D816V variant allele fraction (VAF) of the samples was calculated by dividing the number of mutated KIT D816V copies by the total number of KIT copies, and VAF results were expressed as percent mutant alleles.
TPSAB1 CNV was assessed by ddPCR with probes targeting specifically α- and β-tryptase sequences as described by Lyons et al.30 In brief, genomic DNA was quantified with the Nanodrop 2000 instrument (Thermo Fisher Scientific, Uppsala, Sweden) and adjusted to 200 ng per sample. After DNA digestion with high-fidelity restriction enzyme BamHI (New England BioLabs, Ipswich, MA), ddPCR reaction mix (reagents from Bio-Rad Laboratories, Munich, Germany) was prepared using published primers and probes for α- and β-tryptase genes and primers and probes for AP3B1 as a reference gene.30 Droplets were generated with the QX200 AutoDG Droplet Generator, and PCR was performed on C1000 Touch Thermal Cycler (Bio-Rad Laboratories). Quantification of the allelic α- and β-tryptase copies was performed on the QX-200 Droplet Reader and calculated using QuantaSoft Software (Bio-Rad Laboratories) as described.30 Increased α-tryptase encoding TPSAB1 copy number consistent with HαT was defined as ≥3 α-tryptase copies or 2 α-tryptase copies in the presence of 3 β-tryptase copies.28,30
Statistical analyses
Statistical analysis was performed using R (version 3.4.2, R, Vienna, Austria) and GraphPad Prism (GraphPad Software, La Jolla, CA). Metric data are given as mean or median as indicated, and group differences were evaluated by the Mann-Whitney U test. Categorical data were assessed by Fisher’s exact test. The correlation was assessed by applying Spearman’s rank correlation coefficient (ρ). Differences were considered to be significant when P < .05.
Results
Hereditary α tryptasemia is frequently detected in mastocytosis and associated with high serum tryptase levels in patients
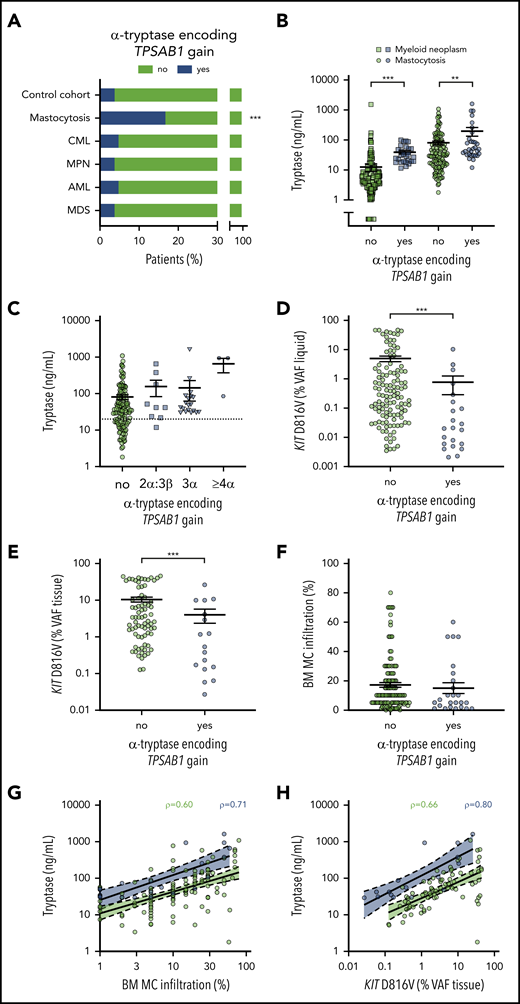
The TPSAB1 CNV was studied by ddPCR on DNA from 180 patients with mastocytosis (Table 1). A total of 148 mastocytosis patients (82.2%) displayed a canonical TPSAB1 genotype, 1 patient (0.6%) showed a TPSAB1 genotype consistent with a tryptase gene deletion, and 31 patients (17.2%) showed a α-tryptase encoding TPSAB1 copy number gain consistent with HαT (supplemental Table 2). In control experiments, the TPSAB1 CNV was tested in 180 anonymized samples of sex-matched individuals from the same geographic area without hematologic neoplasm and 720 samples of patients with different myeloid malignancies (chronic myeloid leukemia, MPN, acute myeloid leukemia, MDS including MDS/MPN, 180 each; supplemental Table 3). The prevalence of α-tryptase encoding TPSAB1 gains in these cohorts ranged from 3.9% to 5.0% (supplemental Tables 1 and 3). The observed prevalence of HαT in mastocytosis patients was significantly higher compared with the control cohort and compared with all other disease groups examined (17.2% vs 4.4%; P < .001, supplemental Table 4). In other words, mastocytosis was the only condition with increased prevalence of α-tryptase encoding TPSAB1 copy number gain, suggesting a specific association between HαT and mastocytosis (Figure 1A).
Prevalence of hereditary α tryptasemia and association of TPSAB1 genotype with biomarkers of disease burden in mastocytosis. (A) Prevalence of α-tryptase encoding TPSAB1 copy number gain in mastocytosis compared with the control cohort and other myeloid neoplasms. (B) Serum tryptase in patients with (blue) or without (green) α-tryptase encoding TPSAB1 gain; circles indicate mastocytosis and squares other myeloid neoplasms. (C) Serum tryptase in mastocytosis patients with (blue) or without (green) α-tryptase encoding TPSAB1 gain stratified according to the tryptase genotype; squares indicate 2α:3β, triangles 3α:anyβ, and hexagons ≥4α:anyβ. The dotted line indicates 20 ng/mL as minor diagnostic criterion for systemic mastocytosis. (D-F) KIT D816V mutant allele burden (VAF) in peripheral blood or bone marrow aspirate samples (VAF liquid, D) or formalin-fixed paraffin-embedded bone marrow biopsy sections (VAF tissue, E), and histologically determined bone marrow mast cell infiltration (F) in patients with (blue) or without (green) α-tryptase encoding TPSAB1 gain. Vertical black lines indicate mean ± standard error of the mean. (G-H) Linear regression for the relation of serum tryptase and bone marrow mast cell infiltration (G) or KIT D816V mutant allele burden (VAF tissue, H) stratified to presence (blue) or absence (green) of TPSAB1 gain. ρ indicates Spearman’s correlation coefficient. **P < .01; ***P < .001.
Prevalence of hereditary α tryptasemia and association of TPSAB1 genotype with biomarkers of disease burden in mastocytosis. (A) Prevalence of α-tryptase encoding TPSAB1 copy number gain in mastocytosis compared with the control cohort and other myeloid neoplasms. (B) Serum tryptase in patients with (blue) or without (green) α-tryptase encoding TPSAB1 gain; circles indicate mastocytosis and squares other myeloid neoplasms. (C) Serum tryptase in mastocytosis patients with (blue) or without (green) α-tryptase encoding TPSAB1 gain stratified according to the tryptase genotype; squares indicate 2α:3β, triangles 3α:anyβ, and hexagons ≥4α:anyβ. The dotted line indicates 20 ng/mL as minor diagnostic criterion for systemic mastocytosis. (D-F) KIT D816V mutant allele burden (VAF) in peripheral blood or bone marrow aspirate samples (VAF liquid, D) or formalin-fixed paraffin-embedded bone marrow biopsy sections (VAF tissue, E), and histologically determined bone marrow mast cell infiltration (F) in patients with (blue) or without (green) α-tryptase encoding TPSAB1 gain. Vertical black lines indicate mean ± standard error of the mean. (G-H) Linear regression for the relation of serum tryptase and bone marrow mast cell infiltration (G) or KIT D816V mutant allele burden (VAF tissue, H) stratified to presence (blue) or absence (green) of TPSAB1 gain. ρ indicates Spearman’s correlation coefficient. **P < .01; ***P < .001.
Serum tryptase levels were available from all mastocytosis patients and 453 patients with other myeloid neoplasms. As expected, mastocytosis patients had higher serum tryptase levels compared with other myeloid neoplasms (median, 36.6 vs 7.0 ng/mL, P < .001; supplemental Figure 1A). Higher serum tryptase levels were found in patients with HαT compared with patients without HαT, both within the mastocytosis cohort (median, 49.6 vs 34.5 ng/mL, P = .004; Figure 1B) and in the cohort of other myeloid neoplasms (median, 28.1 vs 5.3 ng/mL, P < .001; Figure 1B; supplemental Figure 1B). Importantly, basal serum tryptase was >20 ng/mL (meeting the minor diagnostic criterion for SM) in all but 1 patient with a genotype consistent with HαT (supplemental Table 5). When we stratified the patients according to the TPSAB1 gene copy number, a trend toward a gene-dose effect was observed (Figure 1C). In particular, a patient with a 4α:2β genotype and a patient with a 7α:2β genotype displayed excessively high basal serum tryptase levels >900 ng/mL, contrasting the relatively low infiltration grade of MCs in the BM.
When we compared mastocytosis patients with or without α-tryptase encoding TPSAB1 copy number gain, no significant differences in age, sex, positivity for KIT D816V, or presence of AHN were observed (Table 2). Interestingly, mastocytosis patients with HαT showed a significantly lower KIT D816V VAF than patients with normal TPSAB1 genotype (median, 0.27% vs 0.02% for blood/BM aspirate, P < .001; Figure 1D; and median 3.37% vs 0.54% for BM biopsy, P = .007; Figure 1E, respectively) and a not significant trend toward lower MC infiltration in the BM (median, 10% vs 5%, P = .207; Figure 1F) despite the higher levels of serum tryptase. When we stratified the patients according to the TPSAB1 genotype, an improved correlation between serum tryptase and BM MC infiltration and KIT D816V mutant allele burden was observed (Figure 1G-H). Similar results were observed when omitting mastocytosis cases with an AHN (supplemental Figure 1C-D). In summary, an increased α-tryptase encoding TPSAB1 copy number consistent with HαT was associated with high serum tryptase independent of the clonal MC burden in patients with mastocytosis.
Clinical characteristics of mastocytosis patients with and without α-tryptase encoding TPSAB1 gain
| Clinical characteristics . | No HαT (n = 149) . | HαT (n = 31) . | P . |
|---|---|---|---|
| Age at diagnosis, median (range), y | 48 (11-91) | 47 (20-72) | .845 |
| Sex, female/male, n | 84/65 | 14/17 | .322 |
| Mast cell infiltration (% BM histology), median (range) | 10 (0-80) | 5 (1-60) | .207 |
| KIT D816V mutation, n (% positive) | 132/147 (89.9) | 28/30 (93.3) | .741 |
| KIT D816V mutant allele burden, median (range), % | 0.267 (0-46.9) | 0.021 (0.002-10.38) | .001 |
| Serum tryptase level, median (range), ng/mL | 34.5 (1.8-1090) | 49.6 (11.9-1617) | .004 |
| Presence of associated hematologic neoplasm, n (%) | 16 (10.7) | 3 (9.7) | 1.000 |
| Allergy, n (% positive) | 28/142 (19.7) | 11/30 (36.7) | .055 |
| Hymenoptera venom allergy | 14/28 (50) | 9/11 (81.8) | .007 |
| Pollen allergy | 3/28 (10.7) | 1/11 (9.1) | n.t. |
| Drug allergy | 8/28 (28.6) | 1/11 (9.1) | n.t. |
| Food allergy | 6/28 (21.4) | 1/11 (9.1) | n.t. |
| Other type of allergy | 3/28 (10.7) | 0/11 (0) | n.t. |
| Mediator symptoms, n (% positive) | 62/146 (42.5) | 22/31 (71.0) | .005 |
| Skin symptoms | 23/62 (37.1) | 6/22 (27.3) | n.t. |
| Cardiovascular symptoms | 28/62 (45.2) | 14/22 (63.6) | .004 |
| Gastrointestinal symptoms | 19/62 (30.6) | 4/22 (18.2) | n.t. |
| Skeletal symptoms | 7/62 (11.3) | 0/22 (0) | n.t. |
| Neurologic symptoms | 11/62 (17.7) | 3/22 (13.6) | n.t. |
| Clinical characteristics . | No HαT (n = 149) . | HαT (n = 31) . | P . |
|---|---|---|---|
| Age at diagnosis, median (range), y | 48 (11-91) | 47 (20-72) | .845 |
| Sex, female/male, n | 84/65 | 14/17 | .322 |
| Mast cell infiltration (% BM histology), median (range) | 10 (0-80) | 5 (1-60) | .207 |
| KIT D816V mutation, n (% positive) | 132/147 (89.9) | 28/30 (93.3) | .741 |
| KIT D816V mutant allele burden, median (range), % | 0.267 (0-46.9) | 0.021 (0.002-10.38) | .001 |
| Serum tryptase level, median (range), ng/mL | 34.5 (1.8-1090) | 49.6 (11.9-1617) | .004 |
| Presence of associated hematologic neoplasm, n (%) | 16 (10.7) | 3 (9.7) | 1.000 |
| Allergy, n (% positive) | 28/142 (19.7) | 11/30 (36.7) | .055 |
| Hymenoptera venom allergy | 14/28 (50) | 9/11 (81.8) | .007 |
| Pollen allergy | 3/28 (10.7) | 1/11 (9.1) | n.t. |
| Drug allergy | 8/28 (28.6) | 1/11 (9.1) | n.t. |
| Food allergy | 6/28 (21.4) | 1/11 (9.1) | n.t. |
| Other type of allergy | 3/28 (10.7) | 0/11 (0) | n.t. |
| Mediator symptoms, n (% positive) | 62/146 (42.5) | 22/31 (71.0) | .005 |
| Skin symptoms | 23/62 (37.1) | 6/22 (27.3) | n.t. |
| Cardiovascular symptoms | 28/62 (45.2) | 14/22 (63.6) | .004 |
| Gastrointestinal symptoms | 19/62 (30.6) | 4/22 (18.2) | n.t. |
| Skeletal symptoms | 7/62 (11.3) | 0/22 (0) | n.t. |
| Neurologic symptoms | 11/62 (17.7) | 3/22 (13.6) | n.t. |
n.t., not tested.
Hereditary α tryptasemia is most prevalent in ISM/SSM
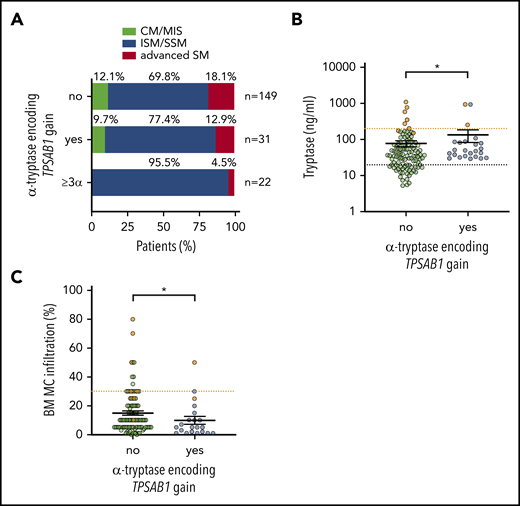
We next asked whether the HαT-mediated hereditary increase in serum tryptase is associated with the subtype of mastocytosis: 28 patients with HαT were diagnosed with SM (90.3%), 2 with CM (6.5%), and 1 with MIS (3.2%). Within SM, the highest prevalence of α-tryptase encoding TPSAB1 copy number gain was observed in patients with ISM/SSM (Table 1; Figure 2A). In particular, when we restricted the analysis to patients with ≥3 α-alleles (n = 22, 12.2%), 19 patients were diagnosed with ISM and 2 with SSM. Together, all but 1 patient (21 of 22, 95.5%) harboring ≥3 α-alleles were diagnosed with ISM/SSM compared with 69.8% (104 of 149) of mastocytosis patients without HαT (Figure 2A).
Hereditary α tryptasemia is associated with indolent systemic mastocytosis. (A) Distribution of the mastocytosis disease subtype in patients without α-tryptase encoding TPSAB1 gain (upper bar), any α allele TPSAB1 gain (middle bar), and ≥3 α-allelic copies (lower bar). Serum tryptase (B) and bone marrow mast cell infiltration (C) in ISM/SSM patients with (blue) and without (green) α-tryptase encoding TPSAB1 gain. Samples of SSM patients are shown in orange. Vertical black lines indicate mean ± standard error of the mean. The dotted black line indicates 20 ng/mL serum tryptase as minor diagnostic criterion for SM, and the dotted orange lines indicate B-finding defining cutoffs (200 ng/mL serum tryptase and 30% mast cell infiltration, respectively). *P < .05.
Hereditary α tryptasemia is associated with indolent systemic mastocytosis. (A) Distribution of the mastocytosis disease subtype in patients without α-tryptase encoding TPSAB1 gain (upper bar), any α allele TPSAB1 gain (middle bar), and ≥3 α-allelic copies (lower bar). Serum tryptase (B) and bone marrow mast cell infiltration (C) in ISM/SSM patients with (blue) and without (green) α-tryptase encoding TPSAB1 gain. Samples of SSM patients are shown in orange. Vertical black lines indicate mean ± standard error of the mean. The dotted black line indicates 20 ng/mL serum tryptase as minor diagnostic criterion for SM, and the dotted orange lines indicate B-finding defining cutoffs (200 ng/mL serum tryptase and 30% mast cell infiltration, respectively). *P < .05.
Within the ISM/SSM cohort (n = 128), the association of HαT with high serum tryptase was confirmed despite a lower BM MC infiltration (Figure 2B-C). A total of 12.5% (3 of 24) of nonadvanced SM (ISM/SSM) patients with HαT had a basal tryptase level >200 ng/mL, compatible with the B-finding of a high MC burden for SSM.1 Two of these patients (ie, 8.3% of the cases with nonadvanced SM) were diagnosed as SSM. One patient with the 7α:2β genotype did not meet the diagnostic criteria for SSM despite excessively high serum tryptase. In comparison, 5.8% (6 of 104) of nonadvanced SM (ISM/SSM) patients without α-tryptase encoding TPSAB1 gain had a basal tryptase level >200 ng/mL, and all of them were diagnosed with SSM (Figure 2B). In summary, increased α-tryptase encoding TPSAB1 copy number consistent with HαT was associated with the diagnosis of ISM/SSM.
Hereditary α tryptasemia is associated with severe mediator-related symptoms in patients with mastocytosis
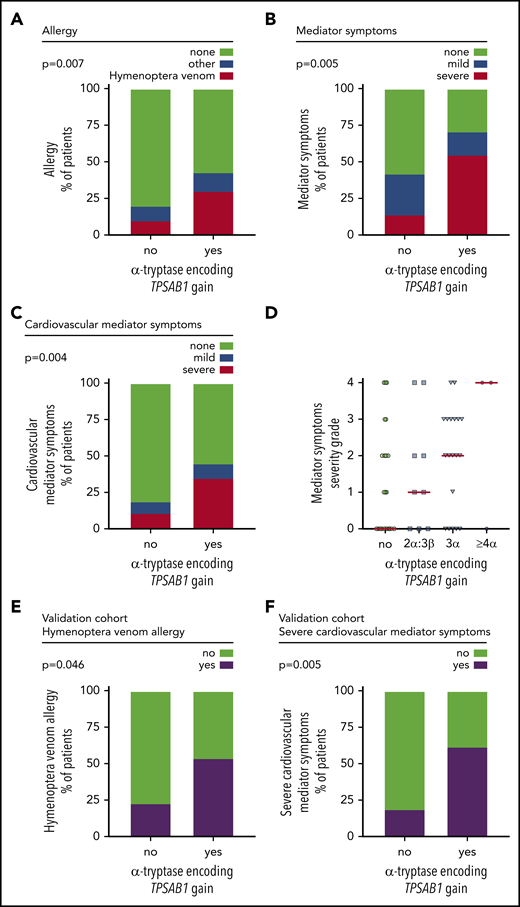
Finally, we investigated the effect of a genotype consistent with HαT on the presence of allergy or mediator-related symptoms in patients with mastocytosis. Detailed data on allergy were available in 95.6% (172 of 180) of patients. In total, 22.7% (39 of 172) of the patients had an allergy, mainly hymenoptera venom allergy (59%; Table 2). Hymenoptera venom allergy was significantly more prevalent in mastocytosis patients with α-tryptase encoding TPSAB1 copy number gain than in those without HαT (30.0% vs 9.9%, P = .007; Figure 3A; supplemental Table 6).
Hereditary α tryptasemia predisposes to hymenoptera venom allergy and severe mediator symptoms in patients with mastocytosis. (A) Frequency of allergy in patients with or without α-tryptase encoding TPSAB1 gain. Hymenoptera venom allergy is depicted in red; other types of allergy are in blue. (B-C) Frequency of any mediator-related symptoms (B) and cardiovascular mediator-related symptoms (C) in patients with or without α-tryptase encoding TPSAB1 gain. Mild symptoms (grade 1-2) are depicted in blue; severe symptoms (grade 3-4) are in red. (D) Severity grade of mediator-related symptoms in patients without α-tryptase encoding TPSAB1 gain (green), 2α:3β (blue squares), 3α:anyβ (blue triangles), or ≥4α:anyβ (blue hexagons) tryptase genotype. Horizontal red lines indicate the median severity grade. (E-F) Frequency of hymenoptera venom allergy (E) and severe cardiovascular mediator-related symptoms (F) in patients with or without α-tryptase encoding TPSAB1 gain of an independent validation cohort.
Hereditary α tryptasemia predisposes to hymenoptera venom allergy and severe mediator symptoms in patients with mastocytosis. (A) Frequency of allergy in patients with or without α-tryptase encoding TPSAB1 gain. Hymenoptera venom allergy is depicted in red; other types of allergy are in blue. (B-C) Frequency of any mediator-related symptoms (B) and cardiovascular mediator-related symptoms (C) in patients with or without α-tryptase encoding TPSAB1 gain. Mild symptoms (grade 1-2) are depicted in blue; severe symptoms (grade 3-4) are in red. (D) Severity grade of mediator-related symptoms in patients without α-tryptase encoding TPSAB1 gain (green), 2α:3β (blue squares), 3α:anyβ (blue triangles), or ≥4α:anyβ (blue hexagons) tryptase genotype. Horizontal red lines indicate the median severity grade. (E-F) Frequency of hymenoptera venom allergy (E) and severe cardiovascular mediator-related symptoms (F) in patients with or without α-tryptase encoding TPSAB1 gain of an independent validation cohort.
Detailed data on mediator-related signs and symptoms were available in 98.3% (177 of 180) of patients. Overall, 47.5% (84 of 177) of the patients had mediator-related symptoms, and severe mediator-related symptoms (grade 3 or 4) were present in 18.7% (33 of 177) of patients (Table 2; supplemental Tables 7 and 8). A total of 71.0% (22 of 31) of mastocytosis patients with HαT reported at least 1 mediator-related symptom compared with 42.4% (62 of 146) without α-tryptase encoding TPSAB1 gain (Figure 3B). This difference was highly significant (P = .005; supplemental Table 7), with an odds ratio (OR) for mediator-related symptoms in the presence of HαT of 3.3 (confidence interval [CI]: 1.43-7.7). In particular, a significantly higher proportion of patients with α-tryptase encoding TPSAB1 gain had severe symptoms (38.7% vs 14.4%, P = .004; OR: 3.76, CI: 1.6-8.9; Figure 3B; supplemental Table 8). The most frequently reported type of mediator-related symptoms in patients with HαT were symptoms reflecting vascular instability, including severe hypotension and anaphylaxis (Table 2). A total of 35.5% (11 of 31) of the mastocytosis patients with HαT had severe cardiovascular mediator-related symptoms compared with 11.0% (16 of 146) without HαT (P = .002; OR: 4.5, CI: 1.81-11.0; Figure 3C; supplemental Table 9). Six of these 11 patients (54.5%) with HαT and severe cardiovascular mediator-related symptoms also had hymenoptera venom allergy.
Next, we analyzed the severity of symptoms according to the TPSAB1 copy number. Patients harboring ≥3 α-alleles showed particularly high mediator symptom grading (Figure 3D). Two of 3 patients with ≥4 α-alleles had grade 4 mediator-related symptoms. These 2 female patients were diagnosed with KIT D816V-positive ISM but had a low KIT D816V allele burden of 0.02% and 0.002% VAF, respectively. The patient with the 4α:2β genotype presented with severe anaphylactic reaction in response to hymenoptera venom and had recurrent dizziness and diarrhea. The patient with the 7α:2β genotype showed excessively high basal serum tryptase levels, had allergic reactions to hymenoptera venom, pollen, drugs, and various cosmetics, and experienced recurrent episodes of severe hypotension requiring hospitalization.
To confirm the relevance of HαT as a biomarker in mastocytosis, we finally studied 61 ISM patients from Medical University of Gdańsk as an independent validation cohort (Table 1). A copy number gain of α-tryptase encoding TPSAB1 was observed in 13 of 61 (21.3%) patients, confirming the high prevalence of HαT in mastocytosis (supplemental Table 10). Both hymenoptera venom allergy and severe cardiovascular mediator-related symptoms were significantly more frequent in the independent validation cohort in patients with HαT compared with those without extra α-tryptase encoding TPSAB1 copies (hymenoptera venom allergy: 53.8% vs 23.4%, P = .046; Figure 3E; supplemental Table 11; severe cardiovascular mediator-related symptoms: 61.5% vs 18.8%, P = .005; Figure 3F; supplemental Table 12). In summary, we observed a strong association of HαT with hymenoptera venom allergy, hypotension, and anaphylaxis as severe mediator-related cardiovascular symptoms in patients with mastocytosis.
Discussion
Mastocytosis is a hematologic neoplasm with complex pathology and a variable clinical course.1-6 Many patients have mediator-related events, but little is known about predictive variables. Recent data suggest that extra copy numbers of TPSAB1 reflecting HαT correlate with MC activation-related events. We studied TPSAB1 CNV in a larger cohort of mastocytosis patients and detected HαT in 17.2% of all cases tested. Genetic studies on smaller cohorts suggested that up to 8% of the general population could be affected.30,34 A recently published larger study found a frequency of 5% in an unselected British birth cohort which is in the same range as our control cohort (4.4%), suggesting that the prevalence of HαT is approximately 5% in the European population.38,39 Likewise, a modest elevation of basal serum tryptase defined as >11.4 ng/mL has been reported to be common in the general population with a prevalence of 4% to 6%.38,40,41 We observed a significantly higher prevalence of HαT-defining genotypes (including a number of triplications or even higher copy numbers) in our mastocytosis cohort compared with a sex-matched control cohort and compared with a large cohort of patients (n = 720) with other myeloid neoplasms. Importantly, mastocytosis remained the only condition with increased prevalence of α-tryptase encoding TPSAB1 copy number gains, providing robust evidence that HαT is a novel emerging and rather specific genetic biomarker of symptomatic mastocytosis. In parallel to our study, Lyons et al39 also reported an increased prevalence of HαT in mastocytosis.
In patients with mastocytosis, the presence of a HαT was associated with high serum tryptase independent of the clonal MC burden. The influence of the TPSAB1 genotype might confound the use of tryptase as a surrogate of MC burden in mastocytosis. Indeed, the correlation of serum tryptase, KIT D816V allele burden, and BM MC infiltration is rather rough and seems to improve when taking the TPSAB1 genotype into account.22,35 Despite the higher serum tryptase levels, α-tryptase encoding TPSAB1 copy number gain was associated with lower KIT D816V allele burden, a trend toward lower MC burden, and indolent disease in our cohort. However, we did not observe an association of TPSAB1 copy number gain with KIT D816V-negative disease as reported elsewhere.39 Taking the low KIT D816V mutant allele burden in mastocytosis patients with HαT into account, the analytical sensitivity of the assay needs to be considered, and a number of them would have been tested negative for KIT D816V with a less sensitive assay.35,37 It is tempting to speculate that patients with α-tryptase encoding TPSAB1 copy number gain were diagnosed at an earlier stage of disease because of the higher serum tryptase level or a higher symptom burden, although no significant difference in the age at diagnosis or the time between symptom onset and diagnosis were observed when comparing mastocytosis patients with or without HαT. Screening for HαT before BM biopsy has been suggested for patients with mildly elevated basal serum tryptase.33 We asked whether the 20-ng/mL serum tryptase level cutoff for diagnosis of SM or the 200-ng/mL cutoff as B-finding for SSM might be influenced by the TPSAB1 genotype.1 Although we did not observe an influence of the TPSAB1 copy number on the discrimination between CM and SM in our cohort, we suggest a specific validation of these cutoffs in large multicentric trials taking the TPSAB1 genotype into account. Likewise, a reevaluation of the normal reference range for basal serum tryptase according to the TPSAB1 genotype has been suggested.28 In addition, the data on HαT in patients with and without mastocytosis point at the necessity to use the increase from the individual baseline of serum tryptase as criterion for severe systemic MC activation in patients with MC activation syndromes.24-27,30,42
Clinically, we observed a strong association between α-tryptase encoding TPSAB1 copy number gain with hymenoptera venom allergy and hypotension and anaphylaxis (severe cardiovascular symptoms) in patients with mastocytosis. In the general population, increased basal serum tryptase levels have been associated with severe anaphylaxis to stinging insects.40,43-45 This has been largely attributed to an underlying clonal MC disease, and a diagnostic workup for mastocytosis has been suggested in these patients.42,46-48 However, in HαT patients without mastocytosis, systemic reactions consistent with IgE-mediated immediate hypersensitivity to stinging insects have also been reported with an approximately three- to fourfold increased prevalence.28,30 In our patients with mastocytosis and HαT, we found a prevalence of 30% for hymenoptera venom allergy and 35.5% for severe cardiovascular mediator-related symptoms (mainly anaphylaxis) corresponding to a three- to fourfold increased risk caused by the TPSAB1 genotype even in mastocytosis patients. Validation of our findings in an independent cohort of mastocytosis patients suggests the assessment of TPSAB1 copy number gain as a novel genetic biomarker to identify mastocytosis patients with very high risk of anaphylaxis. In parallel to our study, Lyons et al39 also reported a substantially increased risk for severe anaphylaxis in patients with mastocytosis and HαT. Recently, a risk score including serum tryptase has been proposed for discriminating SM patients at high risk of anaphylaxis from those at low risk.49 Interestingly, a tryptase level of <40 ng/mL was included as a risk factor for anaphylaxis in this model. However, in that study, the TPSAB1 genotype was not tested, and in other studies, higher tryptase levels were reported to correlate with anaphylaxis in SM.50-52 The TPSAB1 genotype represents a more robust biomarker that is not affected by episodes of MC activation and should be included into a multiparametric model for individualized risk assessment in mastocytosis.
The mechanism underlying the association between mastocytosis and HαT remains unknown. One possibility could be that extra tryptase (variants) exert mitogenic activity on cells of the BM microenvironment and also on hematopoietic stem cells, thereby facilitating the development of SM.53-55 It is thus tempting to speculate that an increased tryptase load in individuals with HαT might support the initiation of SM. Further studies are needed to directly assess autocrine and paracrine effects of tryptase on mast cells, hematopoietic stem cells, and cells of the bone marrow microenvironment in vitro and in vivo. An alternative explanation could be that a highly prevalent genetic variant predisposes for both HαT and an increased risk for mastocytosis (at a low penetrance). A third explanation would be that the tryptase-species formed in HαT patients introduce DNA damage and thus increase mutagenesis, which in turn predisposes for the development of a BM neoplasm. This seems unlikely, however, as we tested a number of other myeloid neoplasms, and mastocytosis remained the only condition with increased prevalence of α-tryptase encoding TPSAB1 copy number gains. Together, our data provide robust evidence for the association between HαT and mastocytosis and warrant further studies on a potential direct role of TPSAB1 copy number gain in the origin of mastocytosis. Thus far, it also remains unknown how α-tryptase encoding TPSAB1 copy number gains lead to the clinical features observed in HαT carriers with mastocytosis. In individuals with HαT but without mastocytosis, an increased expression and secretion of pro-tryptases by MC and basophils in a gene-dose dependent manner has been reported.28,30 α/β-Tryptase hetero-tetramers were also found to activate protease-activated receptor-2 and to cause MC susceptibility to vibration-triggered degranulation.32,39 The management of patients with HαT is currently based on symptomatic treatment.28 Recently, clinical response to the monoclonal anti-IgE-antibody omalizumab has been described in a small series of HαT patients.56 In this regard, it is worth noting that omalizumab has also been reported to be effective in SM patients with severe IgE-dependent anaphylaxis.57 Furthermore, a noncompetitive inhibitory anti-tryptase antibody showed promising results in a preclinical model for severe asthma establishing MC tryptase as a potential therapeutic target.58 A detailed understanding if and how α-tryptase encoding TPSAB1 copy number gains contribute to the clonal expansion of KIT D816V-positive neoplastic MC might provide new insight into the pathophysiology of SM and help to exploit novel targeting strategies.
In summary, α-tryptase encoding TPSAB1 copy number gains typical for HαT are highly prevalent in patients with mastocytosis and are associated with high serum tryptase and with disease-related symptomatology independent of the MC burden. Our data also show that the presence of HαT substantially increases the risk for hymenoptera venom allergy and severe mediator-related symptoms in these patients. We propose the assessment of TPSAB1 copy number gains as a novel genetic biomarker to predict the risk of severe anaphylaxis in patients with mastocytosis.
For original data, please e-mail the corresponding author at gregor.hoermann@meduniwien.ac.at.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by Austria Science Fund (FWF) project P26079-B13, Sonderforschungsbereich (SFB) projects F4701-B20 and F4704-B20, the Medical-Scientific Fund of the Mayor of Vienna, and Polish Ministry of Science and Higher Education grants 02-0066/07/253 and ST 02-0141/07/231.
Authorship
Contribution: G.G., B.S., M.G., N.W., K.G.S., and G.H. performed molecular tests and analyzed the data; G.G., B.S., A.G., B.G., G.U., E.H., H.E., K.V.G., M.T.K., M.P., F.K., H.G., M.N., B.N., W.R.S., P.V., and G.H. obtained and analyzed clinical data; G.G., F.R., and W.R.S. performed statistical analyses; G.G., B.S., P.V., and G.H. designed the study and wrote the paper; and all authors revised and approved the manuscript.
Conflict-of-interest disclosure: G.H. received honoraria and research grants from Novartis, Roche, Beckman Coulter, Pfizer, Celgene, and Bristol-Myers Squibb. P.V. served as a consultant in a global Novartis trial investigating the effects of midostaurin in patients with advanced systemic mastocytosis and received honoraria and research grants from Novartis, Blueprint, Pfizer, Ariad, Incyte, Celgene, and Deciphera. W.R.S. received honoraria from Novartis. G.U. received honoraria and grants from Ariad, Astellas, AstraZeneca, Incyte, Novartis, and Pfizer. K.G.V. received honoraria from Pfizer, Novartis, Incyte, and Sanofi-Aventis. H.G. received honoraria from Novartis, Celgene, Janssen, and AOP Orphan. The remaining authors declare no competing financial interests.
Correspondence: Gregor Hoermann, Department of Laboratory Medicine, Medical University of Vienna, Waehringer Guertel 18-20, A-1090 Vienna, Austria; e-mail: gregor.hoermann@meduniwien.ac.at or gregor.hoermann@mll.com.
REFERENCES
Author notes
G.G. and B.S. contributed equally to this work.