Key Points
Conventional therapy consolidated by auto-SCT remains a promising option for treating patients with T-cell lymphoma.
Patients with relapsing or refractory peripheral T-cell lymphoma should be offered allo-SCT.
Abstract
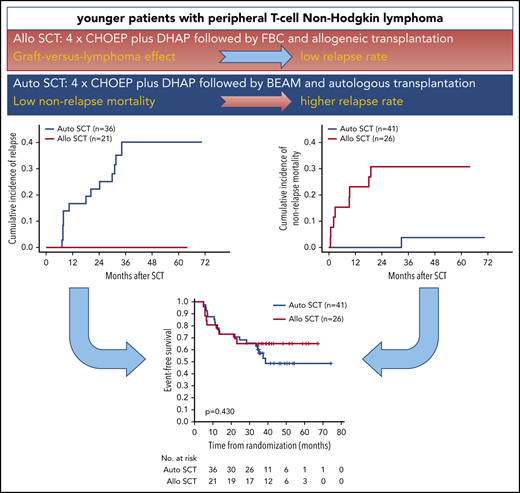
First-line therapy for younger patients with peripheral T-cell non-Hodgkin lymphoma (T-NHL) consists of 6 courses of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) with or without etoposide (CHOEP), consolidated by high-dose therapy and autologous stem cell transplantation (auto-SCT). We hypothesized that allogeneic stem cell transplantation (allo-SCT) could improve outcomes. 104 patients with peripheral T-cell non-Hodgkin lymphoma, except ALK+ anaplastic large cell lymphoma, 18 to 60 years, all stages, and all age adjusted International Prognostic Index scores, except 0 and stage I, were randomized to 4 cycles of CHOEP and 1 cycle of dexamethasone, cytosine-arabinoside, and platinum (DHAP) followed by high-dose therapy and auto-SCT or myeloablative conditioning and allo-SCT. The primary end point was event-free survival (EFS) at 3 years. After a median follow-up of 42 months, the 3-year EFS after allo-SCT was 43%, as compared with 38% after auto-SCT. Overall survival at 3 years was 57% vs 70% after allo- or auto-SCT, without significant differences between treatment arms. None of the 21 responding patients proceeding to allo-SCT relapsed, as opposed to 13 of 36 patients (36%) proceeding to auto-SCT. Eight of 26 patients (31%) and none of 41 patients died of transplant-related toxicity after allo- and auto-SCT, respectively. The strong graft-versus-lymphoma effect after allo-SCT was counterbalanced by transplant-related mortality. This trial is registered at www.clinicaltrials.gov as #NCT00984412.
Introduction
Peripheral T-cell neoplasms comprise a growing number of entities with diverse clinical, morphological, immunohistochemical, and molecular characteristics.1 Except for ALK+ anaplastic large-cell lymphoma (ALCL) they mostly carry a poor prognosis.2 In younger patients with T-cell lymphoma, retrospective studies reported event-free survival (EFS) rates at 3 years of 48% after cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) and 61% after CHOP+etoposide (CHOEP) treatment,3 registry data from Sweden showed progression-free survival (PFS) and overall survival (OS) rates of 44% and 51% for transplant-eligible patients treated with CHOP or CHOEP,4 and the prospective cohort study COMPLETE5 reported a 2-year OS rate of 59% for patients of all ages (median, 63 years) treated with doxorubicin-based, etoposide-based, or single-agent chemotherapy. Auto- or allo-SCT was part of the first-line therapy in 21% of these patients. All studies have reported significantly better survival of patients with low International Prognosis Index (IPI) scores (0-1), whereas the beneficial effect of adding etoposide to CHOP remains controversial. First-line studies combining conventional and targeted therapies either failed to show improvement6 or preferentially included patients who had ALCL, leaving unanswered the important question of which patients with other T-cell lymphoma entities could benefit from this approach.7 Hence, CHO(E)P consolidated with auto-SCT remains the preferred option for younger patients.8,9 The largest phase 2 studies integrating auto-SCT into first-line therapy of younger T-cell lymphoma patients reported OS rates of 51% at 5 years10 and 48% at 3 years.11 Phase 3 studies comparing auto-SCT to alternative therapies or observation, however, have not been undertaken, and it remains unclear which patients actually benefit from this approach. Recent retrospective analyses12 and data from the COMPLETE study5 shed some doubts on whether auto-SCT should be offered to all patients who achieve remission after induction chemotherapy. Because allo-SCT performed in patients with relapsed or refractory T-cell lymphoma gave favorable results,13 with approximately half of the patients becoming long-term survivors, we set out to compare auto- to allo-SCT for consolidation treatment in patients with T-cell lymphoma.
Methods
Study design and participants
This was a 2-arm, prospective, randomized, multicenter, phase 3 trial conducted at 44 trial sites in France and Germany. It was coordinated by the French Lymphoma Study Association (LYSA) and the German Lymphoma Alliance (GLA) (former German High-grade Non-Hodgkin Lymphoma Study Group).
The study was conducted in accordance with the Declaration of Helsinki. The protocol and its amendment were approved by the central ethics committees in Hamburg, Germany, and by the Agence Française de Sécurité Sanitaire des Médicaments et des Produits Biologiques (AFSSAPS ref. 2009-A00947) and Comité de Protection des Personnes, Sud-Est 6 (Ref AU 826), France, as well as local ethics committees. All patients gave written informed consent.
Patients between 18 and 60 years of age with poor prognosis (stage II-IV or age-adjusted IPI [aaIPI] >0) were eligible if they had untreated, biopsy-confirmed peripheral T-cell lymphoma according to the World Health Organization (WHO) classification 2008.14 Local diagnoses were reviewed by expert pathologists from LYSA and GLA. Only patients with peripheral T-cell lymphoma not otherwise specified (PTCL-NOS), angioimmunoblastic T-cell lymphoma (AITL), anaplastic lymphoma kinase (ALK)-negative ALCL (ALK− ALCL), intestinal T-/natural killer (NK)-cell lymphoma, hepatosplenic γ/δ T-cell lymphoma, or subcutaneous panniculitis-like PTCL were included. Patients with extranodal NK/T-cell lymphoma, nasal type, were eligible before the amendment dated 1 October 2014, and only in Germany. In France, patients with extranodal NK/T-cell lymphoma were not eligible. Other key inclusion criteria were ECOG (Eastern Cooperative Oncology Group) score 0 to 3, absence of severe cardiac dysfunction, and pulmonary diffusion capacity >40% of normal. Key exclusion criteria were ALCL, ALK-positive, stage I disease with aaIPI 0, primary central nervous system (CNS) involvement, aspartate aminotransferase, alanine aminotransferase, or alkaline phosphatase >2 times normal, creatinine >1.5 times normal, and known HIV positivity. Full inclusion and exclusion criteria are given in supplemental Table 1, available on the Blood Web site.
Randomization
Patients were randomized at a 1:1 ratio using the Pocock minimization algorithm after stratification for center, stage (I-II vs III-IV), performance status (ECOG 0-1 vs 2-3), serum lactase dehydrogenase (less than vs more than upper normal value), number of extranodal sites (0/1 vs >1), and 1 cycle CHO(E)P given before inclusion (no vs yes).15 Patients were registered at the trial office in Hamburg, Germany, and randomized at the data management center (Institute for Medical Informatics, Statistics, and Epidemiology, University of Leipzig) by use of a computer program with an algorithm, using a biased coin approach that accounted for randomizations that had occurred previously. Patients were randomized up front to receive four 14-day cycles of CHOEP, 1 course of DHAP (dexamethasone, cytosine-arabinoside, and cisplatin or carboplatin) and auto- or allo-SCT. Patients with complete response (CR), unconfirmed CR (CRu), partial response (PR), or stable disease (SD) at the time of restaging continued in the study and were scheduled to receive either BEAM (carmustine [BCNU], etoposide, cytosine-arabinoside, and melphalan) high-dose chemotherapy followed by auto-SCT or myeloablative conditioning with fludarabine, busulfan, and cyclophosphamide (FBC) followed by allo-SCT.16
Procedures
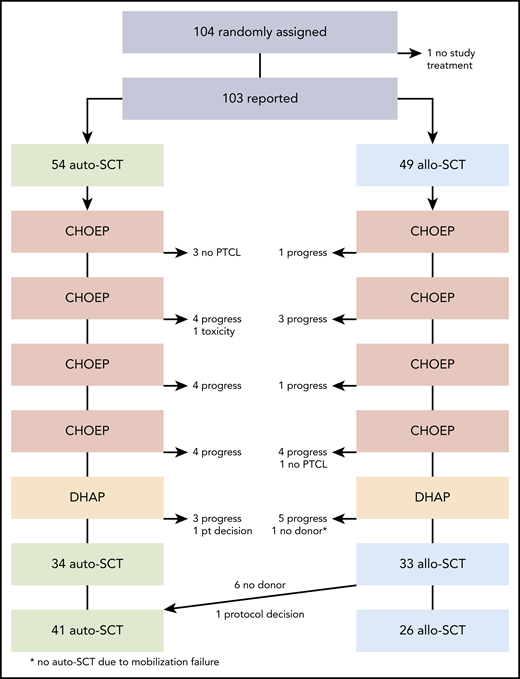
Patients had a baseline assessment including history; clinical characteristics; laboratory tests; magnetic resonance imaging or computed tomographic scans of the neck, thorax, and abdomen; and a bone marrow biopsy. Positron emission tomographic (PET) scans were not mandatory. Figure 1 shows the trial profile. CHOEP comprised cyclophosphamide (750 mg/m2), doxorubicin (50 mg/m2), and vincristine (2 mg), administered IV on day 1; IV etoposide (100 mg/m2) on days 1 to 3; and oral prednisone (100 mg) on days 1 to 5.
All patients received 4 courses of CHOEP at 2-week intervals with G-CSF support from days 4 to 13. Two weeks after cycle 4 of CHOEP, a formal restaging, including physical examination, blood counts and chemistry, electrocardiogram, and CT scans of the neck, thorax, and abdomen were performed. The response was evaluated according the 1999 consensus criteria.17 Patients with CR, CRu, PR, or SD with no active infection or severe organ toxicity proceeded to 1 course of DHAP as soon as leukocytes (>2.5 × 10³/µl) and platelets (>80 × 10³/µl) had recovered. DHAP consisted of dexamethasone (8 mg orally or IV 3 times per day on days 1 to 4), cytosine-arabinoside (2000 mg/m2, IV twice daily on day 2), and cisplatin (100 mg/m2) or carboplatin (area under the curve [concentration vs time]; AUC 5), both IV, on day 1. For patients randomized to allo-SCT, for whom a suitable donor was not found before the planned transplantation date, and for patients randomized to auto-SCT, collection of autologous peripheral blood stem cells was started 2 weeks after DHAP. A minimum of 4 × 106 CD 34+ cells per kg body weight was necessary to continue study treatment. For patients randomized to allo-SCT, the search for an HLA-identical matched sibling or unrelated donor started immediately after randomization. In France, only fully matched (10 of 10 HLA loci) family or unrelated donors were accepted, whereas in Germany, donors compatible at 9 of 10 loci were accepted. Collection of allogeneic stem cells followed local protocols.
High-dose chemotherapy before auto-SCT consisted of carmustine (300 mg/m2) on day −7, cytosine-arabinoside (200 mg/m2, twice daily) on days −6 to −3, etoposide (200 mg/m2) on days −6 to −3, and melphalan (140 mg/m2) on day −2. Patients randomized to allo-SCT who had an HLA-compatible donor were conditioned with fludarabine (25 mg/m2 IV) on days −8 to −4, busulfan (4 × 1 mg/kg body weight orally or 4 × 0.8 mg/kg body weight IV) on days −6 to −4, and cyclophosphamide IV (60 mg/kg body weight) on days −3 and −2. Autologous or allogeneic blood stem cells were transplanted on day 0. Prophylaxis of graft-versus-host disease (GVHD) consisted of anti-thymocyte globulin (10 mg/kg body weight IV) on days −4 to −2, mycophenolate mofetil (1000 mg orally or IV, twice daily) days +1 to +28, and cyclosporine A from days −1 to +100. Tapering of GVHD prophylaxis depended on the presence and severity of acute GVHD. ECOG performance status and all adverse events were retrieved in predefined categories from case report forms, using National Cancer Institute Common Toxicity Criteria (version 3.0).18
Statistical analysis
The trial was planned to detect improvement in EFS in the intent-to-treat population at 3 years, from 35% achieved with auto-SCT to 60% by allo-SCT (full-analysis set). According to the nQuery Advisor, version 2.0, the planned sample size for the primary end point EFS at 3 years was 140 patients (including a 10% loss of patients), to detect a difference at a power of 80% and an α-error of 5%, 2 sided (hazard ratio [HR], 0.487).
Secondary end points included complete remission rate, rate of primary progression, relapse rate, rate of patients proceeding to transplantation, incidence of acute and chronic GVHD after allo-SCT, rate of treatment-related deaths, OS and PFS, as well as safety and tolerability. EFS was calculated as time from randomization to disease progression, to start of salvage treatment, to start of any additional unplanned treatment, or to a response categorized as SD or as unknown, relapse, or death from any cause. PFS was defined as time from randomization to progression, relapse, or death from any cause. OS was defined as time from randomization to death from any cause. Patients with no event reported at the time of analysis were censored at the most recent assessment date. Kaplan-Meier curves were drawn, and log-rank tests were calculated.19,20 Three-year rates of EFS, PFS, and OS with 95% confidence interval (CI) were determined. A Cox multivariate regression model was used to test whether therapeutic effects emerging from univariate analyses remained stable after adjustment for main strata. Estimates are given as HRs with 95% CI and corresponding P-values. Differences between groups were classified as significant at P ≤ .05. Patients’ characteristics were analyzed by χ2 test and, if necessary, by Fisher’s exact test. Statistical analyses were performed with IBM SPSS ver. 25 and 26 software. Cumulative incidence curves for time to relapse and time to nonrelapse mortality are presented using R (version 3.1.0, cuminc).21
The primary end point and major secondary end points were calculated for all patients randomized (intent to treat). Because we expected that 30% to 35% of patients would not reach transplantation or find a compatible donor, additional explorative analyses were planned. First, we analyzed all transplant recipients according to treatment; second, we analyzed all transplant recipients according to randomization.
Results
Patients
From March 2011 to July 2014, 104 patients were included in the trial at 17 German and 27 French centers. The data safety and monitoring board, in agreement with the study steering committee, stopped randomization and recruitment in August 2014, because a planned interim analysis had shown that the study was highly unlikely to meet the primary end point. The transplant-related mortality observed contributed to this decision.
One patient did not receive any study treatment, leaving 103 patients for the intent-to-treat analysis. Fifty-four patients were assigned to auto-SCT and 49 patients to allo-SCT (Figure 1). Baseline characteristics were well balanced without significant differences between treatment arms. According to the primary pathology, 41 patients (40%) had PTCL-NOS, 35 (34%) had AITL, and 15 (15%) had ALK− ALCL. Ten patients (10%) had other T-cell lymphoma subtypes, and 2 had T-cell lymphoma without further specification.
A reference pathology review was performed in 97% of the patients (Table 1).
Demographics and disease characteristics for randomized patients and for transplanted recipients only
| . | Randomized patients . | Transplant recipients . | ||||||
|---|---|---|---|---|---|---|---|---|
| . | Auto-SCT (n = 54) . | Allo-SCT (n = 49) . | Auto-SCT (n = 41*) . | Allo-SCT (n = 26) . | ||||
| Male | 31 | (57) | 34 | (69) | 28 | (68) | 17 | (65) |
| Female | 23 | (43) | 14 | (31) | 13 | (32) | 9 | (35) |
| Age, median (range) | 50 | (28, 60) | 50 | (24, 60) | 51 | (24, 60) | 50 | (35, 60) |
| LDH > Normal | 33 | (61) | 30 | (61) | 22 | (54) | 11 | (42) |
| ECOG > 1 | 11 | (20) | 10 | (20) | 8 | (20) | 5 | (19) |
| Stage III/IV | 47 | (87) | 44 | (90) | 36 | (88) | 23 | (88) |
| aaIPI 0 | 2 | (4) | 3 | (6) | 1 | (2) | 3 | (12) |
| aaIPI 1 | 22 | (41) | 16 | (33) | 20 | (49) | 10 | (38) |
| aaIPI 2 | 21 | (39) | 22 | (45) | 14 | (34) | 10 | (38) |
| aaIPI 3 | 9 | (17) | 8 | (16) | 6 | (15) | 3 | (12) |
| E-involvement | 32 | (59) | 31 | (63) | 24 | (59) | 15 | (58) |
| E > 1 | 16 | (30) | 17 | (35) | 11 | (27) | 6 | (23) |
| IPI 0 | 2 | (4) | 3 | (6) | 1 | (2) | 3 | (12) |
| IPI 1 | 16 | (30) | 10 | (20) | 14 | (34) | 7 | (27) |
| IPI 2 | 22 | (41) | 21 | (43) | 16 | (39) | 11 | (42) |
| IPI 3 | 9 | (17) | 11 | (22) | 9 | (22) | 4 | (15) |
| IPI 4 | 5 | (9) | 4 | (8) | 1 | (2) | 1 | (4) |
| Bulky disease | 10 | (19) | 7 | (14) | 8 | (20) | 3 | (12) |
| B-symptoms | 32 | (59) | 29 | (59) | 23 | (56) | 16 | (62) |
| Bone marrow involved | 17 | (31) | 15 | (31) | 7 | (17) | 9 | (35) |
| Histology | ||||||||
| Reviewed | 54† | (100) | 46 | (94) | 41‡ | (100) | 25 | (96) |
| Peripheral T-cell lymphoma, not otherwise specified (PTCL-NOS) | 16 | (30) | 15 | (33) | 11 | (27) | 8 | (32) |
| Angioimmunoblastic T-cell lymphoma | 17 | (33) | 20 | (43) | 16 | (40) | 12 | (48) |
| Anaplastic large cell lymphoma ALK-negative | 9 | (17) | 5 | (11) | 8 | (20) | 3 | (12) |
| Extranodal NK/T-cell lymphoma, nasal type | 0 | (0) | 1 | (2) | 0 | (0) | 0 | (0) |
| Enteropathy-associated T-cell lymphoma (EATL) types I and II | 3 | (6) | 0 | (0) | 3 | (8) | 0 | (0) |
| Hepatosplenic T-cell lymphoma | 2 | (4) | 1 | (2) | 1 | (2) | 1 | (4) |
| Subcutaneous panniculitis-like PTCL | 1 | (2) | 0 | (0) | 0 | (0) | 0 | (0) |
| Primary cutaneous γ/δ T-cell lymphoma | 0 | (0) | 1 | (2) | 0 | (0) | 0 | (0) |
| T-cell lymphoma, further specification not possible | 1 | (2) | 1 | (2) | 1 | (2) | 1 | (4) |
| Other entities | 3§ | (6) | 2|| | (4) | 0 | (0) | 0 | (0) |
| . | Randomized patients . | Transplant recipients . | ||||||
|---|---|---|---|---|---|---|---|---|
| . | Auto-SCT (n = 54) . | Allo-SCT (n = 49) . | Auto-SCT (n = 41*) . | Allo-SCT (n = 26) . | ||||
| Male | 31 | (57) | 34 | (69) | 28 | (68) | 17 | (65) |
| Female | 23 | (43) | 14 | (31) | 13 | (32) | 9 | (35) |
| Age, median (range) | 50 | (28, 60) | 50 | (24, 60) | 51 | (24, 60) | 50 | (35, 60) |
| LDH > Normal | 33 | (61) | 30 | (61) | 22 | (54) | 11 | (42) |
| ECOG > 1 | 11 | (20) | 10 | (20) | 8 | (20) | 5 | (19) |
| Stage III/IV | 47 | (87) | 44 | (90) | 36 | (88) | 23 | (88) |
| aaIPI 0 | 2 | (4) | 3 | (6) | 1 | (2) | 3 | (12) |
| aaIPI 1 | 22 | (41) | 16 | (33) | 20 | (49) | 10 | (38) |
| aaIPI 2 | 21 | (39) | 22 | (45) | 14 | (34) | 10 | (38) |
| aaIPI 3 | 9 | (17) | 8 | (16) | 6 | (15) | 3 | (12) |
| E-involvement | 32 | (59) | 31 | (63) | 24 | (59) | 15 | (58) |
| E > 1 | 16 | (30) | 17 | (35) | 11 | (27) | 6 | (23) |
| IPI 0 | 2 | (4) | 3 | (6) | 1 | (2) | 3 | (12) |
| IPI 1 | 16 | (30) | 10 | (20) | 14 | (34) | 7 | (27) |
| IPI 2 | 22 | (41) | 21 | (43) | 16 | (39) | 11 | (42) |
| IPI 3 | 9 | (17) | 11 | (22) | 9 | (22) | 4 | (15) |
| IPI 4 | 5 | (9) | 4 | (8) | 1 | (2) | 1 | (4) |
| Bulky disease | 10 | (19) | 7 | (14) | 8 | (20) | 3 | (12) |
| B-symptoms | 32 | (59) | 29 | (59) | 23 | (56) | 16 | (62) |
| Bone marrow involved | 17 | (31) | 15 | (31) | 7 | (17) | 9 | (35) |
| Histology | ||||||||
| Reviewed | 54† | (100) | 46 | (94) | 41‡ | (100) | 25 | (96) |
| Peripheral T-cell lymphoma, not otherwise specified (PTCL-NOS) | 16 | (30) | 15 | (33) | 11 | (27) | 8 | (32) |
| Angioimmunoblastic T-cell lymphoma | 17 | (33) | 20 | (43) | 16 | (40) | 12 | (48) |
| Anaplastic large cell lymphoma ALK-negative | 9 | (17) | 5 | (11) | 8 | (20) | 3 | (12) |
| Extranodal NK/T-cell lymphoma, nasal type | 0 | (0) | 1 | (2) | 0 | (0) | 0 | (0) |
| Enteropathy-associated T-cell lymphoma (EATL) types I and II | 3 | (6) | 0 | (0) | 3 | (8) | 0 | (0) |
| Hepatosplenic T-cell lymphoma | 2 | (4) | 1 | (2) | 1 | (2) | 1 | (4) |
| Subcutaneous panniculitis-like PTCL | 1 | (2) | 0 | (0) | 0 | (0) | 0 | (0) |
| Primary cutaneous γ/δ T-cell lymphoma | 0 | (0) | 1 | (2) | 0 | (0) | 0 | (0) |
| T-cell lymphoma, further specification not possible | 1 | (2) | 1 | (2) | 1 | (2) | 1 | (4) |
| Other entities | 3§ | (6) | 2|| | (4) | 0 | (0) | 0 | (0) |
Data are expressed as number of patients (percentage of total group).
Seven patients randomized to allo-SCT are included.
Two patients and ‡1 patient without definitive diagnosis (PTCL suspected; no definite diagnosis possible).
T-cell histocyte-rich large B-cell lymphoma; histiocytic sarcoma; classic Hodgkin lymphoma.
Lymph node infiltration by primary cutaneous T-cell lymphoma (e.g., mycosis fungoides); Epstein-Barr+ CD30+ lymphoproliferation.
Treatment
Thirty-four of 54 patients (63%) randomized to auto-SCT underwent the procedure; 20 patients were unable to proceed to transplantation because of early progression (15 patients), change of diagnosis (no PTCL; 3 patients), and toxicity or the patient’s decision (1 patient each). Twenty-six of 49 randomized patients (53%) underwent allo-SCT, whereas 15 patients did not complete all chemotherapy because of early progression (14 patients) or change in diagnosis (1 patient). Eight patients randomized to allo-SCT were rescheduled to receive auto-SCT, 1 by decision of the data safety management board and 7 because no compatible donor had been found. The diagnoses of patients without a donor were PTCL-NOS (3 patients), AITL (2 patients), and ALK− ALCL (2 patients). One patient with AITL did not receive auto-SCT because of mobilization failure.
Finally, 41 patients received autografts and 26 had allografts. The median duration of all chemotherapy from day 1 of the first course of CHOEP until the day of transplantation was 107 days in the auto-SCT arm and 119 days in the allo-SCT arm, a significant difference (P = .011). The median time interval between the last course of CHOEP and transplantation was 64 days in the auto-SCT arm and 70 days in the allo-SCT arm. Patients who received an autologous transplant had a median of 5.0 × 106 CD34+ cells per kg body weight (range, 2.3-25.8) and infused and recovered leukocytes up to >1 × 10³/µl on day +10 (quartiles, days 9-12). Platelet recovery >20 × 10³/µl was observed on day 11 (7-13). Twenty-six patients who received allo-SCT had 6.6 × 106 CD34+ cells per kg body weight (2.0-13.6) infused, and leukocyte recovery was observed at day +13 (12-16), and platelet recovery at day +12 (9-14; supplemental Table 2).
Efficacy
By intent-to-treat analysis, 25 of 49 patients (51%) in the allo-SCT arm and 21 of 54 patients (39%) in the auto-SCT arm achieved CR/CRu after the end of all therapy. PR was achieved by 4 patients (8%) in the allo-SCT arm and by 9 (17%) in the auto-SCT arm. SD was diagnosed in 2 patients after auto-SCT, but was not reported in any patient after allo-SCT. After CR, CRu, or PR had been achieved, relapse was recorded in 9 patients in the auto-SCT arm and in 4 in the allo-SCT arm. One patient in the auto-SCT arm and 8 in the allo-SCT arm died after achieving CR, CRu, and untreated PR (Table 2).
Treatment response according to treatment arm for randomized patients and transplant recipients only
| . | Randomized patients . | Transplant recipients . | ||||||
|---|---|---|---|---|---|---|---|---|
| . | Auto-SCT n = 54 . | Allo-SCT n = 49 . | Auto-SCT* n = 41 . | Allo-SCT n = 26 . | ||||
| . | n/group (%) . | 95% CI . | n/group (%) . | 95% CI . | n/group (%) . | 95% CI . | n/group (%) . | 95% CI . |
| Treatment response | ||||||||
| CR/CRu | 21/54 (39) | 26-53 | 25/49 (51) | 36-66 | 26/41 (63) | 47-78 | 19/26 (73) | 52-88 |
| CR/CRu/untreated PR | 30/54 (56) | 41-69 | 28/49 (57) | 42-71 | 36/41 (88) | 74-96 | 21/26 (81) | 61-93 |
| Relapse after CR/CRu | 7/21 (33) | 15-57 | 3/25 (12) | 3-31 | 10/26 (38) | 20-59 | 0/19 (0) | 0-18 |
| Relapse after CR/CRu/untreated PR | 9/30 (30) | 15-49 | 4/28 (14) | 4-33 | 13/36 (36) | 21-54 | 0/21 (0) | 0-16 |
| EFS events | ||||||||
| PD at the end of therapy | 19/54 (35) | 16/49 (33) | 5/41 (12) | 1/26 (4) | ||||
| Relapse after CR, CRu | 7/54 (13) | 3/49 (6) | 10/41 (24) | 0/26 (0) | ||||
| Relapse after untreated PR | 2/54 (4) | 1/49 (2) | 3/41 (7) | 0/26 (0) | ||||
| Treated PR | 0/54 (0) | 1/49 (2)† | 0/41 (0) | 0/26 (0) | ||||
| SD | 2/54 (4)‡ | 0/49 (0) | 0/41 (0) | 0/26 (0) | ||||
| Unknown | 3/54 (6)† | 0/49 (0) | 0/41 (0) | 0/26 (0) | ||||
| Death after CR, CRu, untreated PR | 1/54 (2) | 8/49 (16) | 1/41 (2) | 8/26 (31) | ||||
| EFS, PFS, OS rates with 95 CI | ||||||||
| 3-y EFS | 38 | 25-52 | 43 | 29-57 | 57 | 42-73 | 65 | 47-84 |
| 3-y PFS | 39 | 26-52 | 43 | 29-57 | 57 | 42-73 | 65 | 47-84 |
| 3-y OS | 70 | 57-82 | 57 | 43-71 | 81 | 68-93 | 65 | 47-84 |
| . | Randomized patients . | Transplant recipients . | ||||||
|---|---|---|---|---|---|---|---|---|
| . | Auto-SCT n = 54 . | Allo-SCT n = 49 . | Auto-SCT* n = 41 . | Allo-SCT n = 26 . | ||||
| . | n/group (%) . | 95% CI . | n/group (%) . | 95% CI . | n/group (%) . | 95% CI . | n/group (%) . | 95% CI . |
| Treatment response | ||||||||
| CR/CRu | 21/54 (39) | 26-53 | 25/49 (51) | 36-66 | 26/41 (63) | 47-78 | 19/26 (73) | 52-88 |
| CR/CRu/untreated PR | 30/54 (56) | 41-69 | 28/49 (57) | 42-71 | 36/41 (88) | 74-96 | 21/26 (81) | 61-93 |
| Relapse after CR/CRu | 7/21 (33) | 15-57 | 3/25 (12) | 3-31 | 10/26 (38) | 20-59 | 0/19 (0) | 0-18 |
| Relapse after CR/CRu/untreated PR | 9/30 (30) | 15-49 | 4/28 (14) | 4-33 | 13/36 (36) | 21-54 | 0/21 (0) | 0-16 |
| EFS events | ||||||||
| PD at the end of therapy | 19/54 (35) | 16/49 (33) | 5/41 (12) | 1/26 (4) | ||||
| Relapse after CR, CRu | 7/54 (13) | 3/49 (6) | 10/41 (24) | 0/26 (0) | ||||
| Relapse after untreated PR | 2/54 (4) | 1/49 (2) | 3/41 (7) | 0/26 (0) | ||||
| Treated PR | 0/54 (0) | 1/49 (2)† | 0/41 (0) | 0/26 (0) | ||||
| SD | 2/54 (4)‡ | 0/49 (0) | 0/41 (0) | 0/26 (0) | ||||
| Unknown | 3/54 (6)† | 0/49 (0) | 0/41 (0) | 0/26 (0) | ||||
| Death after CR, CRu, untreated PR | 1/54 (2) | 8/49 (16) | 1/41 (2) | 8/26 (31) | ||||
| EFS, PFS, OS rates with 95 CI | ||||||||
| 3-y EFS | 38 | 25-52 | 43 | 29-57 | 57 | 42-73 | 65 | 47-84 |
| 3-y PFS | 39 | 26-52 | 43 | 29-57 | 57 | 42-73 | 65 | 47-84 |
| 3-y OS | 70 | 57-82 | 57 | 43-71 | 81 | 68-93 | 65 | 47-84 |
Treatment response rates patients in subgroup/total patients (percentage of total group), with the 95% CI in the adjacent column.
Seven patients randomized to allo-SCT are included.
Patients with no PTCL.
Death after salvage treatment.
Overall, 18 of all patients (33%) randomized to auto-SCT and 21 of all patients (43%) randomized to allo-SCT died. Causes of death were progression or relapse of lymphoma in 13 patients (72%) in the auto-SCT arm vs 11 patients (52%) in the allo-SCT arm. Salvage treatment–related death was recorded in 4 patients in the auto-SCT arm and in 2 patients in the allo-SCT arm. No patient death was related to auto-SCT. Eight (38%) deaths in the allo-SCT arm were related to the treatment. No other causes of death were reported, except for 1 patient who died of secondary neoplasia in the auto-SCT arm. For a complete list of causes of death in the intent-to-treat population and patients who actually underwent transplantation, see supplemental Table 3.
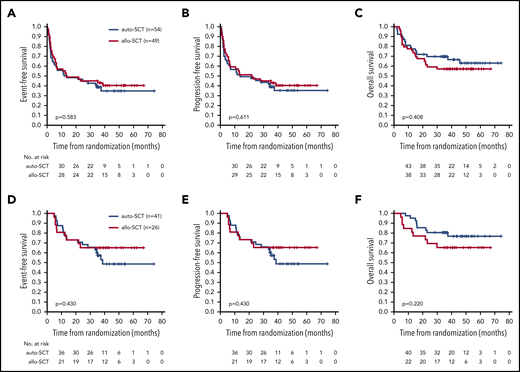
With a median follow-up of 42 months (range, 0.2-74 months) EFS, PFS, and OS showed no significant differences between treatment arms. The 3-year EFS was 43% (95% CI, 29-57) in patients randomized to allo-SCT and 38% (95% CI, 25-52) in patients randomized to auto-SCT (Figure 2A). The 3-year PFS was 43% (95% CI, 29-57) in the allo-SCT arm vs 39% (95% CI, 26-52) in the auto-SCT arm (Figure 2B). The 3-year OS was 57% (95% CI, 43-71) in the allo-SCT vs 70% (95% CI, 57-82) in the auto-SCT arm (Figure 2C).
Outcome according to treatment arm. Event-free (A,D), progression-free (B,E), and overall survival (C,F) for all randomized patients (intent-to-treat population) (A-C) and for transplant recipients only (D-F).
Outcome according to treatment arm. Event-free (A,D), progression-free (B,E), and overall survival (C,F) for all randomized patients (intent-to-treat population) (A-C) and for transplant recipients only (D-F).
Multivariate analyses (allo-SCT vs auto-SCT), adjusted for main strata, confirmed these results: HREFS, 0.9 (95% CI, 0.6-1.5; P = .721); HRPFS, 0.9 (95% CI, 0.5-1.5; P = .702); and HROS, 1.3 (95% CI, 0.7-2.4; P = .421). LDH above normal was found to be a significant factor for EFS (HREFS, 2.3; P = .004) and PFS (HRPFS, 2.4; P = .003) (Table 3).
Multivariate analysis of EFS, PFS, and OS adjusted for strata
| . | EFS HR (95% CI) . | P . | PFS HR (95% CI) . | P . | OS HR (95% CI) . | P . |
|---|---|---|---|---|---|---|
| Randomized patients | ||||||
| Allo-SCT vs auto-SCT | 0.9 (0.6-1.5) | .721 | 0.9 (0.5-1.5) | .702 | 1.3 (0.7-2.4) | .421 |
| LDH > NORMAL | 2.3 (1.3-4.1) | .004 | 2.4 (1.4-4.4) | .003 | 2.0 (1.0-4.3) | .064 |
| ECOG > 1 | 1.0 (0.5-1.8) | .901 | 1.0 (0.5-1.8) | .977 | 1.2 (0.6-2.5) | .648 |
| Stage III/IV | 1.0 (0.4-2.2) | .918 | 1.1 (0.5-2.6) | .844 | 1.4 (0.4-4.8) | .546 |
| E > 1 | 1.2 (0.7-2.1) | .492 | 1.2 (0.7-2.2) | .429 | 1.0 (0.5-1.9) | .896 |
| Transplant recipients | ||||||
| Allo-SCT vs auto-SCT* | 0.8 (0.3-1.7) | .513 | 0.8 (0.3-1.7) | .513 | 1.8 (0.7-4.6) | .218 |
| LDH > NORMAL | 1.4 (0.6-3.1) | .455 | 1.4 (0.6-3.1) | .455 | 1.0 (0.3-3.0) | .977 |
| ECOG > 1 | 1.1 (0.4-2.8) | .886 | 1.1 (0.4-2.8) | .886 | 2.3 (0.8-7.0) | .140 |
| Stage III/IV | 1.3 (0.4-4.5) | .645 | 1.3 (0.4-4.5) | .645 | 1.2 (0.3-5.4) | .807 |
| E > 1 | 0.6 (0.2-1.7) | .341 | 0.6 (0.2-1.7) | .341 | 0.5 (0.1-1.8) | .273 |
| . | EFS HR (95% CI) . | P . | PFS HR (95% CI) . | P . | OS HR (95% CI) . | P . |
|---|---|---|---|---|---|---|
| Randomized patients | ||||||
| Allo-SCT vs auto-SCT | 0.9 (0.6-1.5) | .721 | 0.9 (0.5-1.5) | .702 | 1.3 (0.7-2.4) | .421 |
| LDH > NORMAL | 2.3 (1.3-4.1) | .004 | 2.4 (1.4-4.4) | .003 | 2.0 (1.0-4.3) | .064 |
| ECOG > 1 | 1.0 (0.5-1.8) | .901 | 1.0 (0.5-1.8) | .977 | 1.2 (0.6-2.5) | .648 |
| Stage III/IV | 1.0 (0.4-2.2) | .918 | 1.1 (0.5-2.6) | .844 | 1.4 (0.4-4.8) | .546 |
| E > 1 | 1.2 (0.7-2.1) | .492 | 1.2 (0.7-2.2) | .429 | 1.0 (0.5-1.9) | .896 |
| Transplant recipients | ||||||
| Allo-SCT vs auto-SCT* | 0.8 (0.3-1.7) | .513 | 0.8 (0.3-1.7) | .513 | 1.8 (0.7-4.6) | .218 |
| LDH > NORMAL | 1.4 (0.6-3.1) | .455 | 1.4 (0.6-3.1) | .455 | 1.0 (0.3-3.0) | .977 |
| ECOG > 1 | 1.1 (0.4-2.8) | .886 | 1.1 (0.4-2.8) | .886 | 2.3 (0.8-7.0) | .140 |
| Stage III/IV | 1.3 (0.4-4.5) | .645 | 1.3 (0.4-4.5) | .645 | 1.2 (0.3-5.4) | .807 |
| E > 1 | 0.6 (0.2-1.7) | .341 | 0.6 (0.2-1.7) | .341 | 0.5 (0.1-1.8) | .273 |
Seven patients randomized to allo-SCT are included.
Because only 67 patients (65%) could receive therapy, per protocol, we performed preplanned subgroup analyses restricted to patients who actually underwent autologous or allogeneic transplantation. Forty of 41 patients who proceeded to auto-SCT and 23 of 26 patients who underwent allo-SCT achieved CR, CRu, or PR after 4 courses of CHOEP. Sixteen patients of both treatment arms reached CR or CRu. Three patients who reported SD after 4 courses of CHOEP achieved CRu, PR, and PR after allo-SCT (n = 1 each), whereas the single patient with SD after CHOEP, who underwent auto-SCT, achieved CR after transplantation. The remission status of transplant recipients immediately before transplantation is unknown, because the study protocol did not stipulate another restaging after CHOEP and DHAP chemotherapy.
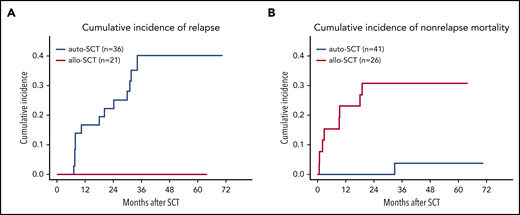
The 3-year EFS in the 26 patients who underwent allo-SCT was 65% (95% CI, 47-84) as compared with 57% (95% CI, 42-73) in the 41 patients who underwent auto-SCT. PFS and OS for patients who received allografts was identical at 65% (95% CI, 47-84), respectively; PFS and OS for those who received autografts was 57% (95% CI, 42-73) and 81% (95% CI, 68-93), respectively. None of the differences was significant (Figure 2D-F). With a median observation time of 42 months, none of the 21 patients who had achieved CR, CRu, or PR relapsed after allo-SCT in contrast to 13 of 36 (36%) patients who relapsed after auto-SCT (Table 2). One patient died of secondary neoplasia after auto-SCT, whereas 8 patients (31%) died of transplantation-related causes after allo-SCT (supplemental Table 3). The cumulative incidence of relapse in patients who had achieved CR, CRu, or PR at final restaging was 17% (95% CI, 4-29) after auto-SCT vs 0% after allo-SCT at 1 year and 40% (95% CI, 22-58) vs 0% at 3 years after transplantation. At 1 year, the cumulative incidence of nonrelapse mortality was 0% in patients who received autografts vs 23% (95% CI, 6-40) in those who received allografts (Figure 3). Treatment-related mortality (TRM) after allo-SCT was mostly associated with acute or chronic GVHD (supplemental Table 4); the incidence and severity are shown in supplemental Table 5.
Relapse and mortality. Cumulative incidence for relapse (A) and nonrelapse mortality (B).
Relapse and mortality. Cumulative incidence for relapse (A) and nonrelapse mortality (B).
Safety
All patients (n = 103) started study treatment with CHOEP chemotherapy. The incidence and severity of adverse events that occurred with CHOEP and DHAP did not differ by treatment arm (supplemental Tables 6 and 7). Adverse events of Common Terminology Criteria for Adverse Events grades 3 to 5 that occurred after BEAM high-dose therapy and auto-SCT or FBC conditioning and allo-SCT are summarized in Table 4. The infections after CHOEP as well as after auto- and allo-SCT are detailed in supplemental Tables 8 and 9.
Nonhematological adverse events grades 3 to 5 after BEAM/auto-SCT and FBC/allo-SCT
| . | Transplant recipients, n (%) . | |||
|---|---|---|---|---|
| . | BEAM/auto-SCT (n = 41)* . | FBC/allo-SCT (n = 26) . | ||
| Nausea | 2/40 | 5 | 2/26 | 8 |
| Vomiting | 1/40 | 2 | 1/26 | 4 |
| Diarrhea | 4/40 | 10 | 3/26 | 12 |
| Constipation | 0/41 | 0 | 0/26 | 0 |
| Mucositis/stomatitis | 13/41 | 32 | 6/26 | 23 |
| Cardiac arrhythmia | 1/40 | 2 | 1/25 | 4 |
| Cardiac general | 1/41 | 2 | 0/26 | 0 |
| Hemorrhage/bleeding | 2/41 | 5 | 1/26 | 4 |
| Renal/genitourinary | 0/41 | 0 | 4/26 | 15 |
| Neuropathy sensory | 0/41 | 0 | 0/26 | 0 |
| Mood alteration | 0/41 | 0 | 1/26 | 4 |
| Allergic reaction/hypersensitivity | 0/40 | 0 | 0/26 | 0 |
| Infections | 13/41 | 32 | 10/26 | 38 |
| Hepatotoxicity (other than VOD) | — | — | 1/26 | 4 |
| VOD | — | — | 0/26 | 0 |
| . | Transplant recipients, n (%) . | |||
|---|---|---|---|---|
| . | BEAM/auto-SCT (n = 41)* . | FBC/allo-SCT (n = 26) . | ||
| Nausea | 2/40 | 5 | 2/26 | 8 |
| Vomiting | 1/40 | 2 | 1/26 | 4 |
| Diarrhea | 4/40 | 10 | 3/26 | 12 |
| Constipation | 0/41 | 0 | 0/26 | 0 |
| Mucositis/stomatitis | 13/41 | 32 | 6/26 | 23 |
| Cardiac arrhythmia | 1/40 | 2 | 1/25 | 4 |
| Cardiac general | 1/41 | 2 | 0/26 | 0 |
| Hemorrhage/bleeding | 2/41 | 5 | 1/26 | 4 |
| Renal/genitourinary | 0/41 | 0 | 4/26 | 15 |
| Neuropathy sensory | 0/41 | 0 | 0/26 | 0 |
| Mood alteration | 0/41 | 0 | 1/26 | 4 |
| Allergic reaction/hypersensitivity | 0/40 | 0 | 0/26 | 0 |
| Infections | 13/41 | 32 | 10/26 | 38 |
| Hepatotoxicity (other than VOD) | — | — | 1/26 | 4 |
| VOD | — | — | 0/26 | 0 |
VOD, venous occlusive disease.
Seven patients randomized to allo-SCT are included.
Nineteen of 26 patients (73%) treated with allo-SCT had GVHD.22 The maximum grade of acute GVHD grade was >2 in 7 patients, 2 of whom died. Chronic GVHD occurred in 8 patients and was described as limited in 7.23 One patient died of chronic GVHD and complications.
Three secondary neoplasms (3%) were observed: 1 aggressive B-cell lymphoma after allo-SCT and 2 solid tumors after auto-SCT.
Discussion
We report that consolidation with high-dose therapy and auto-SCT or myeloablative conditioning and allo-SCT in younger patients with poor-risk T-cell lymphomas showed no significant differences in EFS, PFS, and OS.
For the 54 patients randomized to auto-SCT the 3-year PFS of 39% is between the 36% reported by a German consortium and the 48% reported by the Nordic Lymphoma Group.10,11 The reason for the excellent OS (70%), especially in patients in the auto-SCT arm, may be partly explained by differences in the percentages of T-cell lymphoma entities treated or in the characteristics of individual patients between studies. New drugs that induce further remissions resulting in a tentatively higher percentage of patients who proceed to allo-SCT after failure of auto-SCT may also have their roles. Fourteen of 33 patients (42%) randomized to auto-SCT but refractory to chemotherapy or relapsing after auto-SCT finally received allografts. A long-term follow-up of study patients is planned and will shed further light on the important question of which role allo-SCT has to play in patients with primary refractory disease and those who relapse after auto-SCT.
There is only 1 other study reporting on allogeneic transplantation as part of first-line therapy for T-cell lymphoma. Corradini et al24 treated 61 younger patients (≤60 years of age) with inclusion criteria similar to those in our study. Twenty-three patients who achieved CR or PR after chemotherapy underwent allo-SCT after reduced-intensity conditioning with thiotepa, fludarabine, and cyclophosphamide. Fourteen patients who lacked a suitable donor underwent auto-SCT; all other patients (38%) withdrew from the study before transplantation. In their study, 3 of 23 patients (13%) died of nonrelapse causes, and 4 patients relapsed (17%) after allo-SCT. Lower nonrelapse mortality has repeatedly been reported after reduced-intensity conditioning, in many instances counterbalanced by a higher relapse rate. A retrospective registry study suggested similar outcomes in patients who received allografts after myeloablative or reduced-intensity conditioning.25 In our study, none of the 26 patients who underwent myeloablative conditioning followed by allo-SCT experienced relapse; however, nonrelapse mortality was higher than that reported by Corradini et al.24
This phase 3 study and the phase 2 studies on autologous or allogeneic transplantation24,25 showed comparable OS and PFS rates. Although the survival of our patients did not differ significantly when only patients who underwent auto- or allo-SCT were compared, it is interesting to note that EFS and PFS curves after allo-SCT reached a plateau ∼2 years after transplantation, whereas relapses continued to occur in patients who received autografts. Similar observations have been made in the Nordic trial reporting relapses later than 2 years after auto-SCT in 7% of the intent-to-treat population.10
Although the primary end point was not met, our study has major implications for clinical practice and future studies. First of all, more than one-third of the patients were unable to proceed to transplantation, mostly because of early progression or relapse. Similar observations have been made in all T-cell lymphoma studies investigating first-line chemotherapy.10,11,24 In future trials, patients with up-front chemorefractory disease should be spared toxic but ineffective chemotherapy. Studies that aid in identifying patients with chemorefractory disease by developing innovative molecular approaches will not only contribute to a better understanding of T-cell lymphoma pathophysiology, but will also help in designing new trials involving targeted therapies.
It is important to note that our study did not ask for regular PET scans. Today, PET-CT is routinely used in patients with T-cell lymphoma, and interim PET plays an important role in identifying patients with refractory disease who are in need of an immediate treatment change.26
In our study, many patients experienced progression toward the end of chemotherapy, right before transplantation. Changing chemotherapy from CHOEP to DHAP did not alleviate, but may have aggravated the problem. The ECHELON-2 study reported promising results with CHP (cyclophosphamide, doxorubicin, and prednisone)+brentuximab vedotin (BV), compared with CHOP for first-line therapy for CD30+ ALCL and other T-cell lymphomas.7 To what extent the inclusion of BV in first-line therapy may help more patents to proceed to auto-SCT is not yet clear, because auto-SCT was not part of the study protocol, and few patients received only a transplant. In our study, the median time interval between the last course of CHOEP and transplantation was 64 days in the auto-SCT and 70 days in the allo-SCT arm and thus substantially longer than planned. Such delays seem to be detrimental to patients with T-cell lymphomas and could be reduced by using haploidentical donors for allo-SCT. Early results of haploidentical transplantation in (T-cell) lymphoma seem promising.27,28 Restricting chemotherapy to 2 to 3 cycles followed by immediate allo-SCT could be another option for reducing the number of early treatment failures.
Except for 2 cases of secondary tumors, relapse remains the major problem after auto-SCT. At least for patients with ALCL, this problem may be addressed by the administration of BV after auto-SCT. In patients with Hodgkin lymphoma, this strategy helped to significantly reduce posttransplant relapses.29 After allo-SCT, patients showed a completely different pattern of failure: typical complications of allogeneic transplantation, mostly associated with acute or chronic GVHD, resulted in significant TRM and morbidity. Among others, the myeloablative conditioning used in this study may have contributed to the relatively high TRM observed. Although this study demonstrated a remarkably strong graft vs lymphoma effect in patients with T-cell lymphoma who received allografts in the first remission, we believe that a TRM of 31% is not acceptable today, because new drugs may induce further, albeit short-lived, remission(s) in patients in whom auto-SCT fails, thereby increasing their chance to proceed to allo-SCT during later stages. Thus, although further refinement in donor selection, conditioning, GVHD prophylaxis, and treatment or routine use of haploidentical transplantation may improve results, for the time being, we recommend reserving allo-SCT for patients in whom auto-SCT fails and for those with the earliest signs of progression or relapse. Economic considerations may also support this notion.
Meanwhile, a further search for more effective drugs and cellular therapies in T-cell lymphoma is highly warranted.30
In summary, standard chemotherapy, followed by high-dose therapy and autologous transplantation, remains the preferred option for younger patients with peripheral T-cell lymphoma.
Allogeneic transplantation can also promote long-term survival after failure of autologous transplantation and therefore is considered the treatment of choice for patients with relapsed or refractory disease.
Presented orally at the 55th annual meeting of the American Society of Clinical Oncology, Chicago, IL, 31 May 2019 (abstract 7503), and at the 15th annual International Conference of Malignant Lymphoma (ICML), Lugano, Switzerland, 18 June 2019 (abstract 058).
Original data and protocol may be obtained by e-mail request to the corresponding author.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients, families, caregivers, all investigators from France and Germany who participated in this clinical trial as members of the French Lymphoma Study Association (LYSA) and the German Lymphoma Alliance (GLA), and Fresenius Biotech GmbH, which provided the antithymocyte globulin for the French patients who underwent allogeneic transplantation.
This study was supported by Les Laboratoires Pierre Fabre and Chugai Pharma, which provided research support and by Fresenius Biotech GmbH, which provided the anti-thymocyte globulin for patients who underwent allogeneic transplantation; by Bundesministerium für Bildung und Forschung (BMBF) grant FKZ 01KG0705 (N.S., L.T.) and by Ministère de la Santé et des Solidarités grant PHRC09_05-004-TOURNILHAC (O.T.).
Authorship
Contribution: N.S., O.T., L.T., M.Z., B.G., M.N., and C.G. conceived and designed the study; B.A., M.Z., N.S., and O.T. analyzed and interpreted the data; N.S., B.A., M.Z., M.N., O.T., and B.F. drafted or revised the manuscript; and all authors collected and assembled the data, provided study materials or patients, and reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: N.S. has received personal fees from Riemser, Janssen, Kite/Gilead, Novartis, and Takeda; L.T. has received personal fees from Takeda; J.C. has received personal fees from BMS, Gilead, Takeda, Amgen, Hexal, Celgene, Astra Zeneca; P.R. has received personal fees from Takeda, Novartis, Sanofi-Aventis, and BMS; A.V. has received personal fees from Roche, Kite/Gilead, Pfizer, BMS, and Amgen; V.P. has received personal fees from Abbvie, Amgen, and Roche; and M.H. has received personal feed from Amgen, Roche, Takeda, and Novartis. The remaining authors declare no competing financial interests.
A list of the remaining LYSA and GLA investigators appears in the Appendix.
Correspondence: Norbert Schmitz, Münster University Hospital, Albert-Schweitzer-Campus 1/A1, 48149 Münster, Germany; e-mail: norbert.schmitz@ukmuenster.de.
Appendix
The members of the LYSA and GLA include the expert pathologists who provided histopathological review: Andreas Rosenwald (chairman), Wuerzburg, Germany; Alfred C. Feller, Luebeck, Germany; Martin-Leo Hansmann, Frankfurt, Germany; Wolfram Klapper, Kiel, Germany; Peter Moeller, Ulm, Germany; Hans Konrad Mueller-Hermelink, Wuerzburg, Germany; Harald Stein, Berlin, Germany; Laurence de Leval, Lausanne, Switzerland; Philippe Gaulard, Paris, France; Marie Parrens, Bordeaux, France; Albane Ledoux-Pilon, Clermont-Ferrand, France; Céline Bossard, Nantes, France; Nadine Vailhen, Creteil, France and the LYSA platform (LYSAP); the data and safety monitoring committee, which served as an independent expert advisory group to evaluate safety and efficacy data during the trial: Hildegard Greinix, Graz, Austria; Anthony H. Goldstone, London, United Kingdom; Vincent Levy, Bobigny, France; and Ephraïm P. Hochberg, Boston, MA; all members of the study trial offices including Kristina Kocksch, Hamburg, Germany, Bettina Freymark, Hamburg, Germany, Kerstin Menck, Göttingen, Germany, Elke Stitz, Göttingen, Germany, Christelle Latière, Clermont-Ferrand, France, Aurélie Cabrespine, Clermont-Ferrand, France, Gérald Gouby, Clermont-Ferrand, France, Lise Laclautre, Clermont-Ferrand, France, Richard Lemal, Clermont-Ferrand, France, and Sébastien Bailly, Clermont-Ferrand, France; members of the Clinical Research Organization of the French Innovative Leukemia Organization (FILO): Roselyne Delepine, Tours, France, Alexandra Fayault, Tours, France, and Valérie Rolland, Grenoble, France; the data center: Markus Loeffler, chair, Leipzig, Germany, Sigrid Haupt, Leipzig, Germany, Jürgen Hentschel, Leipzig, Germany, Martina Kunert, Leipzig, Germany, Beate Mann, Leipzig, Germany, Katja Rillich, Leipzig, Germany, Ulrike Schoenwiese, Leipzig, Germany, and Barbara Wicklein Leipzig, Germany; and the principal investigators Marie Pierre Moles, Angers, France; Borhane Slama, Avignon, France; Adrien Chauchet, Besançon, France; Alain Saad, Bézier, France; Olivier Fitoussi, Bordeaux, France; Alain Devidas, Corbeil, France; Mourad Tiab, La Roche Sur Yon, France; Bernard Drenou, Mulhouse, France; Jean Luc Harousseau, Nantes, France; Eric Jourdan, Nimes, France; Pauline Brice, Paris Saint Louis, France; Sylvie Glaisner, Saint Cloud, France; Jérome Cornillon, Saint Etienne, France; Stefan Klein, Mannheim, Germany; Andreas Neubauer, Marburg, Germany; and Ullrich Graeven, Moenchengladbach, Germany.
REFERENCES
Author notes
B.A. and O.T. contributed equally to this study.