Key Points
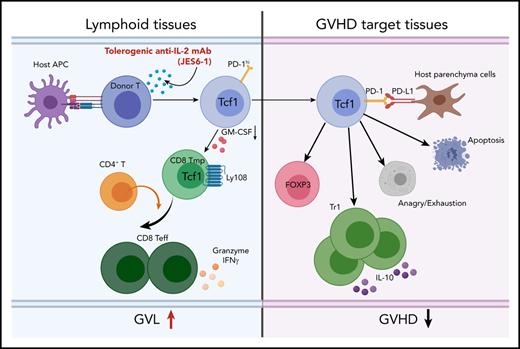
Tolerogenic anti–IL-2 treatment augments tissue PD-L1–dependent depletion of GM-CSF+Th1/Tc1 and expansion of IL-10+Tr1 cells.
Tolerogenic anti–IL-2 preserves strong GVL effect in association with expansion of TCF-1+CD8+ T memory progenitors in lymphoid tissues.
Abstract
Donor T cells mediate both graft-versus-leukemia (GVL) activity and graft-versus-host disease (GVHD) after allogeneic hematopoietic cell transplantation (allo-HCT). Development of methods that preserve GVL activity while preventing GVHD remains a long-sought goal. Tolerogenic anti–interleukin-2 (IL-2) monoclonal antibody (JES6-1) forms anti–IL-2/IL-2 complexes that block IL-2 binding to IL-2Rβ and IL-2Rγ on conventional T cells that have low expression of IL-2Rα. Here, we show that administration of JES6 early after allo-HCT in mice markedly attenuates acute GVHD while preserving GVL activity that is dramatically stronger than observed with tacrolimus (TAC) treatment. The anti–IL-2 treatment downregulated activation of the IL-2-Stat5 pathway and reduced production of granulocyte-macrophage colony-stimulating factor (GM-CSF). In GVHD target tissues, enhanced T-cell programmed cell death protein 1 (PD-1) interaction with tissue–programmed cell death-ligand 1 (PD-L1) led to reduced activation of protein kinase–mammalian target of rapamycin pathway and increased expression of eomesodermin and B-lymphocyte-induced maturation protein-1, increased T-cell anergy/exhaustion, expansion of Foxp3–IL-10–producing type 1 regulatory (Tr1) cells, and depletion of GM-CSF–producing T helper type 1 (Th1)/cytotoxic T cell type 1 (Tc1) cells. In recipient lymphoid tissues, lack of donor T-cell PD-1 interaction with tissue PD-L1 preserved donor PD-1+TCF-1+Ly108+CD8+ T memory progenitors and functional effectors that have strong GVL activity. Anti–IL-2 and TAC treatments have qualitatively distinct effects on donor T cells in the lymphoid tissues, and CD8+ T memory progenitor cells are enriched with anti–IL-2 treatment compared with TAC treatment. We conclude that administration of tolerogenic anti–IL-2 monoclonal antibody early after allo-HCT represents a novel approach for preventing acute GVHD while preserving GVL activity.
Introduction
The success of allogeneic hematopoietic cell transplantation (allo-HCT) for the treatment of hematologic malignancies depends partly on the ability of donor T cells to eliminate residual malignant cells in the recipient after the pretransplant conditioning; the same donor T cells also mediate graft-versus-host-disease (GVHD).1 Prevention of GVHD in patients with immunosuppressants also suppresses graft-versus-leukemia (GVL) activity.2-4 Development of approaches that prevent GVHD while preserving GVL activity remains a long-sought goal.5-8
Interactions of programmed cell death-ligand 1 (PD-L1) with programmed cell death protein 1 (PD-1) and CD80 on activated T cells have an important role in regulating immune responses.9-11 Tumor cell PD-L1 interaction with PD-1 on activated T cells tolerizes antitumor T cells and prevents antitumor immunity.12 Similarly, recipient tissue PD-L1 interactions with PD-1 and CD80 on alloactivated donor CD8+ T cells markedly reduce GVHD severity, although this mechanism is not effective when the graft contains both CD4+ and CD8+ T cells.13 One possible reason is that interleukin-2 (IL-2) produced by CD4+ T cells could prevent tolerance induction by PD-1 signaling,14 although previous studies showed that administration of high-dose IL-2 early after allo-HCT prevents acute GVHD (aGVHD) while preserving GVL activity.15
Regulatory T cells including FoxP3+ Treg and FoxP3–IL-10+ type 1 regulatory (Tr1) cells play important roles in ameliorating aGVHD.16-22 PD-L1 interaction with PD-1 augments conversion of activated T cells into Foxp3+ Treg cells.23 In the pathogenesis of aGVHD, most regulatory T cells are Tr1 cells that require eomesodermin (Eomes) for their development.17 Although PD-L1/PD-1 interaction upregulates expression of Eomes and B-lymphocyte-induced maturation protein-1 (Blimp-1) during induction of anergy and exhaustion of T effector cells (Teff), the role of PD-L1 on Tr1 cell development remains unclear. In addition, persistence of donor CD8+ T cell–induced GVHD was mediated by CD8+ T memory progenitors (Tmp)24 that play a critical role in tumor immunity.25
JES6-1 is a monoclonal antibody (mAb) that binds murine IL-2. JES6 blocks IL-2 interaction with IL-2Rβ and IL-2Rγ on conventional T (Tcon) cells with low or absent expression of IL-2Rα; however, JES6 does not block IL-2 interaction with IL-2Rα, leading to expansion of Foxp3+ Treg cells with high expression of IL-2Rα.26,27 Because our previous studies suggested that IL-2 from donor CD4+ T cells regulates tolerance induction by the interaction of T-cell PD-1 with tissue PD-L1,13 we tested whether inhibition of IL-2 signaling could augment the tolerizing effect of PD-1 interaction with PD-L1 and prevent GVHD while preserving GVL activity.
Materials and methods
Mice
BALB/c (H-2d) and C57BL/6 (H-2b) mice were purchased from the National Cancer Institute (Frederick, Maryland). IL-10−/− C57BL/6 mice (H-2b) were purchased from The Jackson Laboratory (Bar Harbor, Maine). PD-L1−/− BALB/c breeders were provided by L. Chen (Yale University, New Haven, Connecticut). All mice were maintained in a pathogen-free room in the City of Hope Animal Research Center (Duarte, California). All experiments were approved by the Institutional Animal Care and Use Committee at City of Hope National Medical Center.
Methods
Induction and assessment of GVHD, measurement of cytokines in serum, flow cytometry analysis and sorting, histopathology and histoimmunofluorescent staining, single-cell RNA-sequencing library construction using the 10× genomics chromium platform, and statistical analysis are described in previous publications13 and in the supplemental Materials and methods (available on the Blood Web site).
Results
Administration of JES6 mAb prevents aGVHD and preserves GVL activity more effectively than tacrolimus
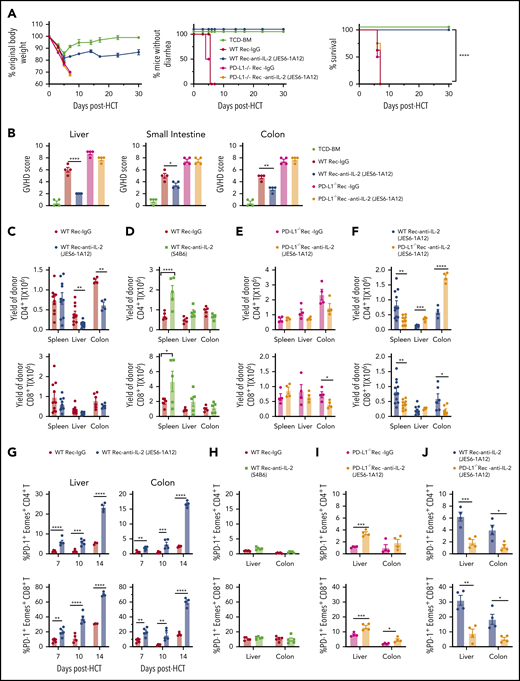
Our recent report proposed that IL-2 from donor CD4+ T cells may make alloreactive donor T cells resistant to induction of tolerance (ie, anergy, exhaustion, apoptosis) by host tissue PD-L1.13 Thus, we tested whether administration of the JES6 (JES6-1A12) mAb that blocks IL-2 interaction with IL-2Rβ and IL-2γ on Tcon cells27 could prevent GVHD and preserve GVL activity. Accordingly, irradiated BALB/c recipients were engrafted with splenocytes (5 × 106) and T cell–depleted bone marrow (TCD-BM) cells (2.5 × 106) from major histocompatibility complex–mismatched C57BL/6 donors. Recipients given TCD-BM cells alone were used as GVHD-free controls. Recipients were treated with JES6 or control rat immunoglobulin G (IgG) at an intraperitoneal (i.p.) dose of 500 µg/mouse on days 0, 2, 4, and 6 after HCT. Compared with IgG treatment, JES6 treatment limited loss of body weight and completely prevented diarrhea, and all recipients survived for >30 days. Moreover, JES6 treatment prevented damage in GVHD target tissues (liver, small intestine, and colon) (Figure 1A-B; supplemental Figure 1A). In contrast, administration of the non-tolerogenic anti–IL-2-S4B6 mAb that augments IL-2 signaling in Tcon cells did not prevent aGVHD (Figure 1C-D; supplemental Figure 1B).
Tolerogenic anti-IL-2 mAb (JES6-1A12) but not non-tolerogenic anti–IL-2 mAb (S4B6) prevents aGVHD and preserves GVL activity more effectively than TAC. (A-D) Lethally irradiated WT BALB/c recipients were given splenocytes (5 × 106) with or without TCD-BM cells (2.5 × 106) from WT C57BL/6 donors. Recipients were given a total of 4 i.p. injections of rat-IgG, anti–IL-2 mAb (JES6-1A12), or anti–IL-2 mAb (S4B6) (500 µg/mouse) at days 0, 2, 4, and 6 after HCT. (A,C) Plots of percent original body weight, diarrhea, and percent survival are shown. n = 7-8 combined from 2 replicated experiments. (B,D) Mean ± standard error of the mean of histopathology scores of liver, small intestine, and colon are shown; n = 4 per group. Combined from 2 replicated experiments. (E) WT BALB/c recipients given splenocytes and TCD-BM cells from WT C57BL/6 donors and injected IgG or anti–IL-2 mAb (JES6-1A12) as described in panels A-D. Recipients were challenged with i.p. injection of BCL1/Luc cells (5 × 106/mouse) on day 0. Mice were monitored for tumor growth by using in vivo bioluminescent imaging (BLI), clinical signs of GVHD, and survival. One representative BLI image from each time point is shown for each group and summary of photons/second, diarrhea, and tumor-free survival of recipients. n = 8 combined from 2 replicated experiments. (F) Lethally irradiated WT BALB/c recipients were given splenocytes (2.5 × 106) and TCD-BM cells (2.5 × 106) from WT C57BL/6 donors. Recipients were challenged with i.p. injection of BCL1/Luc cells (5 × 106/mouse) on day 0 and were given a total of 4 i.p. injections of anti–IL-2 mAb (JES6-1A12) (500 µg/mouse) at days 0, 2, 4, and 6 after HCT, or daily i.p. injections of TAC (0.75 mg/kg) at days 0 to 21 after HCT. One representative BLI image from each time point is shown for the anti–IL-2 mAb (JES6-1A12) and TAC group and summary of photons/second, body weight change, as well as the tumor-free survival of recipients. n = 8-10 combined from 2 replicated experiments. (G) Lethally irradiated BALB/c recipients were given splenocytes (1.25 × 106) and TCD-BM cells (2.5 × 106) from C57BL/6 donors. Recipients were challenged with i.p. injection of BCL1/Luc cells (10 × 106/mouse) on day 0 and were given a total of 4 intravenous injections of IL-2 mAb (500 µg/mouse) at days 0, 2, 4, and 6 after HCT, or i.p. injections of TAC (0.75 mg/kg) once daily until moribund with tumor growth. One representative BLI image from each time point is shown for IL-2 mAb (JES6-1A12) and TAC group and summary of photons/second and tumor-free survival of recipients. n = 8-10 combined from 2 replicated experiments. “+” indicates death. Data represent mean ± standard error. P values were calculated by using ordinary one-way analysis of variance (panels B,D), two-tailed Student t tests (panels F,G), or log-rank test for survival comparison (panels A,E-G). *P < .05, **P < .01, ***P < .001, ****P < .0001.
Tolerogenic anti-IL-2 mAb (JES6-1A12) but not non-tolerogenic anti–IL-2 mAb (S4B6) prevents aGVHD and preserves GVL activity more effectively than TAC. (A-D) Lethally irradiated WT BALB/c recipients were given splenocytes (5 × 106) with or without TCD-BM cells (2.5 × 106) from WT C57BL/6 donors. Recipients were given a total of 4 i.p. injections of rat-IgG, anti–IL-2 mAb (JES6-1A12), or anti–IL-2 mAb (S4B6) (500 µg/mouse) at days 0, 2, 4, and 6 after HCT. (A,C) Plots of percent original body weight, diarrhea, and percent survival are shown. n = 7-8 combined from 2 replicated experiments. (B,D) Mean ± standard error of the mean of histopathology scores of liver, small intestine, and colon are shown; n = 4 per group. Combined from 2 replicated experiments. (E) WT BALB/c recipients given splenocytes and TCD-BM cells from WT C57BL/6 donors and injected IgG or anti–IL-2 mAb (JES6-1A12) as described in panels A-D. Recipients were challenged with i.p. injection of BCL1/Luc cells (5 × 106/mouse) on day 0. Mice were monitored for tumor growth by using in vivo bioluminescent imaging (BLI), clinical signs of GVHD, and survival. One representative BLI image from each time point is shown for each group and summary of photons/second, diarrhea, and tumor-free survival of recipients. n = 8 combined from 2 replicated experiments. (F) Lethally irradiated WT BALB/c recipients were given splenocytes (2.5 × 106) and TCD-BM cells (2.5 × 106) from WT C57BL/6 donors. Recipients were challenged with i.p. injection of BCL1/Luc cells (5 × 106/mouse) on day 0 and were given a total of 4 i.p. injections of anti–IL-2 mAb (JES6-1A12) (500 µg/mouse) at days 0, 2, 4, and 6 after HCT, or daily i.p. injections of TAC (0.75 mg/kg) at days 0 to 21 after HCT. One representative BLI image from each time point is shown for the anti–IL-2 mAb (JES6-1A12) and TAC group and summary of photons/second, body weight change, as well as the tumor-free survival of recipients. n = 8-10 combined from 2 replicated experiments. (G) Lethally irradiated BALB/c recipients were given splenocytes (1.25 × 106) and TCD-BM cells (2.5 × 106) from C57BL/6 donors. Recipients were challenged with i.p. injection of BCL1/Luc cells (10 × 106/mouse) on day 0 and were given a total of 4 intravenous injections of IL-2 mAb (500 µg/mouse) at days 0, 2, 4, and 6 after HCT, or i.p. injections of TAC (0.75 mg/kg) once daily until moribund with tumor growth. One representative BLI image from each time point is shown for IL-2 mAb (JES6-1A12) and TAC group and summary of photons/second and tumor-free survival of recipients. n = 8-10 combined from 2 replicated experiments. “+” indicates death. Data represent mean ± standard error. P values were calculated by using ordinary one-way analysis of variance (panels B,D), two-tailed Student t tests (panels F,G), or log-rank test for survival comparison (panels A,E-G). *P < .05, **P < .01, ***P < .001, ****P < .0001.
To assess the effect of JES6 treatment on GVL activity, BALB/c recipients were inoculated with luciferase-transfected BCL1 cells (BCL1/Luc, 5 × 106/mouse, i.p.) before HCT. BCL1/Luc tumor cells grew rapidly in recipients engrafted with TCD-BM. JES6 treatment did not have a significant impact on the tumor growth, and all recipients died within 20 days after HCT (Figure 1E). BCL1/Luc tumor cells grew transiently in IgG-treated recipients engrafted with splenocytes and TCD-BM cells, but all recipients died with GVHD within 10 days after HCT. BCL1/Luc tumor cells also grew transiently in JES6-treated recipients and were eliminated by day 11, but all mice survived for >30 days without tumor relapse. Treatment with JES6 also eliminated GVL-resistant blast-crisis chronic myelogenous leukemia tumor cells28 while preventing aGVHD (supplemental Figure 2). Although JES6 treatment augmented natural killer cell expansion, depletion of natural cells had no impact on GVL activity (supplemental Figure 3). Taken together, these results show that treatment with JES6 effectively controlled GVHD while preserving strong GVL activity mediated by alloreactive T cells.
The calcineurin inhibitor tacrolimus (TAC) is widely used clinically to prevent aGVHD, in part by inhibiting endogenous IL-2 production in alloactivated donor T cells. Therefore, it was of interest to compare the effects of JES6 and TAC regarding their respective abilities to prevent aGVHD while preserving GVL activity. BALB/c recipients engrafted with spleen (2.5 × 106) and TCD-BM (2.5 × 106) cells from C57BL/6 donors and challenged with 5 × 106 BCL1/Luc cells on day 0 were treated with anti–interleukin-2 (IL-2) on days 0, 2, 4, and 6 after HCT or with an i.p. injection of TAC (0.75 mg/kg) daily for up to 21 days. The 2 groups showed similar loss of body weight, and survival was not statistically different between the 2 groups during the first 30 days after HCT. Both groups cleared tumor cells by 12 to 17 days after HCT (Figure 1F).
In further experiments, BALB/c recipients engrafted with C57BL/6 BM cells and a lower number of spleen cells (1.25 × 106) were challenged with i.p. inoculation of 5 or 10 × 106 Luc/BCL1 cells, with the same regimen of JES6 or TAC. In recipients challenged with 5 × 106 BCL1/Luc cells, tumor cells disappeared before day 12 in all JES6-treated recipients; only 60% of TAC-treated recipients cleared tumor by day 17, however, and the other 40% died with progressive tumor growth by 30 days after HCT (supplemental Figure 4). In recipients challenged with 10 × 106 BCL1/Luc cells, all JES6-treated recipients cleared tumor cells by day 12 after HCT, but all TAC-treated recipients died with progressive tumor growth by 9 days after HCT (Figure 1G). Thus, under the conditions used for these experiments, JES6 and TAC had comparable ability to prevent aGVHD, but JES6 treatment preserved GVL activity, while TAC did not.
Prevention of aGVHD by JES6 requires PD-L1 expression by GVHD target tissues
Prevention of GVHD by depleting donor CD4+ T cells that produced IL-2 was host tissue PD-L1–dependent.13 We therefore tested whether prevention of GVHD by JES6 also depends on recipient PD-L1. JES6 attenuated the severity of GVHD in wild-type (WT) recipients but not in PD-L1−/− recipients (Figure 2A-B; supplemental Figure 5). JES6 treatment markedly ameliorated tissue damage in the liver, small intestine, and colon of WT recipients compared with IgG-treated recipients. In contrast, JES6 treatment did not ameliorate tissue damage at all in PD-L1−/− recipients. Compared with IgG treatment, JES6 treatment reduced the yield of donor CD4+ T cells, although not CD8+ T cells in the liver and colon at day 7 in WT recipients (Figure 2C). In contrast, anti–IL-2-S4B6-treatment expanded donor-type CD4+ and CD8+ T cells in the spleen but produced no significant changes in the liver or colon (Figure 2D). Compared with IgG treatment, the effect of JES6 treatment was not apparent in PD-L1−/− recipients (Figure 2E). However, compared with WT recipients, the yield of CD4+ T cells in the liver and colon at day 7 was higher in PD-L1−/− recipients (Figure 2F). These results also indicate that JES6 augments host tissue PD-L1–mediated protection against aGVHD.
Prevention of aGVHD by tolerogenic anti–IL-2 mAb (JES6-1A12) requires PD-L1 expression by GVHD target tissues. Lethally irradiated WT or PD-L1−/− BALB/c recipients were given splenocytes (2.5 × 106) and TCD-BM cells (2.5 × 106) from C57BL/6 donors. Recipients (Rec) were given a total of 4 i.p. injections of rat-IgG, anti–IL-2 mAb (JES6-1A12), or anti–IL-2 mAb (S4B6) (500 µg/mouse) at days 0, 2, 4, and 6 after HCT. Recipients given TCD-BM cells (2.5 × 106) alone were used as controls. (A) Plots of percent original body weight, diarrhea, and percent survival are shown. n = 8 per group. Combined from 2 replicated experiments. (B) Mean ± standard error of the mean of histopathology scores of liver, small intestine, and colon are shown; n = 4 per group. Combined from 2 replicated experiments. (C-F) At day 6 after HCT, spleen, liver, and colon of recipients were harvested for analysis of donor CD4+ and CD8+ T-cell percentage and yield. Mean ± standard error of the mean of the percentage and yield of H-2Kb+TCRβ+ CD4+ or CD8+ T cells are shown; n = 4 to 11 per group. Combined from 2 to 3 replicated experiments. (G-I) Day 6 post-HCT, percentage of Eomes+PD1+ cells among donor CD4+ and CD8+ T cells in liver and colon of WT or PD-L1−/− recipients treated with anti–IL-2 mAb (JES6-1A12), anti–IL-2 mAb (S4B6), or control IgG; n = 4 to 5 per group. (J) Day 6 post-HCT, percentage of Eomes+PD1+ cells among donor CD4+ and CD8+ T cells in liver and colon of WT and PD-L1−/− recipients treated with anti–IL-2 mAb (JES6-1A12); n = 4 per group. Data represent mean ± standard error combined from 2 replicated experiments. P values were calculated by using the log-rank test (A), one-way analysis of variance (B), or two-way analysis of variance (C-J). *P < .05, **P < .01, ***P < .001, ****P < .0001.
Prevention of aGVHD by tolerogenic anti–IL-2 mAb (JES6-1A12) requires PD-L1 expression by GVHD target tissues. Lethally irradiated WT or PD-L1−/− BALB/c recipients were given splenocytes (2.5 × 106) and TCD-BM cells (2.5 × 106) from C57BL/6 donors. Recipients (Rec) were given a total of 4 i.p. injections of rat-IgG, anti–IL-2 mAb (JES6-1A12), or anti–IL-2 mAb (S4B6) (500 µg/mouse) at days 0, 2, 4, and 6 after HCT. Recipients given TCD-BM cells (2.5 × 106) alone were used as controls. (A) Plots of percent original body weight, diarrhea, and percent survival are shown. n = 8 per group. Combined from 2 replicated experiments. (B) Mean ± standard error of the mean of histopathology scores of liver, small intestine, and colon are shown; n = 4 per group. Combined from 2 replicated experiments. (C-F) At day 6 after HCT, spleen, liver, and colon of recipients were harvested for analysis of donor CD4+ and CD8+ T-cell percentage and yield. Mean ± standard error of the mean of the percentage and yield of H-2Kb+TCRβ+ CD4+ or CD8+ T cells are shown; n = 4 to 11 per group. Combined from 2 to 3 replicated experiments. (G-I) Day 6 post-HCT, percentage of Eomes+PD1+ cells among donor CD4+ and CD8+ T cells in liver and colon of WT or PD-L1−/− recipients treated with anti–IL-2 mAb (JES6-1A12), anti–IL-2 mAb (S4B6), or control IgG; n = 4 to 5 per group. (J) Day 6 post-HCT, percentage of Eomes+PD1+ cells among donor CD4+ and CD8+ T cells in liver and colon of WT and PD-L1−/− recipients treated with anti–IL-2 mAb (JES6-1A12); n = 4 per group. Data represent mean ± standard error combined from 2 replicated experiments. P values were calculated by using the log-rank test (A), one-way analysis of variance (B), or two-way analysis of variance (C-J). *P < .05, **P < .01, ***P < .001, ****P < .0001.
Upregulation of expression of PD-1 and Eomes is a feature of anergic/exhausted T cells.29 JES6 treatment markedly increased the percentages of PD-1+Eomes+ CD4+ and CD8+ T cells in the GVHD target tissues liver and colon in a time-dependent manner in the WT recipients (Figure 2G); anti–IL-2-S4B6 did not have these effects (Figure 2H). Compared with IgG treatment, JES6 treatment also increased the percentage of PD-1+Eomes+ CD4+ and CD8+ T cells in the liver and colon tissues at day 6 after HCT in the PD-L1−/− recipients (Figure 2I). Later time points were not available for evaluation, due to death of most of the PD-L1−/− recipients by 7 days after HCT. The percentages of PD-1+Eomes+ CD4+ and CD8+ T cells in the liver and colon tissues of JES6-treated PD-L1−/− recipients were markedly lower than in JES6-treated WT recipients at day 6 after HCT (Figure 2J). These results indicate that JES6 treatment and tissue PD-L1 interaction with PD-1 synergistically augment T-cell anergy/exhaustion of tissue-infiltrating T cells, leading to prevention of aGVHD.
Prevention of aGVHD by JES6 is associated with tissue PD-L1–dependent depletion of GM-CSF–producing Th1 and Tc1 cells
Granulocyte-macrophage colony-stimulating factor (GM-CSF)–producing T helper type 1 (Th1) and cytotoxic T cell type 1 (Tc1) cells play an essential role in aGVHD pathogenesis.30,31 We evaluated GM-CSF–producing (GM-CSF+) Th1 and Tc1 cells at day 6 after HCT. Compared with IgG treatment, JES6 treatment reduced the percentages and yield of GM-CSF+IFN-γ+ Th1 in the liver and colon of WT recipients and reduced the percentages but not the yields of Tc1 in those tissues (Figure 3A; supplemental Figure 6), whereas anti–IL-2-S4B6 did not have these effects (Figure 3B; supplemental Figure 7). JES6 treatment also reduced the percentage and yield of GM-CSF+ Th1 and Tc1 cells in the liver tissues of PD-L1−/− recipients, but the effect in the spleen and colon tissues was variable (Figure 3C; supplemental Figure 8). The percentage and yield of GM-CSF+ Th1 and Tc1 cells in the spleen, liver, and colon of JES6-treated PD-L1−/− recipients were markedly higher than in JES6-treated WT recipients (Figure 3D; supplemental Figure 9). The lower percentages of Th1 and Tc1 cells expressing GM-CSF induced by JES6 treatment in WT recipients were associated with lower infiltration of neutrophils and monocytes in the liver and colon and with their lower production of pro–IL-1β and reactive oxygen species (supplemental Figure 10). These results indicate that administration of JES6 and tissue PD-L1 interaction with PD-1 on T cells synergistically augment depletion of GM-CSF–producing Th1 and Tc1 cells in GVHD target tissues.
Prevention of GVHD by tolerogenic anti–IL-2 mAb (JES6-1A12) but not non-tolerogenic anti–IL-2 mAb (S4B6) is associated with tissue PD-L1–dependent depletion of GM-CSF–producing Th1 and Tc1 cells. Lethally irradiated WT and PD-L1−/− BALB/c recipients were given splenocytes (2.5 × 106) and TCD-BM cells (2.5 × 106) from C57BL/6 donors. Recipients were given a total of 3 i.p. injections of rat-IgG, anti–IL-2 mAb (JES6-1A12), or anti–IL-2 mAb (S4B6) (500 µg/mouse) at days 0, 2, and 4 after HCT. On day 6, donor cells in the spleen, liver, and colon were analyzed for cytokine profile. (A-B) Percentage and yield of GM-CSF+ cells among donor IFN-γ+ CD4+ and CD8+ T cells in spleen, liver, and colon of WT recipients treated with anti–IL-2 mAb (JES6-1A12 or S4B6) or control IgG. (C) Percentage and yield of GM-CSF+ cells among donor IFN-γ+ CD4+ and CD8+ T cells in spleen, liver, and colon of PD-L1−/− recipients treated with anti–IL-2 mAb (JES6-1A12) or control IgG; n = 4 per group. (D) Percentage and yield of GM-CSF+ cells among donor IFN-γ+ CD4+ and CD8+ T cells in spleen, liver, and colon of WT or PD-L1−/− recipients treated with anti–IL-2 mAb (JES6-1A12); n = 5 per group. Data represent mean ± standard error combined from 2 replicate experiments. P values were calculated by using unpaired two-tailed Student t tests. *P < .05, **P < .01, ***P < .001, ****P < .0001.
Prevention of GVHD by tolerogenic anti–IL-2 mAb (JES6-1A12) but not non-tolerogenic anti–IL-2 mAb (S4B6) is associated with tissue PD-L1–dependent depletion of GM-CSF–producing Th1 and Tc1 cells. Lethally irradiated WT and PD-L1−/− BALB/c recipients were given splenocytes (2.5 × 106) and TCD-BM cells (2.5 × 106) from C57BL/6 donors. Recipients were given a total of 3 i.p. injections of rat-IgG, anti–IL-2 mAb (JES6-1A12), or anti–IL-2 mAb (S4B6) (500 µg/mouse) at days 0, 2, and 4 after HCT. On day 6, donor cells in the spleen, liver, and colon were analyzed for cytokine profile. (A-B) Percentage and yield of GM-CSF+ cells among donor IFN-γ+ CD4+ and CD8+ T cells in spleen, liver, and colon of WT recipients treated with anti–IL-2 mAb (JES6-1A12 or S4B6) or control IgG. (C) Percentage and yield of GM-CSF+ cells among donor IFN-γ+ CD4+ and CD8+ T cells in spleen, liver, and colon of PD-L1−/− recipients treated with anti–IL-2 mAb (JES6-1A12) or control IgG; n = 4 per group. (D) Percentage and yield of GM-CSF+ cells among donor IFN-γ+ CD4+ and CD8+ T cells in spleen, liver, and colon of WT or PD-L1−/− recipients treated with anti–IL-2 mAb (JES6-1A12); n = 5 per group. Data represent mean ± standard error combined from 2 replicate experiments. P values were calculated by using unpaired two-tailed Student t tests. *P < .05, **P < .01, ***P < .001, ****P < .0001.
Prevention of aGVHD by JES6 requires PD-L1–dependent expansion of Tr1 cells
JES6 but not anti–IL-2-S4B6 increased the percentage of Foxp3+ Treg cells in the liver of WT recipients (supplemental Figures 11A-B). Neither mAb had any effect on the percentage or yield of Foxp3+ Treg cells in the colon. JES6 treatment did not have a significant effect on Treg expansion in PD-L1−/− recipients (supplemental Figure 11C).
Foxp3–IL-10+ Tr1 cells represent the majority of regulatory T cells in allo-HCT recipients, and Tr1 cell expression of Eomes and Blimp-1 are required for Tr1 cell differentiation.17 Because tissue infiltrating CD4+ T cells expressed higher levels of Eomes (Figure 2G), we evaluated the impact of JES6 treatment on Foxp3–IL-10+ Tr1 cell expansion at day 6 after HCT. Compared with IgG treatment, JES6, but not anti–IL-2-S4B6, significantly increased the percentage of IL-10+ Tr1 cells among donor-type CD4+ T cells in the liver and colon of WT recipients (Figure 4A-B). However, JES6 treatment did not increase the percentage of Tr1 cells in PD-L1−/− recipients (Figure 4C). The IL-10+ Tr1 cells were also IFN-γ+ but GM-CSF– (supplemental Figure 12).
Prevention of aGVHD by tolerogenic anti–IL-2 mAb (JES6-1A12) but not non-tolerogenic anti–IL-2 mAb (S4B6) requires PD-L1–dependent expansion of Tr1 cells. (A-E) Lethally irradiated WT or PD-L1−/− BALB/c recipients were given splenocytes (2.5 × 106) and TCD-BM cells (2.5 × 106) from C57BL/6 donors. Recipients were given a total of 3 i.p. injections of rat-IgG or anti–IL-2 mAb (JES6-1A12 or S4B6) (500 µg/mouse) at days 0, 2, and 4 after HCT. Day 6 after HCT, donor-type T cells from liver and colon were analyzed for Tr1 cells. (A-B) Representative staining pattern with percentage and yield of donor IL-10+ Foxp3– CD4+ T cells in liver and colon of WT recipients treated with anti–IL-2 mAb (JES6-1A12 or S4B6) or control IgG; n = 5 per group. (C) Representative staining pattern with percentage and yield of donor IL-10+ Foxp3– CD4+ T cells in liver and colon of PD-L1−/− recipients treated with anti–IL-2 mAb (JES6-1A12) or control IgG; n = 4 per group. (D-E) Blimp-1 and Eomes expression of donor CD4+ T cells in liver and colon of WT recipients treated with anti–IL-2 mAb (JES6-1A12 or S4B6) or control IgG; n = 4 per group. (F) Blimp-1 and Eomes expression of donor CD4+ T cells in liver and colon of PD-L1−/− recipients treated with anti–IL-2 mAb (JES6-1A12) or control IgG; n = 4 per group. (G) Lethally irradiated WT BALB/c recipients were given T cells (1.0 × 106) from WT or IL-10−/− C57BL/6 donors and TCD-BM cells (2.5 × 106) from WT C57BL/6 donors. Recipients were given a total of 4 i.p. injections of anti–IL-2 mAb (JES6-1A12) (500 µg/mouse) at days 0, 2, 4, and 6 after HCT. Plots of percent original body weight, diarrhea, and survival are shown. n = 8 per group. Data represent mean ± standard error combined from 2 replicate experiments. P values were calculated by using two-way analysis of variance (A-F) or log-rank test (G). *P < .05, **P < .01, ***P < .001, ****P < .0001. MFI, mean fluorescence intensity.
Prevention of aGVHD by tolerogenic anti–IL-2 mAb (JES6-1A12) but not non-tolerogenic anti–IL-2 mAb (S4B6) requires PD-L1–dependent expansion of Tr1 cells. (A-E) Lethally irradiated WT or PD-L1−/− BALB/c recipients were given splenocytes (2.5 × 106) and TCD-BM cells (2.5 × 106) from C57BL/6 donors. Recipients were given a total of 3 i.p. injections of rat-IgG or anti–IL-2 mAb (JES6-1A12 or S4B6) (500 µg/mouse) at days 0, 2, and 4 after HCT. Day 6 after HCT, donor-type T cells from liver and colon were analyzed for Tr1 cells. (A-B) Representative staining pattern with percentage and yield of donor IL-10+ Foxp3– CD4+ T cells in liver and colon of WT recipients treated with anti–IL-2 mAb (JES6-1A12 or S4B6) or control IgG; n = 5 per group. (C) Representative staining pattern with percentage and yield of donor IL-10+ Foxp3– CD4+ T cells in liver and colon of PD-L1−/− recipients treated with anti–IL-2 mAb (JES6-1A12) or control IgG; n = 4 per group. (D-E) Blimp-1 and Eomes expression of donor CD4+ T cells in liver and colon of WT recipients treated with anti–IL-2 mAb (JES6-1A12 or S4B6) or control IgG; n = 4 per group. (F) Blimp-1 and Eomes expression of donor CD4+ T cells in liver and colon of PD-L1−/− recipients treated with anti–IL-2 mAb (JES6-1A12) or control IgG; n = 4 per group. (G) Lethally irradiated WT BALB/c recipients were given T cells (1.0 × 106) from WT or IL-10−/− C57BL/6 donors and TCD-BM cells (2.5 × 106) from WT C57BL/6 donors. Recipients were given a total of 4 i.p. injections of anti–IL-2 mAb (JES6-1A12) (500 µg/mouse) at days 0, 2, 4, and 6 after HCT. Plots of percent original body weight, diarrhea, and survival are shown. n = 8 per group. Data represent mean ± standard error combined from 2 replicate experiments. P values were calculated by using two-way analysis of variance (A-F) or log-rank test (G). *P < .05, **P < .01, ***P < .001, ****P < .0001. MFI, mean fluorescence intensity.
Compared with IgG treatment, JES6, but not anti–IL-2-S4B6, upregulated expression of Eomes and Blimp-1 by CD4+ T cells in the liver and colon of WT recipients (Figure 4D-E). JES6 did not upregulate expression of Eomes or Blimp-1 in PD-L1−/− recipients (Figure 4F). Finally, JES6 treatment did not prevent aGVHD induced by IL-10−/− donor T cells (Figure 4G); although IL-10 deficiency in donor T cells did not expand GM-CSF+ Th1 or Tc1 cells (supplemental Figure 13). In addition, JES6 reduced the percentages of granulocytic myeloid-derived suppressor cells in the liver but not in the gut or the percentages of monocytic myeloid-derived suppressor cells in the gut or liver (supplemental Figure 14). Therefore, JES6 treatment augments T-cell expression of Eomes and Blimp-1 and T-cell differentiation into IL-10–producing Tr1 cells in a tissue PD-L1–dependent manner, and IL-10 from Tr1 cells also plays an important role in JES6-mediated prevention of GVHD.
Prevention of GVHD by JES6 requires expression of PD-L1 by GVHD target tissues to inhibit activation of IL-2-Stat-5 and AKT-mTOR pathways in donor T cells
Gene Set Enrichment Analysis at day 6 showed inhibition of the IL-2-Stat5 pathway in the CD4+ T and CD8+ T cells from the spleen and colon of recipients treated with JES6 compared with control IgG (Figure 5A). JES6 but not anti–IL-2-S4B6 decreased the expression of phosphorylated protein kinase (p-AKT) and phosphorylated mammalian target of rapamycin (mTOR) in CD4+ T cells from the colon tissue but not the spleen of WT recipients (Figure 5B-C). JES6 treatment did not change p-AKT or phosphorylated mTOR expression in PD-L1−/− recipients (Figure 5D). Consistent with PD-1–mediated inhibition in WT recipients, AKT activation was higher in donor CD4+ T cells from the colon and spleen of JES6-treated PD-L1−/− recipients than in JES6-treated WT recipients (Figure 5E).
Prevention of GVHD by tolerogenic anti–IL-2 mAb (JES6-1A12) but not non-tolerogenic anti–IL-2 mAb (S4B6) requires expression of PD-L1 by GVHD target tissues to inhibit activation of IL-2-Stat-5 and AKT-mTOR pathways in donor T cells. Lethally irradiated WT and PD-L1−/− BALB/c recipients were given splenocytes (2.5 × 106) and TCD-BM cells (2.5 × 106) from C57BL/6 donors. Recipients were given a total of 3 i.v. injections of rat-IgG or anti–IL-2 mAb (JES6-1A12 or S4B6) (500 µg/mouse) at days 0, 2, and 4 after HCT. At day 6 after HCT, spleen and colon were harvested for analysis. (A) Representative Gene Set Enrichment Analysis plots are shown of IL-2-STAT5 pathway-related gene set expression of CD4+ T and CD8+ T cells in the spleen or colon of the WT recipients treated with anti–IL-2 mAb (JES6-1A12) vs IgG cohorts. P values were calculated by using the bioconductor package “clusterProfiler” version 3.10.1. (B-C) p-AKT and phosphorylated mTOR (pmTOR) expression of donor CD4+ T cells in the spleen (SPL) and colon of WT recipients treated with anti–IL-2 mAb (JES6-1A12 or S4B6) or control IgG; n = 5 per group. (D) p-AKT and pmTOR expression of donor CD4+ T cells in the SPL and colon of PD-L1−/− recipients treated with anti–IL-2 mAb (JES6-1A12) or control IgG; n = 4 per group. (E) p-AKT and pmTOR expression of donor CD4+ T cells in the SPL and colon of WT or PD-L1−/− recipients treated with anti–IL-2 mAb (JES6-1A12); n = 4 to 5 per group. Data represent mean ± standard error combined from two replicate experiments. P values were calculated by unpaired two-tailed Student t tests. *P < .05, **P < .01, ****P < .0001. MFI, mean fluorescence intensity.
Prevention of GVHD by tolerogenic anti–IL-2 mAb (JES6-1A12) but not non-tolerogenic anti–IL-2 mAb (S4B6) requires expression of PD-L1 by GVHD target tissues to inhibit activation of IL-2-Stat-5 and AKT-mTOR pathways in donor T cells. Lethally irradiated WT and PD-L1−/− BALB/c recipients were given splenocytes (2.5 × 106) and TCD-BM cells (2.5 × 106) from C57BL/6 donors. Recipients were given a total of 3 i.v. injections of rat-IgG or anti–IL-2 mAb (JES6-1A12 or S4B6) (500 µg/mouse) at days 0, 2, and 4 after HCT. At day 6 after HCT, spleen and colon were harvested for analysis. (A) Representative Gene Set Enrichment Analysis plots are shown of IL-2-STAT5 pathway-related gene set expression of CD4+ T and CD8+ T cells in the spleen or colon of the WT recipients treated with anti–IL-2 mAb (JES6-1A12) vs IgG cohorts. P values were calculated by using the bioconductor package “clusterProfiler” version 3.10.1. (B-C) p-AKT and phosphorylated mTOR (pmTOR) expression of donor CD4+ T cells in the spleen (SPL) and colon of WT recipients treated with anti–IL-2 mAb (JES6-1A12 or S4B6) or control IgG; n = 5 per group. (D) p-AKT and pmTOR expression of donor CD4+ T cells in the SPL and colon of PD-L1−/− recipients treated with anti–IL-2 mAb (JES6-1A12) or control IgG; n = 4 per group. (E) p-AKT and pmTOR expression of donor CD4+ T cells in the SPL and colon of WT or PD-L1−/− recipients treated with anti–IL-2 mAb (JES6-1A12); n = 4 to 5 per group. Data represent mean ± standard error combined from two replicate experiments. P values were calculated by unpaired two-tailed Student t tests. *P < .05, **P < .01, ****P < .0001. MFI, mean fluorescence intensity.
As shown in Figure 5B, mTOR activation was not inhibited in WT CD4+ cells from the spleen of JES6-treated recipients, most likely reflecting the lack of PD-1 interaction with PD-L1 expressed by host parenchymal tissues, because the ratio of CD45– parenchymal cells to donor T cells was >50-fold lower in the spleen than in the colon (supplemental Figure 15). Similar results were observed with the CD8+ T cells (supplemental Figure 16). Taken together, JES6 treatment inhibits IL-2-Stat5 signaling in donor T cells from both spleen and GVHD target tissues and inhibits AKT-mTOR signaling in donor T cells via PD-1 interaction with the tissue PD-L1 only in GVHD target tissues.
JES6 and TAC induce distinct T-cell subpopulations and T-cell transcriptional signatures
JES6 treatment prevented aGVHD while preserving a strong GVL effect that was markedly better than observed with TAC treatment (Figure 1). Single-cell RNA-sequencing was used to characterize the donor T-cell subsets from the spleen of BCL1/Luc tumor-bearing recipients treated with JES6 or TAC at day 7 after HCT. Donor CD4+ and CD8+ T cells were grouped in 10 distinct clusters (Figure 6A). Clusters 0, 1, 2, 5, and 6 contain only CD8+ T cells, whereas cluster 4 contains only CD4+ T cells. Cluster 3 contains both CD4+ and CD8+ T cells in JES6-treated recipients but only CD4+ T cells in TAC-treated recipients (Figure 6B). The general cluster distribution of splenic T cells was similar in recipients treated with JES6 or TAC, and CD8+ T cells were more heterogeneous than CD4+ T cells.
Tolerogenic anti–IL-2 mAb (JES6-1A12) and TAC induce distinct T-cell subpopulations and T-cell transcriptional signatures. (A) t-SNE plot displaying clusters identified in spleen cells from recipients treated with anti–IL-2 mAb (JES6-1A12) or TAC on day 7 after HCT. (B) Violin plots showing CD3e, CD4, and CD8a distribution on individual clusters. (C) Violin plot showing Tcf7 expression level in individual clusters under anti–IL-2 mAb (JES6-1A12) or TAC treatment. (D) Compared T-cell percentage in individual clusters under anti–IL-2 mAb (JES6-1A12) or TAC treatment.
Tolerogenic anti–IL-2 mAb (JES6-1A12) and TAC induce distinct T-cell subpopulations and T-cell transcriptional signatures. (A) t-SNE plot displaying clusters identified in spleen cells from recipients treated with anti–IL-2 mAb (JES6-1A12) or TAC on day 7 after HCT. (B) Violin plots showing CD3e, CD4, and CD8a distribution on individual clusters. (C) Violin plot showing Tcf7 expression level in individual clusters under anti–IL-2 mAb (JES6-1A12) or TAC treatment. (D) Compared T-cell percentage in individual clusters under anti–IL-2 mAb (JES6-1A12) or TAC treatment.
Gene expression profiles differed between the CD8+ T cell–enriched clusters. Clusters 0 and 6 had high expression of G2/M phase markers, whereas clusters 1 and 2 had high expression of S phase markers (supplemental Figure 17). Cluster 3 had high expression of Il7r, Tcf7 (encoding TCF-1) (Figure 6C), and Bcl-2, with low expression of Gzma, Gzmk, and markers of the cell cycle, suggesting that this cluster is enriched for newly described TCF-1+ Tmp cells with unequivocal self-renewal potential.32 Cluster 5 had high expression of Klrd1, Gzma, Gzmk, Id2, and Itgax, suggesting that Teff cells are enriched in this cluster. Although the gene expression profiles of different clusters in the JES6-treated and TAC-treated groups were similar, the relative cluster sizes differed between the 2 groups. Clusters 0, 1, and 2 containing CD8+ T cells in the S phase were larger in the TAC-treated group than in the anti–IL-2-treated group, whereas clusters 3 and 4 containing TCF-1+ CD4+ and CD8+ self-renewing memory progenitors were larger in the JES6-treated group than in the TAC group (Figure 6C-D). TCF-1+CD8+ T progenitors can further differentiate into cytolytic CD8+ T cells under CD4+ T-cell help. These results suggest that self-renewing CD8+ Tmp cells in lymphoid tissues are better preserved during JES6 treatment compared with TAC treatment.
JES6 preserves CD8+ Tmp and functional effectors that mediate GVL activity in lymphoid tissues more effectively than TAC
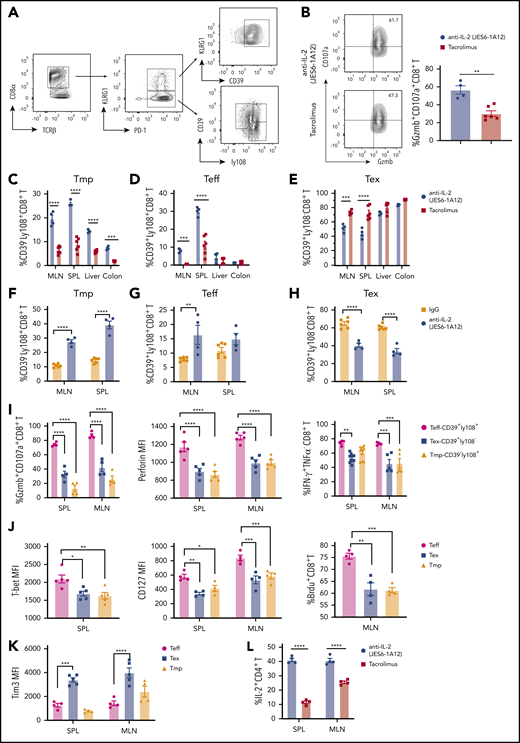
Ly108 can be used as a surrogate to identify TCF-1+CD8+ T progenitors,33 and CD8+ T cells can be divided into KLRG1+PD1+ and KLRG1–PD1+ subsets. The KLRG1+PD-1+ subset are mostly KLRG1+CD39+ terminally differentiated CD8+ T effectors (Ter-Teff). The KLRG1–PD-1+ subset can be further divided into CD39–Ly108+CD8+ Tmp, CD39+Ly108+CD8+ Teff, and CD39+Ly108–CD8+ exhausted T effector cells (Tex). All subsets were observed in the spleen of recipients treated with JES6 (Figure 7A) or TAC (supplemental Figure 18) at day 7 after HCT. The numbers of donor CD8+ cells and Ter-Teff cells in the spleen were lower in JES6-treated recipients than in TAC-treated recipients (supplemental Figure 19A). However, the percentage of cells in the CD107a+/Granzyme B+ subset with stronger cytolytic function among Ter-Teff cells was much higher in JES6-treated recipients than in TAC-treated recipients (Figure 7B).
Tolerogenic anti–IL-2 mAb (JES6-1A12) preserves CD8+ Tmp and functional effectors that mediate GVL activity in lymphoid tissues more effectively than TAC. Lethally irradiated WT BALB/c recipients were given splenocytes (2.5 × 106) and TCD-BM cells (2.5 × 106) from C57BL/6 donors. Recipients were given a total of 4 i.p. injections of anti–IL-2 mAb (JES6-1A12) (500 µg/mouse) at days 0, 2, 4, and 6 after HCT, or once-daily i.p. injections of TAC (0.75 mg/kg) at days 0 to 6 after HCT. On day 7 after HCT, mesenteric lymph node (MLN), spleen (SPL), liver, and colon were harvested for analysis. (A) Representative pattern of gating strategy in recipients given anti–IL-2 treatment. (B) Representative flow cytometry pattern showing the expression of granzyme B and CD107a on CD8+ T cells and percentage of CD107a+granzymeB+ CD8+ T cells in the SPL from IL-2 mAb– or TAC-treated recipients are shown; n = 4 to 6, combined from 2 experiments. (C-H) Percentage of Tmp (CD39–Ly108+), Teff (CD39+Ly108+), and Tex (CD39+Ly108–) among donor CD8+ T cells in MLN, SPL, liver, and colon of WT recipients treated with anti–IL-2 mAb (JES6-1A12), TAC, or control IgG; n = 4-6 per group. (I) Percentage of granzymeB+CD107a+ CD8+T, IFN-γ+ CD8+T cells and mean fluorescence intensity (MFI) of perforin among Teff, Tex, and Tmp in SPL and MLN of WT recipients treated with anti–IL-2 mAb (JES6-1A12). n = 4-6 per group. (J-K) Expression of T-bet, CD127, 5-bromo-2′-deoxyuridine (BrdU), and Tim3 on Teff, Tex, and Tmp in SPL and MLN of WT recipients treated with anti–IL-2 mAb (JES6-1A12). n = 4 to 6 per group. (L) Percentage of IL-2+ CD4+ T cells in SPL and MLN of WT recipients treated with anti–IL-2 mAb (JES6-1A12) or TAC. n = 4 per group. P values were calculated by unpaired two-tailed Student t tests, one-way analysis of variance, or two-way analysis of variance. *P < .05, **P < .01, ***P < .001, ****P < .0001.
Tolerogenic anti–IL-2 mAb (JES6-1A12) preserves CD8+ Tmp and functional effectors that mediate GVL activity in lymphoid tissues more effectively than TAC. Lethally irradiated WT BALB/c recipients were given splenocytes (2.5 × 106) and TCD-BM cells (2.5 × 106) from C57BL/6 donors. Recipients were given a total of 4 i.p. injections of anti–IL-2 mAb (JES6-1A12) (500 µg/mouse) at days 0, 2, 4, and 6 after HCT, or once-daily i.p. injections of TAC (0.75 mg/kg) at days 0 to 6 after HCT. On day 7 after HCT, mesenteric lymph node (MLN), spleen (SPL), liver, and colon were harvested for analysis. (A) Representative pattern of gating strategy in recipients given anti–IL-2 treatment. (B) Representative flow cytometry pattern showing the expression of granzyme B and CD107a on CD8+ T cells and percentage of CD107a+granzymeB+ CD8+ T cells in the SPL from IL-2 mAb– or TAC-treated recipients are shown; n = 4 to 6, combined from 2 experiments. (C-H) Percentage of Tmp (CD39–Ly108+), Teff (CD39+Ly108+), and Tex (CD39+Ly108–) among donor CD8+ T cells in MLN, SPL, liver, and colon of WT recipients treated with anti–IL-2 mAb (JES6-1A12), TAC, or control IgG; n = 4-6 per group. (I) Percentage of granzymeB+CD107a+ CD8+T, IFN-γ+ CD8+T cells and mean fluorescence intensity (MFI) of perforin among Teff, Tex, and Tmp in SPL and MLN of WT recipients treated with anti–IL-2 mAb (JES6-1A12). n = 4-6 per group. (J-K) Expression of T-bet, CD127, 5-bromo-2′-deoxyuridine (BrdU), and Tim3 on Teff, Tex, and Tmp in SPL and MLN of WT recipients treated with anti–IL-2 mAb (JES6-1A12). n = 4 to 6 per group. (L) Percentage of IL-2+ CD4+ T cells in SPL and MLN of WT recipients treated with anti–IL-2 mAb (JES6-1A12) or TAC. n = 4 per group. P values were calculated by unpaired two-tailed Student t tests, one-way analysis of variance, or two-way analysis of variance. *P < .05, **P < .01, ***P < .001, ****P < .0001.
Consistent with single-cell RNA-sequencing analysis, the percentage of Tmp cells within the CD8+ T-cell population was higher in the lymph node, spleen, liver, and colon of JES6-treated recipients at day 7 after HCT than in TAC-treated recipients (Figure 7C; supplemental Figure 19B). The percentage of Teff cells within the CD8+ population was higher in the lymph node and spleen of JES6-treated recipients than in TAC-treated recipients, with no differences in the liver or colon between the 2 groups (Figure 7D). The percentage of Tex cells within the CD8+ population in the lymph node and spleen of JES6-treated recipients was significantly lower than in TAC-treated recipients, with no difference in the liver or colon between the 2 groups (Figure 7E).
Compared with anti-IgG treatment, JES6 treatment increased the percentages of Tmp and Teff cells within CD8+ cells in lymphoid tissues at day 7, whereas the percentage of Tex cells was lower in JES6-treated recipients than in IgG-treated controls (Figure 7F-H). Compared with Teff cells, Tex cells and Tmp cells had lower expression of granzyme B, CD107a, perforin, IFN-γ, T-bet, CD127, and lower 5-bromo-2′-deoxyuridine labeling (Figure 7I-J). Tim3 expression was higher in Tex than in Teff and Tmp (Figure 7K). In addition, the percentage of IL-2–producing CD4+ T cells in spleen and lymph node was higher at day 7 after HCT in recipients treated with JES6 than in those treated with TAC (Figure 7L). Taken together, these results indicate that JES6 treatment augments donor CD8+ T differentiation into Tmp cells and their Teff progeny, and that JES6 treatment is more effective than TAC treatment in preserving the function of donor CD8+ T cells in recipient lymphoid tissues.
Discussion
Immunosuppressive medications such as TAC are routinely used to prevent GVHD in patients after allo-HCT, but they can also inhibit GVL activity.34,35 Here, we have shown that administration of JES6, but not anti–IL-2-S4B6, early after HCT enables GVHD target tissue PD-L1 to effectively tolerize infiltrating T cells, leading to effective prevention of aGVHD, while preserving strong GVL activity that is much more effective than TAC under conditions in which anti–IL-2 and TAC have similar effects on GVHD.
Prevention of aGVHD by administration of JES6 depends on expression of PD-L1 in GVHD target tissues, because the protective effect disappeared in PD-L1−/− recipients. Trotta et al36 showed that an mAb against human IL-2 (F5111.2) similar to JES6 expanded human Foxp+CD4+ Treg cells in ameliorating GVHD in a xenogeneic model. In our model, JES6 had only a small effect on expansion of FoxP3+CD4+ Treg cells, and the percentages of Foxp3+ Treg cells remained <3% in the liver and colon tissues. Instead, we observed downregulation of IL-2-Stat5 signaling by JES6 in donor CD4+ and CD8+ T cells, consistent with predominant effects on Tcon cells. In the absence of IL-2 signaling, the interaction of PD-1 expressed by Tcon cells with PD-L1 expressed in GVHD target tissues depleted GM-CSF+ Th/Tc1 cells, induced T-cell anergy/exhaustion, and augmented differentiation of Tcon cells into IL-10–producing Tr1 cells.
The mechanisms whereby JES6 treatment preserves GVL activity while preventing GVHD involve multiple steps as depicted in the visual abstract. First, JES6 treatment inhibits activation of the IL-2-Stat5 signaling pathway in donor T cells and reduces their production of GM-CSF. Second, due to relative lack of PD-L1 expression in recipient lymphoid tissues, the PD-1+TCF-1+Ly108+CD4+ and CD8+ Tmp cells are well preserved. Moreover, CD4+ T cells help the CD8+ Tmp differentiate into CD39+Ly108+ functional Teff cells to mediate persistent GVL activity. Third, in GVHD target tissues, donor T-cell PD-1 interacts with host tissue PD-L1, leading to downregulated activation of the AKT-mTOR pathway and upregulated activation of anergy/exhaustion–related nuclear factors, including Eomes and Blimp-1. Consequently, many donor T cells in GVHD target tissues become anergic, exhausted, or apoptotic, with depletion of GM-CSF+ T cells. At the same time, some T cells differentiate into Foxp3–IL-10–producing Tr1 cells that further suppress pathogenic T-cell function in GVHD target tissues.
JES6 treatment would be expected to affect both naive and memory T cells in the graft. Conventional memory T cells express IL-2Rβ, and naive T cells upregulate IL-2Rβ expression after activation.37 Memory T cells in the graft have reduced GVHD capacity with preserved GVL activity in mice38-40 and in patients.41 We expect that administration of anti–IL-2 mAb could prevent GVHD while preserving GVL effects mediated by both naive and memory T cells in the graft.
These observations provide new insights into how to separate GVHD from GVL activity mediated by the same alloreactive donor T-cell population. JES6 treatment reduced IL-2-Stat5 activation independent of host tissue PD-L1, but the treatment upregulated T-cell expression of PD-1 and reduced activation of AKT-mTOR pathways in the T cells in GVHD target tissues in the host tissue PD-L1–dependent manner. Therefore, simultaneous blocking of IL-2 signaling and augmentation of PD-1 signaling inhibits the AKT-mTOR pathway in the T cells in GVHD target tissues, leading to prevention of GVHD. Lack of PD-1 interaction with host tissue PD-L1 in lymphoid tissues allows alloreactive T-cell survival, leading to stronger GVL activity.
We observed that PD-L1/PD-1 interaction augments differentiation and expansion of Foxp3–IL-10–producing Tr1 cells. IL-10+ Tr1 cells represent the major regulatory T-cell population in allo-HCT recipients. Moreover, Eomes is required for donor T-cell differentiation into FoxP3–IL-10+ Tr1 cells, and Blimp-1 augments expansion of Tr1 cells.17 We observed that JES6 treatment upregulated donor CD4+ T expression of Eomes in both WT and PD-L1−/− recipients but upregulated expression of Blimp-1 only in WT but not in PD-L1−/− recipients. These findings suggest that reduction of AKT-mTOR activation by blocking IL-2 signaling alone is sufficient to upregulate Eomes in the absence of PD-1 signaling; however, simultaneous reduction of AKT-mTOR activation by blocking IL-2 signaling and inhibition of AKT-mTOR activation by PD-1 signaling triggered by tissue PD-L1 is required to upregulate expression of Blimp-1. Therefore, JES6 enables tissue PD-L1 to mediate differentiation and expansion of Tr1 cells in GVHD target tissues.
JES6 treatment exploits differences in expression of PD-L1 by recipient GVHD target and lymphoid tissues that affect the ability of PD1+TCF-1+Ly108+CD8+ Tmp cells to cause GVHD and mediate GVL activity. Blockade of PD-1 interaction with PD-L1 can revive the function of TCF-1+ Tmp and Teff cells.33 The paucity of PD-L1–expressing cells in recipient lymphoid tissues preserves donor-type PD-1+TCF-1+Ly108+CD8+ Tmp and their derivatives locally, where they mediate GVL activity. In contrast, the abundance of PD-L1–expressing cells in the GVHD target tissues such as colon and liver tolerizes donor-type PD-1+TCF-1+Ly108+ Tmp and their derivative Teff cells locally, thereby preventing GVHD. Thus, JES6 treatment allows donor-type PD-1+TCF-1+Ly108+CD8+ Tmp cells to mediate GVL activity in the lympho-hematopoietic compartment without causing aGVHD in parenchymal tissues, even though CD8+ Tmp cells can mediate persistence of GVHD.24
In summary, an anti–IL-2 mAb that forms complexes with IL-2 and blocks IL-2 binding to IL-2Rβ and IL-2Rγ in Tcon cells prevents GVHD while preserving strong GVL activity in mice. Whether a similar mAb against human IL-2 such as F5111.2 also augments induction of T-cell tolerance by PD-1 interaction with tissue PD-L1 remains to be determined. Confirmation of this hypothesis would support clinical trials to determine whether this antibody could prevent GVHD without impairing GVL activity.
The data reported in this article have been deposited in the Gene Expression Omnibus database (accession number GSE149138).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Lieping Chen at Yale University School of Medicine for providing PD-L1−/− BALB/c breeders. They also thank Xiwei Wu and his staff at the COH Integrative Genomics Core, Lucy Brown and her staff at the COH Flow Cytometry Facility, Peiguo Chu and his staff at COH Solid Tumor Core, and Richard Ermel and his staff at COH Animal Research Center for providing excellent service.
This work was supported by National Institutes of Health grants R01 CA228465 (National Cancer Institute) and R01 AI066008 (National Institute of Allergy and Infectious Diseases) (D.Z.).
Authorship
Contribution: Q.S. designed and performed research, as well as prepared the manuscript; X. Wang and Y.L. performed experiments; X. Wu and H.Q. performed RNA and single-cell RNA-sequencing and analyzed data; A.D.R. provide advice and additional financial support for the project, as well as critical review of the manuscript; P.J.M. provided advice on experimental design and critically reviewed and edited the manuscript; Y.-Z.C. is the PhD advisor of Q.S.; and D.Z. designed and supervised the research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Defu Zeng, Beckman Research Institute of City of Hope, 1500 East Duarte Rd, Duarte, CA 91010; e-mail: dzeng@coh.org; and Yuan-Zhong Chen, Fujian Medical University Union Hospital, 29 Xinquan Rd, Fuzhou, Fujian 350001, China; e-mail: chenyz@fjmu.edu.cn.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal