TO THE EDITOR:
B-cell precursor (BCP) acute lymphoblastic leukemia (BCP-ALL) in infants (ie, children age <1 year) is a rare disease traditionally subdivided into MLL-rearranged (alias KMT2A; MLL-R) and MLL germ line (MLL-G) subtypes. MLL gene rearrangements occur in ∼75% of infants with BCP-ALL1-3 and are typically associated with a mixed-lineage phenotype.4 MLL rearrangements originate prenatally in utero5 and play a role in transcription factor dysregulation.6 MLL-R BCP-ALL in infants is associated with dismal outcomes.1-3
Infants with MLL-G ALL treated with intensive therapies in the Interfant-06 protocol may have a relatively favorable prognosis, with a 6-year event-free survival (EFS) rate of 73.9%,2 slightly inferior to that observed in older children with BCP-ALL.1,2 Of interest, a recent study from the Japanese Pediatric Leukemia/Lymphoma Study Group MLL-10 trial reported more favorable outcomes in infants with MLL-G ALL, with a 3-year EFS rate of 93.3%.7 However, it must be noted that in the Japanese study, the cohort of patients was relatively small (15 patients with MLL-G ALL were enrolled, compared with 167 patients in the Interfant-06 Consortium, which includes many national groups from different countries with different outcomes), and the chemotherapy treatment was overall more intensive, with additional high-dose cytarabine and L-asparaginase in early consolidation. Despite the great interest and efforts in clarifying the biology of MLL-G BCP-ALL in infants, the information available on genetic alterations is still rather limited.8 Conversely, BCP-ALL in noninfants is MLL-G in 98% of the cases, and it is characterized by a great diversity of chromosomal aberrations and gene rearrangements, which correlate with favorable or unfavorable early treatment response and prognosis and are currently used, in association with minimal residual disease (MRD) assessment, for treatment stratification.9-12 The identification of specific genetic abnormalities in MLL-G BCP-ALL in infants might be crucial for selecting appropriate personalized treatments and improving outcomes.13 Recently, the NUTM1 gene was found to be rearranged in MLL-G BCP-ALL in pediatric, as well as infant cases, and the presence of NUTM1 fusions was associated with favorable outcomes in both settings.14,15 Herein, we report results on the incidence and associated outcomes of fusion genes in a cohort of 30 infants with MLL-G BCP-ALL.
We retrospectively analyzed 30 of 37 consecutive infant patients with MLL-G BCP-ALL treated in centers of the Italian Association of Pediatric Hemato-Oncology from 2006 to 2019 within the Interfant-06 (n = 29)2 or Italian Association of Pediatric Hemato-Oncology/Berlin-Frankfurt-Münster ALL2017 (n = 1) protocols for whom biological material was available. The study was approved by the ethics committees of the participating institutions, and written consent was obtained from parents. Split-signal fluorescence in situ hybridization analysis was mandatory to assess MLL rearrangements; multiplex reverse transcription polymerase chain reaction and genomic breakpoint cloning were performed to identify MLL fusion partners.16 RNA samples were analyzed using a custom next-generation sequencing (NGS) panel, with probes capturing 95 leukemia-related genes, including ABL1, JAK2, PAX5, EBF1, PDGFRB, CRLF2, and TCF3 (Nugen, Tecan, CA). Sequencing analysis was performed in 2 × 150 paired ends on NextSeq550 (Illumina, San Diego, CA). Fusion genes were identified by STAR-Fusion, Dragen RNA, and an in-house bioinformatic pipeline.17 In a subset of cases, whole-transcriptome RNA sequencing analysis was also performed (Nugen). All identified fusions were validated by reverse transcription polymerase chain reaction and Sanger sequencing (supplemental Figure 1; supplemental Tables 1-2, available on the Blood Web site). EFS was defined as time from diagnosis to first event (ie, resistance, relapse, death resulting from any cause, or second malignancy, whichever occurred first). Observation periods were censored at time of last contact when no events were reported. EFS was estimated according to the Kaplan-Meier method (with Greenwood standard error). Median follow-up was 4.0 years (range, 0.4-10.2 years). Analyses were performed using SAS software (version 9.4).
Overall, 30 infant patients with MLL-G BCP-ALL were screened. Table 1 describes their main features: 24 patients were aged >6 months, 29 had white blood cell count <300 × 109/L at presentation, and two thirds were female. In contrast to MLL-R cases, which are typically CD10−, a CD10+ immunophenotype was observed in 28 (93%) of 30 MLL-G cases. Supplemental Table 3 reports the details of cytogenetic data. In addition, 26 of 30 cases were prednisone good responders, and all achieved complete remission at the end of induction therapy. Of 21 patients with available data, MRD at the end of induction therapy was negative in 7 (33%), low (<5 × 10−4) in 11 (52%), and high (≥5 × 10−4) only in 3 cases (14%). Only 1 (5%) of 19 patients had high MRD (≥5 × 10−4) at the end of consolidation phase 1B. These data confirm that MLL-G BCP-ALL in infants is associated with favorable clinical features at diagnosis (ie, age >6 months and low white blood cell count) and favorable initial response to therapy (ie, prednisone good response and low MRD level).2
Clinical and biological features of patients
| . | Fusion class, no. of patients . | ||||
|---|---|---|---|---|---|
| Negative . | NUTM1 class . | PAX5 class . | Other . | Total . | |
| Sex | |||||
| Male | 4 | 2 | 2 | 1 | 9 |
| Female | 4 | 7 | 4 | 6 | 21 |
| Age at diagnosis, mo | |||||
| 0 to <3 | 1 | 3 | 0 | 0 | 4 |
| 3 to <6 | 0 | 1 | 0 | 1 | 2 |
| 6 to <9 | 0 | 2 | 1 | 2 | 5 |
| 9 to <12 | 7 | 3 | 5 | 4 | 19 |
| WBC count, ×109/L | |||||
| ≤100 | 8 | 6 | 5 | 4 | 23 |
| 100-300 | 0 | 2 | 1 | 3 | 6 |
| ≥300 | 0 | 1 | 0 | 0 | 1 |
| Immunophenotype | |||||
| CD10− | 0 | 1 | 0 | 1 | 2 |
| CD10+ | 8 | 8 | 6 | 6 | 28 |
| Prednisone response | |||||
| PGR | 6 | 8 | 5 | 7 | 26 |
| PPR | 1 | 1 | 1 | 0 | 3 |
| NK | 1 | 0 | 0 | 0 | 1 |
| MRD at EOI, ×10−4* | |||||
| Negative | 4 (0) | 2 (0) | 0 | 1 (0) | 7 (0) |
| <5 | 0 | 5 (0) | 2 (1) | 4 (2) | 11 (3) |
| ≥5 | 0 | 0 | 2 (2) | 1 (1) | 3 (3) |
| Total | 8 | 9 | 6 | 7 | 30 |
| . | Fusion class, no. of patients . | ||||
|---|---|---|---|---|---|
| Negative . | NUTM1 class . | PAX5 class . | Other . | Total . | |
| Sex | |||||
| Male | 4 | 2 | 2 | 1 | 9 |
| Female | 4 | 7 | 4 | 6 | 21 |
| Age at diagnosis, mo | |||||
| 0 to <3 | 1 | 3 | 0 | 0 | 4 |
| 3 to <6 | 0 | 1 | 0 | 1 | 2 |
| 6 to <9 | 0 | 2 | 1 | 2 | 5 |
| 9 to <12 | 7 | 3 | 5 | 4 | 19 |
| WBC count, ×109/L | |||||
| ≤100 | 8 | 6 | 5 | 4 | 23 |
| 100-300 | 0 | 2 | 1 | 3 | 6 |
| ≥300 | 0 | 1 | 0 | 0 | 1 |
| Immunophenotype | |||||
| CD10− | 0 | 1 | 0 | 1 | 2 |
| CD10+ | 8 | 8 | 6 | 6 | 28 |
| Prednisone response | |||||
| PGR | 6 | 8 | 5 | 7 | 26 |
| PPR | 1 | 1 | 1 | 0 | 3 |
| NK | 1 | 0 | 0 | 0 | 1 |
| MRD at EOI, ×10−4* | |||||
| Negative | 4 (0) | 2 (0) | 0 | 1 (0) | 7 (0) |
| <5 | 0 | 5 (0) | 2 (1) | 4 (2) | 11 (3) |
| ≥5 | 0 | 0 | 2 (2) | 1 (1) | 3 (3) |
| Total | 8 | 9 | 6 | 7 | 30 |
EOI, end of induction therapy; NK, not known; PGR, prednisone good responder; PPR, prednisone poor responder; WBC, white blood cell.
Values in parentheses indicate no. of relapses. MRD data were available for 21 patients.
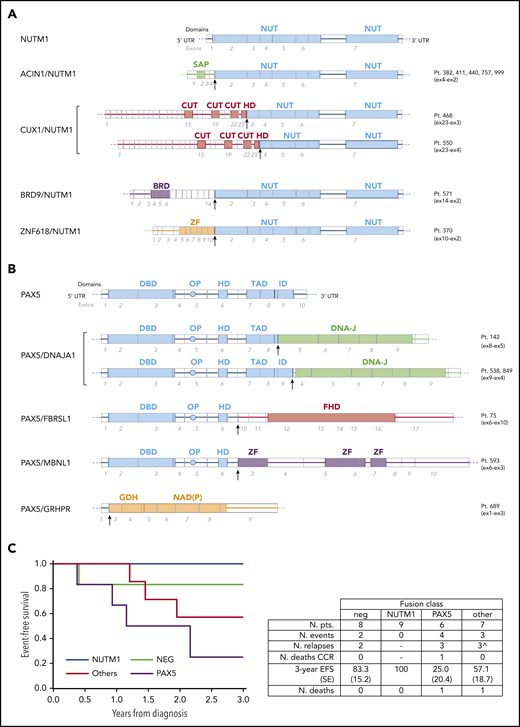
Strikingly, the NGS analysis revealed that MLL-G BCP-ALL in infants is characterized by a high rate of fusion genes. Indeed, among these 30 patients, as many as 22 (73%) carried a fusion gene. NUTM1 fusions were the most frequent, identified in 9 cases (30%), with ACIN1 (n = 5), CUX1 (n = 2), BRD9 (n = 1), and ZNF618 (n = 1) as fusion partners (Figure 1A). NUTM1 class patients (mean age at diagnosis, 8.8 months) had a 3-year EFS rate of 100% (Figure 1C). This finding suggests that NUTM1 fusions are associated with very good outcomes, in agreement with recent data from an ongoing International collaborative 'Ponte di Legno' Childhood ALL Working Group project.15 Remarkably, we found that rearrangement of the PAX5 gene was also recurrent, detected in 6 (20%) of 30 cases, with DNAJA1 (n = 3), FBRSL1 (n = 1), MBNL1 (n = 1), and GRHPR (n = 1) as fusion partner genes (Figure 1B). To our knowledge, these PAX5 fusion partners have not been reported in the literature or databases as common PAX5 partners in older patients.18 As in older children, the PAX5 fusion genes found in most of the infant patients retained the DNA binding domain and the homeodomain, with PAX5/GRHPR as the only exception, where the first exon of PAX5 is fused with almost the entire structure of GRHPR. Whether the disruption of PAX5 or the expression of GRPHR under the PAX5 promoter might represent the driver event for leukemogenesis must be further explored. In contrast with NUTM1 class patients, patients carrying PAX5 fusions had a mean age at diagnosis of 11.4 months and had poorer outcomes, with 4 events (bone marrow relapse, n = 3; death in first remission, n = 1) occurring in 6 patients, leading to a 3-year EFS rate of 25.0% (±20.4%; Figure 1C). Notably, the outcomes of PAX5 patients did not differ from those of 65 concurrent MLL-R BCP-ALL patients, whose 3-year EFS rate was 43.6% (±6.5%; supplemental Figure 2). Moreover, in 7 cases (23%), other fusion transcripts were detected, including TCF3 (with different partner genes) and ABL class fusions. We identified the following: TCF3/PBX1 (n = 2), TCF3/ZNF384 (n = 1), ETV6/ABL1 (n = 1), P2RY8/CRLF2 (n = 1), and a new KDM2B/GATAD2B fusion in a pair of monozygotic twins. None of the cases was positive for the fusions more commonly found in patients age >1 year (ie, ETV6/RUNX1 or BCR/ABL1). The 3-year EFS rate in this heterogeneous group was 57.1% (±18.7%; Figure 1C). Finally, no fusion genes were detectable in the remaining 8 cases (27%), neither by the RNA-targeted NGS approach nor by whole-transcriptome RNA sequencing screening. Interestingly, 3 of 4 patients for whom we had cytogenetic data were hyperdiploid. These negative cases had a 3-year EFS rate of 83.3% (±15.2%; Figure 1C), with 1 testis relapse occurring 3.1 years after diagnosis in this subgroup.
Genomics and outcome of infant patients with MLL-G BCP-ALL. Schematic representation of NUTM1 (A) and PAX5 (B) fusion genes identified in infant patients with MLL-G B-ALL compared with the respective wild-type genes. For each gene, exons and relevant protein domains are indicated at the bottom and top, respectively. Arrows indicate the fusion breakpoints. (C) EFS according to the class of fusion transcript detected in 30 infant patients with MLL-G BCP-ALL. The negative (NEG; no fusion gene detected) curve does not depict 1 relapse (testis) that occurred at 3.1 years. Table reports the details of patients and events. ^Relapses occurred in: TCF3 class (n = 1; died after relapse), ABL class (n = 1), and CRLF2 class (n = 1). BRD, bromodomain; CUT, CUT DNA binding motif; DBD, DNA binding domain; DNA-J, DNA-J peptide binding; FHD, fibrosin homology domain; GDH, glycerate dehydrogenase catalytic domain; HD, homeodomain; ID, inhibitory domain; NAD(P), NAD(P) binding domain; NUT, NUT domain; NUTM1, NUTM1 class fusions; other, other fusions; PAX5, PAX5 fusions; OP, octapepdtie domain; SAP, SAP motif; TAD, transactivation domain; ZF, zinc finger.
Genomics and outcome of infant patients with MLL-G BCP-ALL. Schematic representation of NUTM1 (A) and PAX5 (B) fusion genes identified in infant patients with MLL-G B-ALL compared with the respective wild-type genes. For each gene, exons and relevant protein domains are indicated at the bottom and top, respectively. Arrows indicate the fusion breakpoints. (C) EFS according to the class of fusion transcript detected in 30 infant patients with MLL-G BCP-ALL. The negative (NEG; no fusion gene detected) curve does not depict 1 relapse (testis) that occurred at 3.1 years. Table reports the details of patients and events. ^Relapses occurred in: TCF3 class (n = 1; died after relapse), ABL class (n = 1), and CRLF2 class (n = 1). BRD, bromodomain; CUT, CUT DNA binding motif; DBD, DNA binding domain; DNA-J, DNA-J peptide binding; FHD, fibrosin homology domain; GDH, glycerate dehydrogenase catalytic domain; HD, homeodomain; ID, inhibitory domain; NAD(P), NAD(P) binding domain; NUT, NUT domain; NUTM1, NUTM1 class fusions; other, other fusions; PAX5, PAX5 fusions; OP, octapepdtie domain; SAP, SAP motif; TAD, transactivation domain; ZF, zinc finger.
Overall, this study shows that MLL-G BCP-ALL in infants is characterized by remarkable genetic heterogeneity. An unexpectedly high rate of fusion genes was found, associated with distinct treatment responses and outcomes. This study shows for the first time that PAX5 fusions are recurrent in MLL-G BCP-ALL in infants and associated with worse outcomes compared with NUTM1 class fusions. The major limitation in this study is the small size of the patient cohort and subgroups. Because of the rarity of the disease, confirmation of these findings can only be pursued through large international collaborations. If confirmed, the detection of PAX5 and NUTM1 class (and potentially other) fusions by either fluorescence in situ hybridization and/or NGS targeted strategies17 might be applied prospectively to stratify more properly infant patients with ALL in the context of a dedicated clinical protocol. The dismal prognosis observed in infant ALL with PAX5 fusions is of particular interest, because these patients may benefit from novel therapeutic approaches, such as the kinase inhibitor nintedanib. This compound, also known as BIBF1120, Vargatef, or Ofev (Boehringer Ingelheim, Ingelheim am Rhein, Germany), has antiangiogenic and antitumoral effects in several cancers19,20 and is already used in patients with pulmonary fibrosis.21 Of note, we previously demonstrated the in vitro and ex vivo antileukemic activity of nintedanib in PAX5+ ALL cells from pediatric patients (age >1 year at diagnosis), both alone and in combination with standard chemotherapy.22,23 Other innovative therapies (eg, immunotherapy) may be considered in infants with MLL-G BCP-ALL not associated with NUTM1 fusions, because prognosis in these patients seems unfavorable. In summary, if confirmed in a larger cohort, these data would support the redefinition of the infant MLL-G BCP-ALL subgroup, where specific genetic features might have prominent biological and prognostic roles, with a rationale for risk stratification and potential benefit from genetically driven or other innovative treatments.
Genomic data have been uploaded to ArrayExpress: targeted allele sequencing of BCP-ALL pediatric patients (E-MTAB-9971) and whole-transcriptome analysis to detect fusion genes in pediatric BCP-ALL (E-MTAB-9925).
Original data are available upon request; please contact gianni.cazzaniga@hsgerardo.org.
The online version of this article contains a data supplement.
Acknowledgments
This work was partially supported by the Comitato Maria Letizia Verga, grant IG 2017-20564 from the Italian Association for Cancer Research (AIRC; A.B.), grant PRIN 2017-N. 20178S4EK9 from the Ministero dell’Università e della Ricerca (M.G.V.), an AIRC fellowship (A.G.), the Italian Ministry of Health, and grant GR-2016-02364753 from the Ricerca Finalizzata-GR (C.P., G.F., M.B.).
Authorship
Contribution: G.F., M.B., S.P., C.P., and M.Q. performed NGS and molecular analyses; G.C. initiated the project and supervised the team’s work; A.G. performed bioinformatic analyses; P.D.L. and M.G.V. collected clinical data and performed survival analyses; M.Q. performed reverse transcription polymerase chain reaction validation tests; L.P. analyzed the twin patients; L.C.A. performed MRD monitoring; B.B. performed immunophenotypic analyses; R.P., E.B., M.Z., C.F., F.L., L.V., C.R., and A.B. provided information on patients treated in their centers; and G.F., M.B., V.C., P.D.L., and G.C. analyzed data and wrote the paper, with contributions from all authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Giovanni Cazzaniga, Centro Ricerca Tettamanti/University of Milano-Bicocca, Centro Maria Letizia Verga, via Cadore snc, 20900 Monza (MB) Italy; e-mail: giovanni.cazzaniga@unimib.it.
REFERENCES
Author notes
G.F. and M.B. contributed equally to this work.
G.C. and A.B. contributed equally as senior authors.