In this issue of Blood, Yuan et al identified TMEM163 as a key regulator of platelet zinc homeostasis, playing a critical role in dense granule biogenesis in megakaryocytes.1
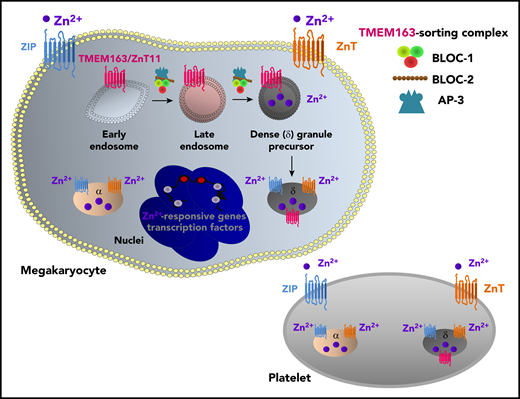
Zn2+ homeostasis in megakaryocytes and platelets.Several Zn2+-responsive genes and transcription factors are expressed in megakaryocytes and involved in diverse processes, including granule biogenesis and platelet production. ZIP/ZnT transporters in megakaryocytes and platelets regulate Zn2+ influx/efflux and Zn2+ content in secretory granules, thereby facilitating Zn2+-dependent metabolic and signaling pathways. Granular-resident Zn2+ in platelets contributes to the activation of the coagulation cascade. The newly identified platelet Zn2+ transporter TMEM163 (also called ZnT11) is located in the δ-granule membrane and regulates the biogenesis of δ-granules in megakaryocytes. BLOC-1, BLOC-2, and AP-3 are involved in this process and transport TMEM163 from early endosomes to perinuclear δ-granules and late-endosome–positive compartments.
Zn2+ homeostasis in megakaryocytes and platelets.Several Zn2+-responsive genes and transcription factors are expressed in megakaryocytes and involved in diverse processes, including granule biogenesis and platelet production. ZIP/ZnT transporters in megakaryocytes and platelets regulate Zn2+ influx/efflux and Zn2+ content in secretory granules, thereby facilitating Zn2+-dependent metabolic and signaling pathways. Granular-resident Zn2+ in platelets contributes to the activation of the coagulation cascade. The newly identified platelet Zn2+ transporter TMEM163 (also called ZnT11) is located in the δ-granule membrane and regulates the biogenesis of δ-granules in megakaryocytes. BLOC-1, BLOC-2, and AP-3 are involved in this process and transport TMEM163 from early endosomes to perinuclear δ-granules and late-endosome–positive compartments.
Hermansky-Pudlak syndrome (HPS) is an inherited disease that is caused by mutations in genes that have an essential role in the assembly of cellular organelles, platelet dense (δ-delta) granules, melanosomes, lysosomes, lung lamellar bodies and cytotoxic T-cell lymphocyte granules.2 The abnormalities associated with HPS include hypopigmentation, visual deficiencies, prolonged bleeding times, and ceroid deposition in the lung and other organs. Adaptor protein-3 (AP-3) and biogenesis of lysosome-related organelles complex (BLOC) were identified as key players in the synthesis of lysosomal-related organelles. Platelet δ-granules are lysosomal-related organelles that are derived from endosomes and multivesicular bodies in megakaryocytes. Similar to their action in melanosomes, AP-3– and BLOC-dependent pathways play an important role in platelet δ-granule biogenesis.2 However, the exact molecular mechanisms involved in this process have not been determined in HPS and other platelet storage pool diseases.
Recently, transmembrane protein 163 (TMEM163) was identified as a new member of the zinc (Zn2+) transporter Slc30 family with a protein structure similar to that of ZnT3 and ZnT9.3 TMEM163 regulates Zn2+ transport in mammalian cells,3 particularly in cellular organelles, such as lysosomes, endosomes, and synaptic vesicles.4,5 Transient receptor potential mucolipin 1 interacts with TMEM163, supporting translocation of TMEM163 from the plasma membrane to the lysosome, building a functional unit to regulate Zn2+ and proton (H+) transport. TMEM163 also regulates the protein expression and activity of the P2X purinergic receptors, indicating that TMEM163 has a diverse role in Zn2+-dependent signaling and gene regulation.5,6
Yuan et al showed that, in a megakaryocytic cell line, TMEM163 is selectively localized in precursors and maturated δ-granules, but not in α-granules. TMEM163 levels were significantly decreased in platelets isolated from mouse models and human patients lacking HPS-associated proteins (BLOC-1, BLOC-2, and AP-3). TMEM163-deficient platelets lack δ-granules, and Zn2+ abnormally accumulated in TMEM163-deficient megakaryocytic cells and platelets, indicating a crucial role for TMEM163-mediated Zn2+ transport in platelet δ-granule biogenesis. Furthermore, TMEM163 interacted with BLOC-1. Therefore, it was postulated that this interplay may regulate the trafficking of TMEM163 from early endosomes to maturated δ-granules (see figure). Consistent with the lack of δ-granules, TMEM163-deficient mice had reduced secretion of δ-granule–specific components, such as adenosine triphosphate and serotonin. The reduction in these second-wave mediators and consequent amplification of platelet reactivity through purinergic and serotonin receptors resulted in impaired α-granule secretion and platelet-aggregation responses. These platelet abnormalities influenced hemostasis, as shown by prolonged bleeding times in TMEM163-deficient mice. Previous studies have shown similar pathological symptoms using mouse or human platelets with storage pool deficiency; conversely, extracellular Zn2+ supplementation could rescue many hemostatic defects.7 Altogether, these results highlighted TMEM163 as an important Zn2+ transporter, contributing its function to platelet pathology of HPS and storage pool disease with δ-granule abnormalities.
Interestingly, TMEM163-deficient mice displayed normal platelet count and size with normal α-granule content, indicating that TMEM163 is not essential for platelet production or α-granule biogenesis. Although abnormal accumulation of Zn2+ was detected in TMEM163-deficient platelets, this did not influence the gene expression of other ZIP/ZnT transporters. Combined deficiency of Rab32/Rab38 small guanosine triphosphatases in mice results in HPS with profound defects in hemostasis.8 Rab32/Rab38 levels were normal in TMEM163-deficient mice, indicating a unique role for TMEM163-dependent pathways in δ-granule biogenesis.
In addition to TMEM163, other ZIP/ZnT isoforms may be involved in megakaryocyte and platelet Zn2+ homeostasis, which regulate distinct Zn2+-dependent signaling pathways.7 The expression profile and function of ZIP/ZnT isoforms could be dynamic during megakaryopoiesis, changing to optimize Zn2+ levels in the cytoplasm and intracellular organelles and regulate Zn2+-responsive genes and transcription factors to support platelet production (see figure). Granular-resident Zn2+ store in platelets is also involved in the regulation of the coagulation cascade.7,9 These complex processes can be further analyzed in mouse models with ZIP/ZnT and HPS/TMEM163 deficiency to determine how defective Zn2+ transport and granular Zn2+ content are involved in platelet signaling and hemostatic complications in HPS and other storage pool diseases.
The study by Yuan et al has provided new insights into the regulatory mechanism of Zn2+ transport in δ-granules and suggests a link between abnormal δ-granule biogenesis and hemostasis. These findings may provide the basis for exploring Zn2+-dependent therapeutic strategies in HPS and other storage pool diseases.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal