TO THE EDITOR:
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is the only curative option for several hematologic malignancies.1,2 Recent advances have significantly reduced treatment-related mortality; nevertheless, infections and graft-versus-host disease (GVHD) still represent major transplant-related complications.
Recent studies indicate that the intestinal microbiota may be an important factor for transplant outcome.3,4 Diversity and stability of the intestinal flora are disrupted during allo-HSCT.5 Low diversity of the intestinal microbiota at engraftment is an independent predictor of mortality.3,6,7
Moreover, intestinal inflammation secondary to GVHD was associated with major shifts in the composition of intestinal microbiota able to modulate the severity of intestinal inflammation.8-12 High relative amounts of proinflammatory enteric bacteria (ie, Enterococcus spp.),7,13,14 paralleled by a decrease of anaerobic commensals belonging to Lachnospiraceae and Ruminococcaceae (ie, Blautia spp. and Faecalibacterium spp.), have been associated with higher risk of GVHD.5-7,15-17
Our recent preliminary study indicates that longitudinal analysis of microbiome profile allows early identification of patients at risk for major transplant-related complications.18 Herein we deeply investigate the role of intestinal microbiota in the setting of acute GVHD.
Between October 2014 and March 2016, we conducted a prospective single-center observational study to examine the intestinal microbiota by 16S next-generation sequencing techniques in 100 consecutive adult patients (Table 1), who received allo-HSCT for high-risk hematologic malignancies.18 This study was approved by the Institutional Ethical Committee before sample collection.
Transplant characteristics
| . | N . |
|---|---|
| Donor type | |
| MRD | 15 |
| MUD | 35 |
| MMRD | 45 |
| CBU | 5 |
| Stem cell source | |
| PB | 87 |
| BM | 8 |
| CBU | 5 |
| Conditioning regimen | |
| MAC | 78 |
| RIC | 22 |
| GvHD prophylaxis (A) | |
| PT-Cy | 59 |
| ATG | 25 |
| PT-Cy and ATG | 7 |
| None | 9 |
| GvHD prophylaxis (B) | |
| Rapamycin | 81 |
| Cyclosporine | 19 |
| . | N . |
|---|---|
| Donor type | |
| MRD | 15 |
| MUD | 35 |
| MMRD | 45 |
| CBU | 5 |
| Stem cell source | |
| PB | 87 |
| BM | 8 |
| CBU | 5 |
| Conditioning regimen | |
| MAC | 78 |
| RIC | 22 |
| GvHD prophylaxis (A) | |
| PT-Cy | 59 |
| ATG | 25 |
| PT-Cy and ATG | 7 |
| None | 9 |
| GvHD prophylaxis (B) | |
| Rapamycin | 81 |
| Cyclosporine | 19 |
ATG, antithymocyte globulin; BM, bone marrow; CBU, cord blood unit; GVHD, graft-versus-host disease; MAC, myeloablative conditioning; MMRD, mismatched related donors; MRD, matched related donor; MUD, matched unrelated donor; PB, peripheral blood; PT-Cy, posttransplant cyclophosphamide; RIC, reduced intensity conditioning.
All adult patients were treated according to current institutional programs on written informed consent. Stem cell donors were family haploidentical (n = 45), HLA identical sibling (n = 15), unrelated volunteer (n = 35), and cord blood (n = 5). Stem cell source was mainly unmanipulated peripheral blood stem cells. GVHD prophylaxis was mostly posttransplant cyclophosphamide (PTCy; 59%) and rapamycin (81%).19,20 All patients received levofloxacin and trimethoprim-sulfamethoxazole as prophylaxis and piperacillin-tazobactam as first-line empirical treatment of febrile neutropenia according to institutional guidelines.18
Fecal specimens were collected before conditioning regimen (T0), during aplasia (T1), and after engraftment (T2). The fecal microbiome was analyzed using the 454 GS Junior System (Roche Applied Science, Mannheim, Germany), with polymerase chain reaction primers targeting the V3 to V5 regions of the 16S ribosomal RNA gene, with a mean of 16 800 (8000-40 700) reads per sample, and Quantitative Insights Into Microbial Ecology (QIIME) software.21
Acute GVHD was defined and scored following the International Bone Marrow Transplant Registry Severity Index and the Glucksberg criteria.22 We used the statistical program SPSS Statistics (version 23; IBM) to perform all statistical analyses and GraphPad Prism Software (version 6) for receiver operating characteristic (ROC) curves, cumulative probability curves comparison, and Kaplan-Meier survival curves. ROC curves, calculated at the family level for all outcomes, were plotted at all studied time points to identify possible early microbiome-based risk stratification markers. Curves with an area under the curve >0.6 and P ≤ .05 were considered significant; from significant curves, the points with the best accuracy were chosen and used to estimate their association with the different outcomes using Cox regression.18
In this population, cumulative incidence of grade II to IV and III to IV acute GVHD at 100 days was 25% and 19%, respectively. Skin-only involvement was observed in 30 patients, gut-only involvement in 4, skin and gut involvement in 4, liver and gut involvement in 1, and skin with liver and gut involvement in 7 patients.
Development of acute GVHD was preceded by significant changes in intestinal microbiota composition, with a predominant role played by gram-positive bacteria belonging to Firmicutes. The presence of Lachnospiraceae was associated to a decreased risk of developing acute GVHD (P = .04 and relative risk [RR] = 4.35), whereas a relative dominance of Enterococcaceae (P < .01 and RR = 3.23) and Staphylococcaceae (P < .01 and RR = 3.5) was associated to its increased incidence.
A decrease in bacterial α-diversity (Shannon Index ≤1.3) between T0 and T1 was observed in patients developing acute GVHD, with a relative loss of Lachnospiraceae (P = .022) and relative increase in Staphylococcaceae (P = .005) in patients with early onset GVHD (within 30 days).
However, microbiome markers were more informative at 10 days after transplant (T1). Of note, 19 patients were under prophylaxis, whereas 77 were receiving antibiotic therapy at T1.
At T1, a decrease in α-diversity (hazard ratio [HR], 4.08; P = .008) and relative enteric dominance by Enterococcaceae >90% (HR, 4.77; P = .004) were associated with the development of early acute GVHD. A relative decrease in the protective family of Lachnospiraceae <10% showed a trend toward increased risk of early acute GVHD (HR, 3.75; P = .066). Analogously, low α-diversity (HR, 2.27; P = .027) and Enterococcaceae >90% (HR, 2.67; P = .012) were predictive for late acute GVHD (within 100 days).
Low α-diversity (P = .014) and Enterococcaceae >90% (P = .002) were similarly reported in patients who developed grade II to IV acute GVHD at 100 days. Of notice, microbiome biomarkers also predicted grade III to IV acute GVHD at 100 days: in particular, low α-diversity (P = .0008), Lachnospiraceae < 10% (P = .062), and Enterococcaceae >90% (P = .001).
Interestingly, microbiome biomarkers could predict risk of organ-specific acute GVHD involvement. In particular, a relative dominance of Staphylococcaceae (>40%) predicted acute GVHD with liver involvement (early: HR, 7.99; P = .007; late: HR, 5.14; P = .029).
Uniquely, at T1, Staphylococcaceae >40% was able to predict the occurrence of steroid-refractory acute GVHD (RR, 8.40; P = .007).
Different microbiome biomarkers at T1 were associated with acute GVHD with gastrointestinal (GI) involvement. Remarkably, a relative enteric dominance by Enterococcaceae >90% predicted GI involvement (early: HR, 5.78; P = .005; late: HR, 4.80; P = .001), similar to low α-diversity (early: HR, 6.81; P = .002; late: HR, 4.43; P = .002).
Instead, other T1 microbiome biomarkers were correlated more specifically to a short-term risk of acute GVHD with GI involvement. In particular, Staphylococcaceae >40% predicted early GI GVHD (HR, 4.84; P = .037). Similarly, Lachnospiraceae <10% was associated with GVHD with early GI involvement (HR, 5.68; P = .015); of notice, no cases of early GI GVHD occurred in patients with Lachnospiraceae ≥10% at T1. Instead, patients with Lachnospiraceae <10% showed a trend toward higher risk of late GI GVHD (HR, 2.82; P = .096).
No specific biomarker of skin acute GVHD was identified. Nonetheless, a decreased α-diversity correlated to an increased risk of early GVHD with skin involvement (HR, 2.69; P = .023).
When stratifying patients according to GVHD prophylaxis in the cohort of patients receiving PTCy, a relative dominance by Enterococcaceae predicted grade II to IV and III to IV acute GVHD (P = .009 and P = .03, respectively). No relevant microbiome biomarkers have been identified with antithymocyte globulin.
A deeper knowledge of the molecular mechanisms involved in the initiation phase is potentially relevant in combating acute GVHD. Timely recognition of patients who are at high risk for GVHD may lead to a better preventive care and early introduction of treatments.23
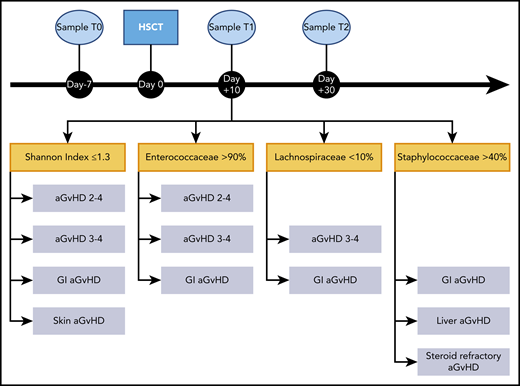
In this setting, the microbiome profile at T1, during aplasia, resulted a reliable predictor of acute GVHD in terms of severity and organ localization (Figure 1). Overall, low α-diversity was significantly associated with increased risk of grade II to IV and III to IV acute GVHD, particularly with skin and gut involvement. We found a relative dominance of Enterococcaceae in patients who developed grade II to IV, III to IV, and GI acute GVHD. A relative dominance of Staphylococcaceae predicted liver, GI, and steroid-refractory GVHD. A relative dominance of Enterococcaceae was associated with grade II to IV, III to IV, and GI GVHD. A relative decrease in Lachnospiraceae predicted gut involvement, showing a trend toward grade III to IV acute GVHD.
Sampling timeline and potential enteric microbiome biomarkers of acute GVHD. Sampling timeline (top). Microbiome samples were collected at 3 timepoints during the transplant course: before conditioning regimen (T0), during aplasia (T1), and after engraftment (T2). Focus on T1, showing details of the microbiome markers associated with the development of acute GVHD (bottom). In particular, low bacterial α-diversity, quantified by Shannon index, relative dominance by Enteococcaceae and Staphylococcaceae, and relative loss of Lachnospiraceae were correlated to acute GVHD, its severity, and organ localization. aGVHD, acute graft-versus-host disease.
Sampling timeline and potential enteric microbiome biomarkers of acute GVHD. Sampling timeline (top). Microbiome samples were collected at 3 timepoints during the transplant course: before conditioning regimen (T0), during aplasia (T1), and after engraftment (T2). Focus on T1, showing details of the microbiome markers associated with the development of acute GVHD (bottom). In particular, low bacterial α-diversity, quantified by Shannon index, relative dominance by Enteococcaceae and Staphylococcaceae, and relative loss of Lachnospiraceae were correlated to acute GVHD, its severity, and organ localization. aGVHD, acute graft-versus-host disease.
A longitudinal study of the microbioma profile may contribute to the early identification of patients at major risk for GVHD, paving the way for rational interventions to restore microbiota integrity, such as with fecal microbiota replacement.24-26
The datasets generated for this study are available on request to the corresponding author at ciceri.fabio@hsr.it; procedures to also have sequencing data available in a centralized repository (ENA and/or NCBI SRA archives) are ongoing at the time of publication.
Acknowledgment
This study was supported by Fondazione Umberto Veronesi (FUV) Post-doctoral Fellowship 2019.
Authorship
Contribution: R.G., R.N., N.M., M.C., and F.C. designed the study; R.G., R.N., N.M., R.P., and F.L. analyzed the data; R.G., R.N., N.M., F.L., M.C., and F.C. wrote the manuscript; and all authors contributed to patient clinical care and data collection and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Fabio Ciceri, Hematology and Bone Marrow Transplantation Unit, IRCCS San Raffaele Scientific Institute, University Vita-Salute San Raffaele, Milan, Italy; e-mail: ciceri.fabio@hsr.it.
REFERENCES
Author notes
R.G. and R.N. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal