Abstract
Acute graft-versus-host disease (GVHD) is 1 of the major life-threating complications after allogeneic cell transplantation. Although steroids remain first-line treatment, roughly one-half of patients will develop steroid-refractory GVHD (SR-GVHD), which portends an extremely poor prognosis. Many agents that have shown encouraging response rates in early phase 1/2 trials for prevention and treatment have been unsuccessful in demonstrating a survival advantage when applied in the setting of SR-GVHD. The discovery of novel treatments has been further complicated by the absence of clinically informative animal models that address what may reflect a distinct pathophysiology. Nonetheless, the combined knowledge of established bone marrow transplantation models and recent human trials in SR-GVHD patients are beginning to illuminate novel mechanisms for inhibiting T-cell signaling and promoting tissue tolerance that provide an increased understanding of the underlying biology of SR-GVHD. Here, we discuss recent findings of newly appreciated cellular and molecular mechanisms and provide novel translational opportunities for advancing the effectiveness of treatment in SR-GVHD.
Introduction
Despite significant progress in allogeneic cell transplantation (HCT; allo-HCT) for the treatment of malignant and nonmalignant disorders, acute graft-versus-host disease (GVHD) remains a major driver of nonrelapse mortality. For decades, high dosages (1-2 mg/kg per day) of prednisone or methylprednisolone have remained a pillar of frontline treatment in the 30% to 50% of allo-HCT recipients who develop GVHD.1 Unfortunately, roughly one-half of patients receiving therapy will not demonstrate an initial response; even fewer (∼30%) will exhibit a durable response that can facilitate withdrawal from glucocorticoids.2,3 Thus, scenarios of nonresponse, progression, or prolonged dependence broadly define steroid-refractory GVHD (SR-GVHD). Overall survival (OS) in SR-GVHD is poor, historically <50% at 6 months, and survival after failure to respond to second-line therapy is dismal (OS < 30%).4,5 Many agents have shown encouraging phase 2 response rates, but none have demonstrated a survival advantage in randomized trials.6,7 The lack of high-quality clinical evidence in the form of controlled trials is compounded by our incomplete understanding of SR-GVHD pathophysiology. To better understand the mechanistic underpinnings of SR-GVHD, we discuss the molecular and intracellular functions of steroids in modulating innate and adaptive immune responses. Second, we discuss potential mechanisms of steroid-mediated regulation of alloreactivity based on our current understanding of GVHD pathophysiology. Here, potential mechanisms of corticosteroid resistance as well as results from basic and translational studies highlight emerging themes in modification of T-cell function, regulation of immune tolerance, and protection of host tissues. Finally, we discuss implications for the prediction, treatment, and prevention of SR-GVHD in the clinic.
Current understanding of the pathophysiology of acute GVHD
Acute GVHD progression can be categorized into 3 phases.8 Host tissue injuries caused by conditioning regimens mediate release of damage-associated molecular patterns (DAMPs), such as adenosine triphosphate,9,10 from injured tissues and pathogen-associated molecular patterns (PAMPs), such as lipopolysaccharide,11 from microbiomes. DAMPs and PAMPs activate recipient and/or donor-derived antigen-presenting cells (APCs), such as dendritic cells (DCs), macrophages (MFs), and host-derived nonhematopoietic cells in epithelial surfaces that in turn produce numerous proinflammatory cytokines (tumor necrosis factor α [TNF-α], interleukin 6 [IL-6]).12-15 Pattern recognition receptors, such as Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain–containing 3 (NLRP3), recognize PAMPs and DAMPs.16,17 Specific subsets of DCs (CD103+ DCs, CD8α+ DCs) are capable of alternating roles in either promoting or ameliorating GVHD depending on the inflammatory milieu.18,19 This contrasts certain committed populations such as myeloid-derived suppressor cells and granulocyte colony-stimulating factor (G-CSF)–induced CD34+ regulatory monocytes that exclusively function to constrain GVHD severity.20,21 More recently, activated neutrophils stimulated by bacteria may also function as APCs in an inflammasome-dependent context.22,23 Taken together, activated APCs stimulate newly infused donor-derived naive T cells to respond to host antigens/tissues that characterize GVHD.
The diversity of gut microbiota and viromes has been shown to be dramatically altered after conditioning and may participate in the development of GVHD, especially in the gastrointestinal (GI) tract.24-27 IL-22 produced by innate lymphoid cells protects intestinal stem cells (ISCs) and ameliorates GVHD.28 The ISC compartment located at the crypt base is the primary target of allogeneic donor T cells regulated by mucosal addressin cell adhesion molecule 1 (MAdCAM-1), which is an important adhesion molecule for T-cell migration into the gut.29 In addition, regenerating islet-derived 3α (REG3α) from Paneth cells, induced by IL-22, has been demonstrated to prevent crypt apoptosis and decrease GVHD.30 Moreover, metabolites, such as butyrate or indole, play an important role in protecting ISCs and maintaining tissue homeostasis.31-33 Therefore, host target tissue–intrinsic mechanisms may be crucial for ameliorating GVHD. We and others have demonstrated that, in intestinal epithelial cells, several molecules and pathways, such as inhibitor of apoptosis proteins, NLRP6, retinoic acid–inducible gene I (RIG-I)/mitochondrial antiviral signaling, and stimulator of interferon genes, act as tissue-intrinsic mechanisms that regulate GVHD pathogenesis.27,34-37
After donor T cells engage APCs they become activated to proliferate. Activated donor CD4+ T cells differentiate into a variety of subsets, such as T-helper 1 (Th1), Th2, Th9, Th17, and Th22 cells whereas CD8+ T cells differentiate into cytotoxic T cells.38 In addition, several transcription factors (TFs) and their regulators, such as MAPK/extracellular signal-regulated kinases, Aurora kinase A/Janus kinase 2 (JAK2), as well as metabolic pathways (glycolysis and mitochondrial oxidative phosphorylation), have been reported to be important in the activation of T cells.38,39 These T cells then migrate into the primary GVHD target organs (intestine, liver, skin) to attack the host. Thus, donor immune cells and host tissue tolerance both emerge as key concepts in regulating GVHD. Because this system uses counterregulatory mechanisms to avoid an excessive immune response, dysfunction in certain cell populations such as regulatory T cells (Tregs), invariant natural killer (NK) T (iNKT) cells, or immune check points (programmed cell death 1 [PD-1]/programmed cell death ligand 1 [PD-L1], CD28/CTLA-4, and CD24/Siglec-G) can disrupt peripheral tolerance and amplify GVHD.40 Decreasing Treg/T effector cell ratios has especially been shown to exacerbate GVHD.39,41,42 Therefore, a major thrust of therapeutics in GVHD has emphasized enhancing Tregs by using ex vivo or in vivo expansion techniques.
How do steroids regulate GVHD?
The mechanisms leading to steroid-induced suppression of inflammation remain to be elucidated in SR-GVHD.43,44 Immunoregulatory functions of synthetic glucocorticoids (dexamethasone, prednisolone) are initiated through binding glucocorticoid receptors (GRs).43 Cytoplasmic GR activation promotes release from chaperones (heat shock protein 90 [HSP90]) and translocation to the nucleus to interact with DNA and proteins that mediate genomic and nongenomic functions.43-45 GRα is the canonical receptor that binds steroids and modulates immune responses. In contrast, GRβ is a splice variant that binds to DNA to antagonize GRα. Steroid-induced immune regulation can be divided into effects that are genomic and nongenomic.44,45 Genomic effects occur in the nucleus and alter gene expression by 3 mechanisms: (1) direct binding to glucocorticoid response elements to modify gene expression, (2) protein-protein interactions (tethering) with other TFs to alter gene expression of nuclear factor κB (NF-κB) and the signal transducer and activator of transcription (STAT), and (3) binding to composite elements containing a glucocorticoid response element and a response element of another TF. Genomic effects are persistent whereas nongenomic effects arise almost immediately after stimulation, are transient, and do not modify gene expression.46 Thus, steroids regulate inflammation-associated TFs in a manner that can reduce the production of proinflammatory cytokines, chemokines, and adhesion molecules.43,44
In GVHD, the primary anti-inflammatory mechanism of steroids is mediated by inhibiting NF-κB pathways in APCs and T cells as well as TLR-mediated signaling.47-50 In addition, glucocorticoids can directly regulate activated DCs and MFs by modulating their differentiation and maturation.51-54 Steroids have been shown to decrease expression of major histocompatibility complex (MHC) class II, costimulatory molecules (CD80, CD40) and production of proinflammatory cytokines while enhancing the production of anti-inflammatory cytokines, such as IL-10 in DCs.55 In T cells, steroids suppress activation and proliferation of T cells by dampening key signaling pathways, such as nuclear factor of activated T cells, STAT, lymphocyte-specific protein tyrosine kinase, and mitogen-activated protein kinase/extracellular signal-regulated kinase.56-59 Also, steroids preferentially repress Th1 and Th17 differentiation60,61 but promote Th2 and Tregs.62-64 Finally, steroids reduce the production of chemokines and expression of adhesion molecules in a manner that decreases the migration of donor T cells into target tissues.65 However, although glucocorticoids have numerous effects that mitigate the allogeneic T-cell response, their impact on wound healing and tissue regeneration44,66 and on ameliorating tissue tolerance may in some contexts antagonize recovery from GVHD.40,67
Cellular mechanisms of steroid resistance: implications for GVHD
The pathophysiology of SR-GVHD is complex and enigmatic. The concept of “glucocorticoid resistance” in immunology was first described in the 1970s68 and remains an important area of investigation in autoimmune disease.43,69 Because steroids impair not only effector T cells but also regulatory cells, it is conceivable that their effects may also limit long-term tolerance mediated by immune-suppressor cells.70 Donor alloreactive T cells play a central role in the development of GVHD. However, one possibility is that donor T cells are less crucial in SR-GVHD than in initiation of GVHD due to previous exposure to the suppressive effects of steroids and/or calcineurin inhibitors (Figure 1A). This reasoning is supported by the results of some clinical trials in which broadly depleting donor T cells (antithymocyte globulin [ATG]) and CD25+ activated T cells (inolimomab) did not show benefit in the treatment of SR-GVHD.7 This is also supported by our data that donor T cells are dispensable in murine SR-GVHD.71 Another possibility is that certain subsets, such as Th17 cells,72,73 may not be sufficiently suppressed,64,74 especially glucocorticoid-resistant pathogenic Th17 cells that express the efflux transporter P-glycoprotein75 or glucocorticoid-induced TNF receptor family-related protein. Activation of glucocorticoid-induced TNF receptor family-related protein mediates the opposite effect on the regulation of alloreactive CD4+ and CD8+ T cells in GVHD.76 A recent study suggests that activated steroid-resistant allogeneic donor T cells in the inflamed tissues also undergo markedly altered gene-expression profiles resulting in upregulation of genes involved in T-cell activation (CD28, Tnfrsf9), migration (Cxcr6), and metabolic reprograming (Hif1α) that facilitate increased glycolytic demands.77
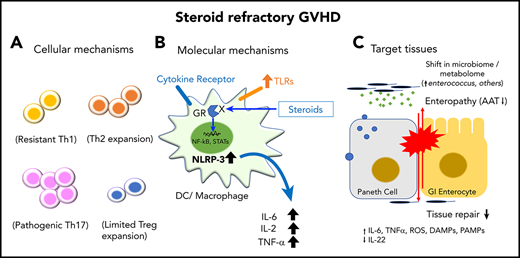
Mechanisms of SR-GVHD. (A) Cellular mechanisms. Steroids regulate the majority of Th1 responses but can paradoxically increase Th2 and pathogenic Th17-mediated immune responses that may promote GVHD. The role of steroids in CD8+ T cells is uncertain. The combination of steroids with calcineurin inhibitor (CNI) may unintentionally blunt induction of Tregs based on their requirement for IL-2 resulting in loss of peripheral tolerance. (B) Molecular mechanisms. Steroids repress expression of TFs necessary for production of proinflammatory cytokines (IL-6, TNFα). In addition, steroids promote induction of regulatory cell subsets, such as CD103+ DCs and M2 MFs that induce immune tolerance. In refractory disease, long-term use of steroids may paradoxically increase expression of TLRs and NLRP3 that perpetuate inflammation. (C) Target tissue–intrinsic mechanisms. In the GI tract, steroids can impede the reparative processes of the host following T-cell–mediated injury that is associated with loss of Paneth cells, ISCs, and immune-regulatory proteins (α-1-antitrypsin [AAT]). Limited tissue regeneration from long-term suppression of inflammation with ongoing mucosal barrier injury is associated with alterations in the intestinal microbiome and metabolome. Dysbiosis results in loss of protective metabolites (butyrate). Ongoing inflammation can eventually stimulate APCs to increase production of proinflammatory cytokines that further damage host tissue. AP-1, activator protein 1; GCR, glucocorticoid receptor; ROS, reactive oxygen species; Tc, cytotoxic T cell; TCR, T-cell receptor.
Mechanisms of SR-GVHD. (A) Cellular mechanisms. Steroids regulate the majority of Th1 responses but can paradoxically increase Th2 and pathogenic Th17-mediated immune responses that may promote GVHD. The role of steroids in CD8+ T cells is uncertain. The combination of steroids with calcineurin inhibitor (CNI) may unintentionally blunt induction of Tregs based on their requirement for IL-2 resulting in loss of peripheral tolerance. (B) Molecular mechanisms. Steroids repress expression of TFs necessary for production of proinflammatory cytokines (IL-6, TNFα). In addition, steroids promote induction of regulatory cell subsets, such as CD103+ DCs and M2 MFs that induce immune tolerance. In refractory disease, long-term use of steroids may paradoxically increase expression of TLRs and NLRP3 that perpetuate inflammation. (C) Target tissue–intrinsic mechanisms. In the GI tract, steroids can impede the reparative processes of the host following T-cell–mediated injury that is associated with loss of Paneth cells, ISCs, and immune-regulatory proteins (α-1-antitrypsin [AAT]). Limited tissue regeneration from long-term suppression of inflammation with ongoing mucosal barrier injury is associated with alterations in the intestinal microbiome and metabolome. Dysbiosis results in loss of protective metabolites (butyrate). Ongoing inflammation can eventually stimulate APCs to increase production of proinflammatory cytokines that further damage host tissue. AP-1, activator protein 1; GCR, glucocorticoid receptor; ROS, reactive oxygen species; Tc, cytotoxic T cell; TCR, T-cell receptor.
Although the initiating factors of steroid resistance remain obscure, cytokine-induced mechanisms are well investigated in chronic inflammation. Inflammatory cytokines, such as interferon γ, which upregulate the expression of MHC class II and increased antigen presentation in the intestinal epithelium, may be correlated with SR-GVHD.15,78 IL-6, a potential mediator of SR-GVHD, is elevated in the serum during GVHD79 and is also known to directly damage intestinal epithelial tissues80 with IL-6/IL-6 receptor levels enriched in the colonic microenvironment.81 Downstream of IL-6, JAKs possess pleomorphic functions in regulating the immune response and have demonstrated strong rationale in the therapy of SR-GVHD.82-85 The representative cellular mechanisms of SR-GVHD are summarized in Figure 1A.
Molecular mechanisms of SR-GVHD
Compared with cellular mechanisms, relatively less is understood about the underlying molecular features of SR-GVHD. However, recent insights into the immunological impact of steroids, some that paradoxically are immune enhancing, have shed light on these processes (Figure 1B). For example, the TLR4 agonist lipopolysaccharide (LPS) regulates the expression of GRα and β isoforms that prevent the inhibitory effects of glucocorticoids on granulocyte macrophage colony-stimulating factor (GM-CSF) secretion.86 Proinflammatory cytokines also induce glucocorticoid resistance by impairing phosphorylation and function of the GR.87-89 The inflammasome, specifically NLRP3–caspase 1 (CASP1), can deplete cellular levels of GR thus limiting overall cell sensitivity to glucocorticoids.90 Preclinical models suggest that intact recipient GR is necessary to restrain the severity of GVHD,91 and GR single-nucleotide polymorphisms (SNPs) have been associated with clinical outcomes.92 These findings suggest that cytokines, DAMPs, PAMPs, including inflammasome pathways, may drive SR-GVHD.
Target tissue–intrinsic mechanisms of SR-GVHD
Target tissue–intrinsic mechanisms are becoming increasingly implicated in SR-GVHD (Figure 1C). Steroids administrated in GVHD are delivered into damaged tissues infiltrated by T cells, DAMPs, and PAMPs. As mentioned, steroids have bilateral effects, not only immunosuppressive but also immune-stimulatory that potentiate the effects of DAMPs and PAMPs, which in turn mediate GR modifications. In addition, steroids promote expression of TLRs, inflammasomes (NLRP3), and purinergic receptor (P2Y2R), which further sensitize cells to potently respond to DAMPs and PAMPs.44 These results suggest that steroids themselves, in certain contexts, may enhance tissue-specific immune responses that perpetuate GVHD. Steroids and other currently available pharmacologic agents have not been shown to terminate ongoing organ damage. Increased serum cytokeratin-18 fragments, associated with epithelial cell apoptosis, can be detected as persistent organ damage occurs in SR-GVHD.93 Angiopoietin-2 (ANG2), which regulates vessel quiescence, is also increased in patients with SR-GVHD,94,95 suggesting that vascular endothelial injury contributes to SR-GVHD. Interestingly, these findings appear associated with the clinical syndrome of transplant-associated thrombotic microangiopathy (TA-TMA), mediated by alternative and terminal complement activation, which is linked with SR-GVHD.96,97
Disruption of tissue tolerance may explain why intensive immune-suppressive strategies are often ineffective in SR-GVHD, especially involving the GI tract.40,67 Typically, injured tissues have the capacity to regenerate. Once tissue tolerance has been impaired, barrier function, which protects host tissues from invading pathogens and abnormal immune responses, is destroyed. It has recently been recognized that the diversity of intestinal microbiota is altered in GVHD24,27,98 and efforts to correct this dysbiosis with fecal microbiome transplantation (FMT) are being attempted for treatment of SR-GVHD,99-101 suggesting that the microbiota and its associated metabolites such as butyrate may be implicated.31 Long-term exposure to aberrant microbiomes, PAMPs, and DAMPs may mediate prolonged mucosal barrier dysfunction and inflammation. Therefore, disease-promoting tissue-intrinsic mechanisms are becoming an important component to consider in the treatment of SR-GVHD.
Predicting SR-GVHD in the clinic
In the absence of clinical progression, identifying treatment resistance often requires 7 to 14 days of high-dose steroid therapy.2,102 Responses are insidious, as reductions in diarrhea may be fleeting once attempts are made to reduce steroid dosage.6 Because SR-GVHD often reflects advanced organ injury, early detection with prompt treatment intensification has been postulated as a clinical strategy to reduce alloreactivity and constrain organ injury. Although some studies suggest that early institution of second-line therapy can improve outcome,103 the premise that early prediction can improve outcomes remains an open question being addressed in preemptive trials (NCT03459040). In the following sections, we emphasize biology-based markers for SR-GVHD; other comprehensive reviews are available on the topic of GVHD biomarkers.104,105
Clinical and histologic risk factors
Patient characteristics known to heighten the probability of steroid resistance include donor-recipient HLA disparity, advanced age, and lower GI tract or hepatic involvement.106 Not surprisingly, organ involvement and severity at GVHD onset strongly predict response. Patients deemed high risk by refined Minnesota score had a 44% probability of response by day 28 compared with 68% in standard risk, although among 1723 patients only 16% were labeled high risk.107 Despite being a clinical diagnosis, histology at GVHD onset may suggest advanced pathobiology heralding a complicated clinical course. For example, severe crypt loss with denudation of the GI epithelium is correlated with higher clinical grade, steroid resistance, and mortality.108 Paneth cells that support adjacent ISCs in the crypt regulate epithelial regeneration and shape microbial ecology through secretion of antimicrobial peptides (α-defensins, REG3α). In line with these observations, loss of Paneth cells in GI biopsies correlates with lack of response to therapy.109 Because Paneth cell loss can influence ISC populations (and regeneration), these findings support tissue injury being a hallmark of clinically resistant phenotypes.
Cellular byproducts of tissue injury
Given the diagnostic challenges in GVHD, there is interest in generating validated serum biomarkers capable of aiding clinical decisions. The Mount Sinai Acute GVHD International Consortium (MAGIC) recently assessed a validated 2-biomarker algorithm involving suppressor of tumorigenicity-2 (ST2) and REG3α to predict treatment resistance.110 High levels of REG3α (stored in Paneth cells and GI mucous) are released into serum during early crypt damage and soluble ST2 is released by alloreactive T cells in the gut.111-114 Patients with high-risk biomarkers 1 week after initiating corticosteroid treatment had significantly higher treatment failure. Interestingly, a high-risk biomarker profile was suggestive of treatment failure independent of early clinical response. If prospectively validated, this information may guide risk-adapted decision-making (tapering steroids vs second-line therapy).
As GI injury is central to fatal GVHD (and repeat endoscopy is challenging), there is interest in informative stool analytes. Fecal calprotectin (CPT), expressed in MFs, monocytes, and the cytoplasm of granulocytes, may provide a real-time surrogate marker of disease activity. Elevated CPT, activated in the presence of DAMPs or PAMPs, serves as a ligand for TLR4 thus suggesting ongoing inflammatory signaling (NF-κB) not constrained by steroids. CPT itself may contribute to mucosal permeability,115 is elevated in lower GI tract GVHD, and is correlated with histopathologic severity116 ; thus, longitudinal assessment may aid in distinguishing steroid refractoriness from other forms of colitis (cytomegalovirus).117 Combining CPT with analytes such as α-1-antitrypsin (AAT), lost during GVHD-induced enteropathy, may further enhance sensitivity and provide an “actionable” protein to supplement.118
Endothelial cell dysfunction
In addition to epithelial surfaces, inflammation of SR-GVHD involves an endothelium capable of perpetuating injury. TA-TMA is prevalent among patients with SR-GVHD (79% vs 42%; P = .001). Whether TA-TMA is an epiphenomenon of SR-GVHD or an inciting event is unknown, however, damage to the endothelium results in complement activation (BBPlus and C5b-9), release of soluble thrombomodulin, and ANG2 that impedes organ recovery.94,96 In 1 study, elevated levels of ANG2 were detected before onset of SR-GVHD, which may sensitize the endothelium to the injurious effects of proinflammatory cytokines. Variant SNPs in the TM gene have been shown to predict responsiveness to GVHD treatment, suggesting host-intrinsic differences in vulnerability to injury.94
Evaluating treatment strategies for SR-GVHD
At present, ruxolitinib represents the only US Food and Drug Administration (FDA)-approved therapy for SR-GVHD, however, no agent has demonstrated superiority in head-to-head trials.119,120 As SR-GVHD reflects complex biology, successful treatment will likely require targeting alternative pathways or combinatorial treatment approaches that overcome putative mechanisms of corticosteroid resistance. Akin to a marathon, and acknowledging inherent limitations in clinical grading systems based on quantifying stool volume, assessing a given intervention will require careful interpretation of both early and late milestones for success. This might be accomplished through varied end points that quantify not only the frequency but depth and durability of response.121 Most SR-GVHD studies report overall response (overall response rate [ORR] or complete response [CR] plus partial response) by day 28 of treatment onset due to its association with improved treatment-related mortality in upfront treatment settings as an early readout of promising activity, particularly when this end point compares favorably against historical rates (∼50% in second-line therapy).4,122 ORR, however, is imperfect as this end point may not be indicative of improved survival if there are subsequent rises in morbidity, late mortality, or GVHD. Thus, treatment trials should increasingly incorporate other qualifiers of success that measure hard end points such as overall and GVHD-free survival, infection, and relapse to identify the most promising agents for larger controlled studies.121,123
Lessons from existing therapies
Corticosteroids possess potent lymphocytic and anticytokine properties, nonetheless, increasing dosage beyond 2 mg/kg methylprednisolone does not increase response.6,124 T-cell receptor β (TCRβ) sequencing of diagnostic GI-tract biopsies has illuminated the persistence of glucocorticoid-resistant T-cell clonotypes in later stages of GVHD,125 suggesting that targeting alternative or multiple simultaneous signaling pathways may be necessary. T-cell–depleting sera (ATG), CD52 direct antibodies targeting T and B cells (alemtuzumab), or chemotherapies (pentostatin, pulse cyclophosphamide) are all intensive approaches used as second-line therapy.126,127 However, toxicity, namely myelosuppression, opportunistic infection, and interstitial pneumonitis, can reduce their overall efficacy, resulting in <10% long-term OS in some studies.128,129 Therefore, treatment must account for adverse events that accelerate mortality. Due to mucosal barrier injury, ongoing tissue injury from GVHD, and immune suppression, patients are particularly vulnerable to bacteremia, often from pathogenic organisms such as Enterococcus.130,131 Acknowledging these limitations, investigations have also focused on approaches that might selectively block key signaling events in GVHD. Although seemingly innocuous, monoclonal antibodies such as daclizumab, which eliminates activated CD25+ T cells, may heighten infectious mortality or unintentionally deplete Treg populations.132 In a cohort treated with CD25+- or TNFα-directed anticytokine therapy, infection-related mortality was 28% at a median of 88 days.133
These observations suggest that immunologically intensive therapies are not necessarily ideal in refractory settings, as subtler immunomodulatory methods also illicit response. Extracorporeal photopheresis (ECP) is a commonly used technique that limits host tissues to chemotherapy via ex vivo exposure of apheresed mononuclear cells to the DNA crosslinking agent 8-methoxypsoralen in the presence of UVA light.134 ECP has pleotropic effects that include apoptosis of alloreactive lymphocytes, induction of tolerogenic DC subsets, and expansion of Tregs, which may be suppressed in SR-GVHD. In a meta-analysis of prospective studies conducted as second-line treatment, the response rate for ECP was 69%, with the highest organ-specific response rates observed in the skin (84%), followed by visceral organs (65% in GI; 55% in liver).135 Although it is unclear whether this degree of response can be recapitulated as stand-alone therapy in highly refractory settings, early initiation of ECP is a commonly used adjunct that can facilitate salutary steroid-sparing effects.
New approaches: selective targeting of alloreactive T cells
Select strategies for SR-GVHD are summarized in Table 1. Recently, a phase 1/2 trial in GI and liver GHVD evaluated an anti-CD3/CD7 antibody conjugated to ricin toxin that elicited responses of 60% of SR–acute GVHD with 60% of patients alive after 6 months. This molecule selectively targets activated T- and NK-cell signaling that may enhance potency compared with prior anti-CD3 therapies.136-138 Infectious mortality was relatively low (∼20%), possibly due to only brief periods of lymphopenia after administration. This agent is now being evaluated in an open-label trial for SR-GVHD (CTN 1802). Other proteins such as CD30, expressed on activated T cells (particularly central memory CD8+CD45RO+CD62L+ subsets), are also enriched at GVHD onset.139 In a phase 1 study of brentuximab vedotin, an antibody-drug conjugate targeting CD30, 38% of patients responded at day 28 with an additional 25% experiencing CRs at day 56.140 Finally, integrin expression on lymphocytes may be impaired in corticosteroid resistance in a manner that promotes lymphocyte recruitment to target tissues.44 A phase 2 study of natalizumab directed against α4-integrin chains, administered as a single dose with steroids, produced high response rates (∼75%) in the initial treatment of GI GVHD.141 However, natalizumab inhibits α4β1 and α4β7 integrins, the former via interactions with vascular cell adhesion molecule 1 (VCAM1), which impedes central nervous system trafficking, thereby potentially predisposing patients to John Cunningham virus reactivation and progressive multifocal leukoencephalopathy. In contrast, α4β7 integrins, selectively targeted by the monoclonal antibody vedolizumab, interact with GI mucosal addressin MadCAM-1 expressed in Peyer patches, and lamina propria may have a niche role in treating GI GVHD.29 In a pilot study, 6 of 6 patients responded after 7 to 10 days with 4 experiencing durable remissions.142 A separate retrospective study reported 60% response, however, 34% of patients experienced grade 3-4 infection, frequently bacteremia from Staphylococcus or Enterococcus.143 As several patients had received multiple lines of prior therapy, it is unclear whether infections were attributable to advanced GVHD or possible impairments in immune trafficking.
Novel biology-driven strategies for SR-GVHD
| Approach . | Mechanism . | Clinical responses, % . | References . |
|---|---|---|---|
| Modification of alloreactive T cells | |||
| Anti-CD3/CD7 antibody conjugated to ricin toxin | Apoptosis↑ in T and NK cells | ORR, 60; CR, 50 | 136 |
| Brentuximab vedotin | CD30 inhibition, central memory ↓ (CD8+CD45RO+CD62L+ T cells) | ORR, 38.2 at day 28 | 139,140 |
| Vedolizumab | Integrin α4β7 inhibition, donor T-cell homing to GI tract↓ | ORR, 100 | 142 |
| ORR, 79 | 176 | ||
| ORR, 64 | 143 | ||
| Cytokines | |||
| Tocilizumab | IL-6 signaling↓ (Innate/adaptive response↓) | ORR, 67 | 147 |
| ORR, 44 | 146 | ||
| CR, 62.5 | 145 | ||
| F-652 (IL-22 dimer/Fc fusion molecule) | Preserved ISCs | NCT02406651* | |
| Gut regeneration↑ | |||
| Combination | |||
| Ruxolitinib | JAK1/2 inhibition | ORR, 81.5 | 85 |
| ORR, 45 | 177 | ||
| ORR, 57 | 152 | ||
| ORR, 78 | 178 | ||
| ORR, 84 | 153 | ||
| Tissue regeneration | |||
| Lithium | Wnt signaling ↑ by inhibiting GSK3 | CR, 50 | 157 |
| AAT | Serine protease inhibitor, ↑ Treg, | ORR, 66.7 | 163 |
| ↓APC function | |||
| ↓Proinflammatory cytokines (IL-6) | ORR, 65 | 162 | |
| Microbiome | |||
| FMT | Gut microbiome diversity ↑ | ORR, 100 | 99 |
| ORR, 75 | 101 | ||
| Resistant starch | Modification of metabolome | NCT02763033,* NCT02805075* |
| Approach . | Mechanism . | Clinical responses, % . | References . |
|---|---|---|---|
| Modification of alloreactive T cells | |||
| Anti-CD3/CD7 antibody conjugated to ricin toxin | Apoptosis↑ in T and NK cells | ORR, 60; CR, 50 | 136 |
| Brentuximab vedotin | CD30 inhibition, central memory ↓ (CD8+CD45RO+CD62L+ T cells) | ORR, 38.2 at day 28 | 139,140 |
| Vedolizumab | Integrin α4β7 inhibition, donor T-cell homing to GI tract↓ | ORR, 100 | 142 |
| ORR, 79 | 176 | ||
| ORR, 64 | 143 | ||
| Cytokines | |||
| Tocilizumab | IL-6 signaling↓ (Innate/adaptive response↓) | ORR, 67 | 147 |
| ORR, 44 | 146 | ||
| CR, 62.5 | 145 | ||
| F-652 (IL-22 dimer/Fc fusion molecule) | Preserved ISCs | NCT02406651* | |
| Gut regeneration↑ | |||
| Combination | |||
| Ruxolitinib | JAK1/2 inhibition | ORR, 81.5 | 85 |
| ORR, 45 | 177 | ||
| ORR, 57 | 152 | ||
| ORR, 78 | 178 | ||
| ORR, 84 | 153 | ||
| Tissue regeneration | |||
| Lithium | Wnt signaling ↑ by inhibiting GSK3 | CR, 50 | 157 |
| AAT | Serine protease inhibitor, ↑ Treg, | ORR, 66.7 | 163 |
| ↓APC function | |||
| ↓Proinflammatory cytokines (IL-6) | ORR, 65 | 162 | |
| Microbiome | |||
| FMT | Gut microbiome diversity ↑ | ORR, 100 | 99 |
| ORR, 75 | 101 | ||
| Resistant starch | Modification of metabolome | NCT02763033,* NCT02805075* |
↓, decrease; ↑, increase; AAT, α-1-antitrypsin; CR, complete response; FMT, fecal microbiome transplantation; GSK3, glycogen synthase kinase 3; ORR, overall response rate; VGPR, very good partial response.
Clinical trial number.
Cytokine-based approaches
Considering the redundant roles cytokines play, antagonism of single cytokines may be insufficient to downregulate inflammation in SR-GVHD.144 Antagonizing IL-6 signaling with anti–IL-6 receptor antibody (tocilizumab) or circulating IL-6 (siltuximab) may be 1 exception as this cytokine plays multiple roles in innate and adaptive immunity and may possess tissue-projective effects.80 Production of IL-6, which is transcriptionally downregulated by corticosteroids, is likely to be further elevated during SR-GVHD. Several clinical series now report activity of tocilizumab with responses in 44% to 62% of SR-GVHD.145-147 IL-6 antagonism may be less impactful on immunity because experimentally it does not perturb monocyte DC activation or alloreactive T-cell responses.148 Another cytokine with compelling rationale for treating SR-GVHD is IL-22, owing to its tissue-protective effects. In HCT models, IL-22 restored REG3γ production lost after Paneth cell destruction and facilitated regeneration of gut epithelium.30,149 An IL-22 dimer/Fc fusion molecule (F-652) is currently undergoing testing in newly diagnosed lower GI GVHD (NCT02406651).
Combination therapy with tyrosine kinase inhibition
To date, attempts to combine immunosuppressive agents in second-line therapy have been unsuccessful. No significant elevation in response rate was seen when duel cytokines (IL-2 and TNFα) were interrupted, resulting in high rates of infection and a 6-month OS of 29%.150 However, targeting downstream cytokine-induced signal transduction via small molecule tyrosine kinase inhibitors of JAKs (and STATs) is an emerging approach to overcoming steroid resistance. For example, depletion or inactivation of cytoplasmic GRs may limit tethering to STAT3 proteins that limit transcriptional repression of proinflammatory cytokines.151 After reporting impressively high response rates (81%) in a retrospective survey, a prospective, open label, multicenter trial (REACH1) of the JAK1/2 inhibitor ruxolitinib resulted in FDA approval for treating SR-GVHD.85,152 Overall, 57% of SR-GVHD patients met the primary end point of day 28 overall response including 31% with CR. An impressive finding was that a number of responses were reported to be durable, lasting a median of 345 days. In addition to blood cytopenia, infections were the primary adverse event (∼40%). Whether the high proportion of responses justifies this potential risk remains to be determined.153 As ruxolitinib is applied to increasing patients, studies are needed to guide coadministration with CYP3A4 inhibitors (azole antifungals) that dramatically increase drug levels and optimal withdrawal procedures given theoretical risks for cytokine release. More selective JAK-1 inhibitors (itacitinib) have also similarly displayed encouraging results in pilot studies of SR-GVHD154 and are undergoing phase 3 testing (NCT03139604). Other kinase targets that engage signaling distal to immunoreceptors such as spleen tyrosine kinase (Syk) can elicit immune-suppressive effects on alloreactive T cells and APCs in preclinical models, thus warranting study in SR-GVHD.155,156
Regeneration of host tissues
The majority of therapies focus on modifying components of the donor immune system. However, a greater emphasis on damaged target tissues may be especially crucial in SR-GVHD, in which profound injury likely participates in clinical pathology. This alters the focus of treatment toward restoration of organ function necessary for functional recovery (alimentation, reduced infection). In 1 study, lithium, capable of promoting Wnt signaling, produced a 50% CR rate in patients with GI mucosal denudation.157 In subset analysis, all patients receiving lithium within 3 days of identifying denuded mucosa had durable CR. Unfortunately, there is a paucity of available agents capable of inducing Wnt signaling, as clinical development has focused on Wnt inhibition for cancer.158
Dysfunction of the mucosal barrier also promotes loss of immunoregulatory proteins that provide tissue protection. AAT, an acute-phase protein produced by the liver, has increased stool clearance in GI GVHD. In addition to its role as a serine protease inhibitor that prevents organ damage (congenital emphysema) by inhibiting neutrophil elastase, AAT also possesses immunomodulatory functions that suppress proinflammatory cytokines, attenuate DC function, and induce Tregs. Several independent laboratories have demonstrated that exogenous AAT infusion can reduce GVHD-related mortality.159-161 As second-line therapy, 2 trials of AAT demonstrated response rates of 65% (including 50% CR in the GI tract) with low infectious mortality (10% at 6 months).162,163 Placebo-controlled trials of AAT are currently under way in the treatment of high-risk GVHD (NCT04167514). Finally, although we have focused on pharmacological means for treating SR-GVHD, the use of cell-based therapies, Tregs, iNKT, and mesenchymal stem cells (MSCs) hold potential to promote tissue repair.164 Given early success in prevention,165,166 adoptive transfer or in vivo expansion of Tregs remains an attractive strategy, owing to their ability to mitigate GVHD without abrogating CD8+ T-cell–killing function. For example, approaches include targeting TNF superfamily receptor TNFRSF25 using the TL1A-immunoglobulin fusion protein or facilitating iNKT-Treg interactions with a synthetic TCR ligand (α-GalCer),167,168 although generating adequate cell numbers for suppressive activity will remain a challenge. With respect to MSCs, a large meta-analysis composed of nonrandomized studies reported a cumulative survival of 63% at 6 months.169 The immunosuppressive mechanisms remain to be elucidated but may relate to MSC sensitivity to apoptosis, which in turn can promote phagocytosis by host MFs and release of indoleamine 2,3-dioxygenase.170 Further mechanistic and controlled trials are needed to establish the role of MSCs in SR-GVHD.
Can we prevent SR-GVHD?
Treatment of SR-GVHD will remain a challenge despite advances, emphasizing the importance of prevention. Rates of severe GVHD have declined due to use of high-resolution HLA typing, use of ATG, and perhaps the recent application of posttransplant cyclophosphamide.171,172 Although this indirectly impacts SR-GVHD, whether such approaches improve survival is uncertain. Extending concepts of tissue regeneration to tolerance (prevention) may increase thresholds for GVHD, reducing requirements for immune suppression. Approaches that selectively mitigate tissue response to sterile inflammatory mediators (DAMPs) by targeting Siglec-10 on host APCs have been shown to reduce GVHD in murine models.173,174 Use of a CD24 fusion protein (CD24Fc) is now undergoing clinical testing for the reduction of grade III-IV GVHD (NCT02663622). Another approach is to address microbiome-metabolome dysfunction. Here, altering the microbiome to protect vital cell populations (ISCs) may prevent irrevocable injury. For example, preclinical data suggest that short-chain fatty acids, specifically dietary butyrate or butyrate-producing clostridia, are a key energy source for ISCs that prevent GI GVHD.31 Trials testing modification of the host microbiome metabolome through ingestion of resistant starch (NCT02763033) and dietary fructo-oligosaccharides (NCT02805075) are being evaluated for GVHD prevention. Interventions involving transfer of fecal microbiota (FMT) are also associated with responses in SR-GVHD,99,101 but need to address antibiotic use, repeat administrations, and infectious risks in the preventative setting. IL-22 antagonists that induce epithelial regeneration could be logically advanced to the prevention setting because their function depends on intact ISCs. In the future, exploring what combinations of agents (IL-22, Wnt agonists) might best shape the host microbiome to preserve target tissues will be interesting.175
Concluding remarks
After several decades, incremental advances in the fundamental mechanistic underpinnings of GVHD, together with new therapeutic approaches that target dysregulated immune biology, bring renewed optimism for progress in SR-GVHD. Strategies that selectively reshape the composition of alloreactive T cells and APCs by targeting key inflammatory mediators (IL-6), that influence cytokine-driven signal transduction (JAK/STAT), or that target organ homing have displayed impressive response rates coupled with manageable toxicity and are currently undergoing testing in larger controlled trials. Despite these advances, SR-GVHD will remain a vexing challenge, owing to its complex biology and often irreversible organ injury; thus, future directions must continue to emphasize methods to prevent or preemptively treat high-risk disease. Future innovation that can leverage emerging knowledge of the host microbiome, tissue-protective strategies, and cellular engineering as well as noninvasive diagnostics to detect subclinical disease hold significant promise for advancing the field. Finally, although common to HCT, SR-GVHD remains an orphan disease with limited numbers of patients to appropriately power larger efficacy trials. Given these limitations, prioritizing the most promising strategies based on compelling biology, rigorous preclinical data, and well-conducted phase 1/2 designs will be important to advance therapies with the greatest potential for success.
Acknowledgments
The authors thank E. Matsuki for kindly drawing the figure. The authors acknowledge that, given space and citation constraints, some relevant published work was omitted.
This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI Grant-in-Aid for Research Activity Start-up grant number JP17H06537 (T.T.), the Mochida Memorial Foundation for Medical and Pharmaceutical Research (T.T.), the Ichiro Kanehara Foundation (T.T.), the SENSHIN Medical Research Foundation (T.T.), the Japanese Society of Hematology research grant (T.T.), the Friends of Leukemia Research Fund (T.T.), National Institutes of Health National Institute of Allergy and Infectious Diseases grant K23 AI123595-04 (J.M.), and a Rogel Cancer Center Scholarship (J.M.).
Authorship
Contribution: T.T. and J.M. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tomomi Toubai, Division of Hematology and Cell Therapy, Department of Internal Medicine III, Faculty of Medicine, Yamagata University, 2-2-2 Iida-Nishi, Yamagata, 990-9585, Japan; e-mail: toubai@med.id.yamagata-u.ac.jp; or John Magenau, Division of Hematology and Oncology, Department of Internal Medicine, University of Michigan Medical School, 1500 E. Medical Center Dr, Ann Arbor, MI 48109; e-mail: johnmage@med.umich.edu.

![Mechanisms of SR-GVHD. (A) Cellular mechanisms. Steroids regulate the majority of Th1 responses but can paradoxically increase Th2 and pathogenic Th17-mediated immune responses that may promote GVHD. The role of steroids in CD8+ T cells is uncertain. The combination of steroids with calcineurin inhibitor (CNI) may unintentionally blunt induction of Tregs based on their requirement for IL-2 resulting in loss of peripheral tolerance. (B) Molecular mechanisms. Steroids repress expression of TFs necessary for production of proinflammatory cytokines (IL-6, TNFα). In addition, steroids promote induction of regulatory cell subsets, such as CD103+ DCs and M2 MFs that induce immune tolerance. In refractory disease, long-term use of steroids may paradoxically increase expression of TLRs and NLRP3 that perpetuate inflammation. (C) Target tissue–intrinsic mechanisms. In the GI tract, steroids can impede the reparative processes of the host following T-cell–mediated injury that is associated with loss of Paneth cells, ISCs, and immune-regulatory proteins (α-1-antitrypsin [AAT]). Limited tissue regeneration from long-term suppression of inflammation with ongoing mucosal barrier injury is associated with alterations in the intestinal microbiome and metabolome. Dysbiosis results in loss of protective metabolites (butyrate). Ongoing inflammation can eventually stimulate APCs to increase production of proinflammatory cytokines that further damage host tissue. AP-1, activator protein 1; GCR, glucocorticoid receptor; ROS, reactive oxygen species; Tc, cytotoxic T cell; TCR, T-cell receptor.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/136/4/10.1182_blood.2019000953/1/m_bloodbld2019000953cf1.png?Expires=1769154075&Signature=Ki1WG8rmr9jaN0NKKPW~1sQomn6PkHhSAUAKS3d5sKKpA-JfjqfrHNlILi-n2mZZtw04k-WMAOihkNlavhZERmEzFdTzFKiMgDzNYk2vYi9LV2~8WTT3hFFs9q-UgWSPdLWWD8RwL8fr7A1oRumM9JSXWH0jH1Ju~k4t4Qut1s2lYzUhx4Mx0aOOhQq-J0UgCZOTgLCJQGPJFXruNcEfsqsDNEnPzmftnhRXIJj~HURWnd9~5ghjtrvn7~Vlu5fVMRToGJDJCoNVWuduL25vAtyTporxCpDVQTFbXrNSkY1jvEGiVNc~~R-eDgGGEWk0PdzWI~DG-0d2Cc4tGhXkDg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal