Abstract
Allogeneic hematopoietic stem cell transplantation (alloSCT) is an important curative therapy for high-risk hematological malignancies, but the development of severe and/or steroid-refractory acute graft-versus-host disease (aGVHD) remains a significant limitation to optimal outcomes. New approaches to prevent and treat aGVHD remain an unmet need that can be best addressed by understanding the complex disease pathophysiology. It is now clear that chemoradiotherapy used prior to alloSCT induces the release of endogenous alarmins (eg, HMGB-1, ATP, IL-1α, IL-33) from recipient tissue. Exogenous pathogen-derived molecules (eg, lipopolysaccharide, nucleic acids) also translocate from the gastrointestinal tract lumen. Together, these danger signals activate antigen-presenting cells (APCs) to efficiently present alloantigen to donor T cells while releasing cytokines (eg, interleukin-12 [IL-12], IL-23, IL-6, IL-27, IL-10, transforming growth factor-β) that expand and differentiate both pathogenic and regulatory donor T cells. Concurrent costimulatory signals at the APC–T-cell interface (eg, CD80/CD86-CD28, CD40-CD40L, OX40L-OX40, CD155/CD112-DNAM-1) and subsequent coinhibitory signals (eg, CD80/CD86-CTLA4, PDL1/2-PD1, CD155/CD112-TIGIT) are critical to the acquisition of effector T-cell function and ensuing secretion of pathogenic cytokines (eg, IL-17, interferon-γ, tissue necrosis factor, granulocyte-macrophage colony-stimulating factor) and cytolytic degranulation pathway effectors (eg, perforin/granzyme). This review focuses on the combination of cytokine and costimulatory networks at the T-cell surface that culminates in effector function and subsequent aGVHD in target tissue. Together, these pathways now represent robust and clinically tractable targets for preventing the initiation of deleterious immunity after alloSCT.
Introduction
Allogeneic hematopoietic stem cell transplantation (alloSCT) remains a curative therapy for high-risk hematological malignancies but risks of transplant-related mortality (TRM), largely from graft-versus-host disease (GVHD) and infection, remain. Systemic steroids, the standard treatment of acute GVHD (aGVHD), increase the risk of infection,1 and the prognosis of steroid-refractory aGVHD remains poor with a 2-year overall survival of 25%.2 Thus, preventing severe and steroid-refractory aGVHD is crucial to improving survival.
The pathophysiology of aGVHD generally can be thought of in 3 phases.3 In phase 1, conditioning chemoradiotherapy induces tissue damage and the release of inflammatory cytokines (eg, interleukin-6 [IL-6], tissue necrosis factor [TNF]), alarmins (eg, high-mobility group box-1 [HMGB1], IL-1α, IL-33), and pathogen-associated molecular pattern (PAMP) molecules that enhance alloantigen presentation by host antigen-presenting cells (APCs). In phase 2, transplanted donor CD8 and CD4 T cells recognize alloantigens presented within HLA class I and II, respectively, activate, expand, and differentiate into effector T cells. In phase 3, cytokines (eg, interferon-γ [IFN-γ], TNF, IL-2, IL-17) secreted by differentiated effector T cells and phagocytes (including monocytes and macrophages) contribute to target tissue apoptosis.4
We have focused this review on costimulation (both cell surface ligands and soluble cytokines) at the APC–T-cell synapse as outlined in detail in “The role of costimulatory and coinhibitory pathways in aGVHD.” We provide context for the cytokine-dependent activation of the APCs prior to this in “The initiation of inflammation by pretransplant conditioning,”, together with a brief context for the role of (T-cell–derived) cytokines in mediating tissue pathology during GVHD in “The impact of T-cell–derived cytokines on GVHD.” The review focuses on donor T cells, and we direct the reader to excellent reviews on the subject of natural killer cell activation and function after alloSCT.5,6
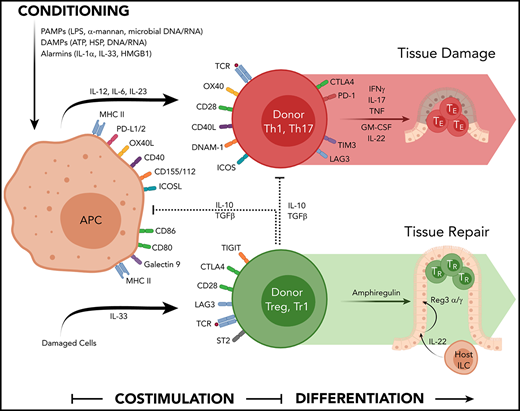
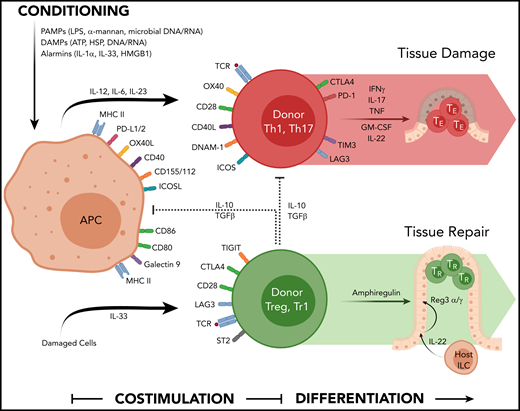
Multiple signals are required in the second phase of the GVHD process to enable full-donor T-cell activation and the acquisition of effector function. Signal 1 to the T cell involves the ligation of the T-cell receptor (TCR) by major histocompatibility complex (MHC)–peptide complex on the recipient APC surface. Signal 2 involves costimulation delivered by interaction of costimulatory molecules on the recipient APC surface to their cognate ligand expressed by the donor T cell (eg, CD80/CD86-CD28, CD40-CD40L). A third signal is mediated by cytokines secreted by APCs and T cells (eg, IL-2, IL-12, IL-23, IL-6)7 that bind their relevant receptor on donor T cells to induce proliferation and differentiation into effector T cells. These pathways are depicted pictorially in Figure 1.
The cytokine and costimulatory pathways involved in acute GVHD. Chemoradiotherapy used in conditioning liberates a number of DAMP and PAMP signals that contribute to hematopoietic and nonhematopoietic APC activation (antigen presentation, costimulation molecule expression, and cytokine secretion). Allogeneic peptides presented in MHC are recognized by the TCR on conventional donor T cells in conjunction with a suite of costimulatory molecules (CD40-CD40L, CD80/86-CD28, OX40L-OX40, ICOSL-ICOS) that together with appropriate cytokine signals (eg, IL-12, IL-6, IL-23) drives T-cell differentiation and their secretion of effector cytokines (eg, IFN-γ, IL-17, TNF, GM-CSF) that invoke local inflammation to mediate target tissue apoptosis directly and by recruitment of additional immune effector cells. The activation of Tregs involves MHC/peptide-TCR interactions, costimulatory and coinhibitory molecule engagement (eg, TIGIT and CTLA-4), and additional cytokine signals from IL-33 that enhance cytokine secretion mediating regulatory and repair function (eg, IL-10/TGF-β and amphiregulin, respectively). Over time, many of these coinhibitory molecules are expressed by conventional T effector cells.
The cytokine and costimulatory pathways involved in acute GVHD. Chemoradiotherapy used in conditioning liberates a number of DAMP and PAMP signals that contribute to hematopoietic and nonhematopoietic APC activation (antigen presentation, costimulation molecule expression, and cytokine secretion). Allogeneic peptides presented in MHC are recognized by the TCR on conventional donor T cells in conjunction with a suite of costimulatory molecules (CD40-CD40L, CD80/86-CD28, OX40L-OX40, ICOSL-ICOS) that together with appropriate cytokine signals (eg, IL-12, IL-6, IL-23) drives T-cell differentiation and their secretion of effector cytokines (eg, IFN-γ, IL-17, TNF, GM-CSF) that invoke local inflammation to mediate target tissue apoptosis directly and by recruitment of additional immune effector cells. The activation of Tregs involves MHC/peptide-TCR interactions, costimulatory and coinhibitory molecule engagement (eg, TIGIT and CTLA-4), and additional cytokine signals from IL-33 that enhance cytokine secretion mediating regulatory and repair function (eg, IL-10/TGF-β and amphiregulin, respectively). Over time, many of these coinhibitory molecules are expressed by conventional T effector cells.
The initiation of inflammation by pretransplant conditioning
Current conditioning therapies employ cytotoxic chemoradiotherapy to eradicate residual malignant cells, generate space for donor cell engraftment, and prevent immunological graft rejection. Although hematopoiesis-targeted conditioning may reduce tissue damage,8-12 in clinical practice, cytotoxic therapy (myeloablative or reduced intensity conditioning) is still required for stable engraftment. Cytotoxic conditioning provokes the release of damage associated molecular patterns (DAMPs) and/or alarmin proteins from damaged tissues and promotes the translocation of PAMP molecules across impaired mucosal barriers, particularly within the gastrointestinal (GI) tract. DAMPs include nucleic acids, intracellular proteins (eg, HMGB1, heat shock proteins, histone, actin, adenosine triphosphate [ATP], and reactive oxygen species), and extracellular proteins (eg, hyaluronic acid and biglycan), which bind to sensing receptors, including toll-like receptors (TLRs), NOD-like receptors (NLRs), retinoic acid inducible gene I (RIG-I)-like receptors, C-type lectin receptors, and the receptor for advanced glycation end products (RAGE).13 Alarmins are constitutively expressed endogenous molecules, including some cytokines (IL-1α and IL-33), that are released by degranulation from injured or dead cells.14 PAMPs include bacterial (eg, lipopolysaccharide [LPS], lipoproteins, peptidoglycan, and flagellin), fungal components (eg, β-glucans, α-mannans), and viral nucleic acids.15 In animal models, GVHD has been shown to be exacerbated by the following DAMPs/PAMPs or their signaling pathways: LPS,16 viral/bacterial DNA-mimicking CpG oligodeoxynucleotides,17 α-mannans,18 ATP,19 uric acid,20 NLRP3,20 the ASC inflammasome adaptor protein,20 RAGE21 which is the receptor for HMGB1 and the TLR-signaling molecules, MyD88 and TRIF.21,22 These tissue and microbiota-derived molecules stimulate recipient cells to secrete further cytokines (eg, TNF, IL-1, IL-6, IL-33, IL-12, IL-23, type1 IFN) and chemokines (eg, CCL5), which enhance alloantigen presentation and the expression of costimulatory molecules and cytokines by recipient APCs. Some of the critical and clinically tractable pathways are described in more detail below and in Table 1.
Potential cytokine inhibition/agonists applicable to the prevention of aGVHD
| Target . | Reagent . | Disease setting . | Clinical trial status/outcome . | Clinical Trial ID . | Reference . |
|---|---|---|---|---|---|
| TNF | TNFR-Fc | GVHD prophylaxis | Phase 2, II-IV aGVHD 46%, but steroid-refractory GI GVHD is 23% | NCT00639717 | 26,27 |
| TNFR-Fc | GVHD treatment (as initial therapy) | Phase 2, TNFR-Fc was inferior to MMF with lower CR | NCT00224874 | 25 | |
| IL-1β | Human rIL-1Ra | GVHD prophylaxis | Phase 3, no reduction in the incidence of aGVHD | 31 | |
| IL-33R | Anti-ST2 Ab | COPD | Phase 2, active, not recruiting | NCT03615040 | |
| IL-6R | Anti-IL-6 receptor Ab | GVHD prophylaxis | Phase 1/2, low rates grade II-IV aGVHD | NCT02206035* | 24,124* |
| IL-12/23 p40 | Anti-IL-12/23 p40 Ab | GVHD prophylaxis | Phase 1/2, improved OS and GRFS | NCT01713400 | 134 |
| IL-23 p19 | Anti-IL-23p19 Ab | Psoriasis | Phase 3, improved efficacy compared with anti-TNFα Ab | NCT02207231 | 135 |
| NCT02207244 | |||||
| Ulcerative colitis | Phase 2/3, active, recruiting | NCT04033445 | |||
| Phase 2, active, recruiting | NCT03662542 | ||||
| Crohn disease | Phase 2/3, active, recruiting | NCT03466411 | |||
| IL-17A | Anti-IL-17A Ab | Active rheumatoid arthritis | Phase 3, secukinumab was inferior to abatacept with lower response rates | NCT01350804 | 154 |
| Ankylosing spondylitis (AS) | Phase 3, improved AS symptoms and low mean progression in spinal radiographic change | NCT01358175 | 153 | ||
| Plaque psoriasis | Phase 3, effective in 2 randomized trials | NCT01365455 | 152 | ||
| NCT01358578 | |||||
| IL-2 | Anti-CD25 Ab | GVHD prophylaxis | Phase 3, no reduction in aGVHD | 144 | |
| IL-22 | Human IL-22 IgG2-FC | GVHD treatment (II to IV lower GI tract GVHD) | Phase 2a, active, not recruiting | NCT02406651 | |
| IL-2 | Human rIL-2 | GVHD prophylaxis | Phase 2, eliminated grade II-IV aGVHD (0% vs 13%) | NCT00539695 | 142 |
| Phase 2, no reduction in the aGVHD | NCT01927120 | 143 |
| Target . | Reagent . | Disease setting . | Clinical trial status/outcome . | Clinical Trial ID . | Reference . |
|---|---|---|---|---|---|
| TNF | TNFR-Fc | GVHD prophylaxis | Phase 2, II-IV aGVHD 46%, but steroid-refractory GI GVHD is 23% | NCT00639717 | 26,27 |
| TNFR-Fc | GVHD treatment (as initial therapy) | Phase 2, TNFR-Fc was inferior to MMF with lower CR | NCT00224874 | 25 | |
| IL-1β | Human rIL-1Ra | GVHD prophylaxis | Phase 3, no reduction in the incidence of aGVHD | 31 | |
| IL-33R | Anti-ST2 Ab | COPD | Phase 2, active, not recruiting | NCT03615040 | |
| IL-6R | Anti-IL-6 receptor Ab | GVHD prophylaxis | Phase 1/2, low rates grade II-IV aGVHD | NCT02206035* | 24,124* |
| IL-12/23 p40 | Anti-IL-12/23 p40 Ab | GVHD prophylaxis | Phase 1/2, improved OS and GRFS | NCT01713400 | 134 |
| IL-23 p19 | Anti-IL-23p19 Ab | Psoriasis | Phase 3, improved efficacy compared with anti-TNFα Ab | NCT02207231 | 135 |
| NCT02207244 | |||||
| Ulcerative colitis | Phase 2/3, active, recruiting | NCT04033445 | |||
| Phase 2, active, recruiting | NCT03662542 | ||||
| Crohn disease | Phase 2/3, active, recruiting | NCT03466411 | |||
| IL-17A | Anti-IL-17A Ab | Active rheumatoid arthritis | Phase 3, secukinumab was inferior to abatacept with lower response rates | NCT01350804 | 154 |
| Ankylosing spondylitis (AS) | Phase 3, improved AS symptoms and low mean progression in spinal radiographic change | NCT01358175 | 153 | ||
| Plaque psoriasis | Phase 3, effective in 2 randomized trials | NCT01365455 | 152 | ||
| NCT01358578 | |||||
| IL-2 | Anti-CD25 Ab | GVHD prophylaxis | Phase 3, no reduction in aGVHD | 144 | |
| IL-22 | Human IL-22 IgG2-FC | GVHD treatment (II to IV lower GI tract GVHD) | Phase 2a, active, not recruiting | NCT02406651 | |
| IL-2 | Human rIL-2 | GVHD prophylaxis | Phase 2, eliminated grade II-IV aGVHD (0% vs 13%) | NCT00539695 | 142 |
| Phase 2, no reduction in the aGVHD | NCT01927120 | 143 |
COPD, chronic obstructive pulmonary disease; CR, complete response; GRFS, GVHD/relapse-free survival; OS, overall survival.
Drobyski et al124 is related to NCT02206035.
TNF is a pivotal dysregulated cytokine in response to total body irradiation (TBI) in preclinical systems, and inhibition during conditioning can potently inhibit GVHD.23 In contrast, systemic TNF dysregulation is generally not seen in patiebly due to the influence of GVHD prophylaxis,24 and results of clinical studies of TNF inhibition25 have been less dramatic than in murine systems. Phase 2 trials comparing the addition of etanercept to standard GVHD prophylaxis (starting at conditioning),26 or the combination of etanercept (starting on the day of transplant) plus extracorporeal photopheresis,27 have suggested possible reductions in the incidence of steroid-refractory aGVHD without reductions in the incidence of grade II to IV aGVHD.
Activation of the inflammasome pathway (eg, by ATP, crystals [eg, uric acid], NLR family members) is required for IL-1β and IL-18 production.28,29 Preclinical studies have demonstrated that IL-1β promotes GVHD,20 and IL-1β blockade can attenuate GVHD, particularly in conditioning containing cyclophosphamide.30 However, IL-1 blockade commencing during conditioning for up to 2 weeks did not reduce GVHD in a randomized clinical study.31 Most IL-18 produced early posttransplant is recipient-derived32 and in concert with IL-12, IL-18 is critical for T helper type 1 cell (Th1) differentiation and IFN-γ secretion.33 Exogenous IL-18 administration prior to conditioning has been demonstrated to invoke Fas-dependent donor CD4+ T-cell death, that attenuates MHC class II–dependent GVHD while preserving MHC class I–dependent graft-versus-leukemia (GVL) effects.34,35 However, opposing effects that exaggerate CD8+-mediated GVHD36 suggest this is unlikely to be clinically tractable. Thus, it remains to be seen whether modulation of the inflammasome pathway is practical in patients.
IL-33 is an IL-1 family member that is constitutively expressed in the nuclei of endothelial and epithelial cells and released in response to tissue injury.37 IL-33 binds to the IL-33 receptor (also known as growth stimulation expressed gene 2 or serum stimulation-2 [ST2]),38 which has membrane-bound and soluble (sST2) forms. In animal models, conditioning induced intraintestinal IL-33 early posttransplant.39 IL-33 administration preconditioning increased the numbers of recipient regulatory T cells (Tregs) and attenuated aGVHD,40 whereas IL-33 administration postconditioning,39 or preconditioning in the absence of Treg,40 exacerbated GVHD lethality due to enhanced expansion of effector T cells. Thus, the regulatory effects of IL-33 are via the expansion of ST2+ Tregs with enhanced regulatory function,41 together with their expression of amphiregulin42 that enhances tissue repair.43 In contrast, IL-33 can promote inflammation via enhancing type 1 differentiation of T cells.44,45 Importantly, sST2 is a biomarker of severe GVHD46 that acts to antagonize IL-33. Indeed, ST2 inhibition with a monoclonal antibody (Ab) that neutralizes sST2 has been shown to attenuate aGVHD by promoting IL-33 signaling in Th2s and Tregs.47 Well-designed studies will be required to ascertain whether these complex contextual effects of IL-33 can be exploited clinically.
Type I IFN can be generated via endogenous nucleic acid–dependent intracellular signaling of the cyclic guanosine monophosphate–adenosine monophosphate synthase (cGAS)/stimulator of interferon genes (STING) pathway or the RIG-I/mitochondrial antiviral-signaling protein (MAVS) pathway. Type I IFN can also be secreted in response to TLR-ligation at the cell surface by exogenous nucleic acids, such as CpG motifs.48 The absence of MAVS in alloSCT recipients results in enhanced TBI-induced intestinal damage, suggesting that TBI-driven host type I IFN production is protective in tissue.49 Type I IFN signaling of recipient hematopoietic cells (putatively dendritic cells [DC]) also protects recipients from CD4+-mediated GVHD but enhances CD8+ T-cell–mediated GVHD and GVL.50 At this point, further experimental information is needed to understand how type I IFNs modulate GVHD, and the outcome of type I IFNs or RIG-I agonists on clinical GVHD are difficult to predict. Indeed, the administration of type I IFNs after alloSCT are useful to enhance GVL in patients with relapsed leukemia but do so at the expense of GVHD.51
The role of costimulatory and coinhibitory pathways in aGVHD
The activation and expansion of alloreactive donor T cells requires cognate costimulation at the APC–T-cell synapse. Many of these costimulatory molecules are members of the immunoglobulin superfamily (IgSF) or TNF/TNF receptor superfamilies (TNFSF/TNFRSF).52-54 IgSF members can mediate stimulatory or inhibitory signaling, whereas most TNFSF/TNFRSF signals are stimulatory only, and these pathways are described in detail below and in Figure 1 and Table 2.
Potential costimulatory molecule inhibition in aGVHD
| Target . | Reagent . | Disease setting . | Study or clinical trial status/outcome . | Clinical Trial ID . | Reference . |
|---|---|---|---|---|---|
| CD80/86 | CTLA4-Ig | GVHD prophylaxis | Phase 2, low rate of II-IV aGVHD (2 of 10 patients) | NCT01012492 | 65 |
| Phase 2 (randomized), reduced grade III/IV aGVHD and improved GFS, particularly after 7/8 MUD | NCT01743131 | 66 | |||
| CD28 | Anti-CD28 Fab Ab | NHP: GVHD prophylaxis | Preclinical, 100% GVHD-free survival in combination with sirolimus, but infection-related death is high | 68 | |
| Anti-CD28 Fab Ab | Healthy subjects | Phase 1, safe and tolerated | NCT02800811 | 69 | |
| ICOSL | Anti-ICOS ligand Ab | SLE | Phase 1b, safe, not powered for efficacy | NCT01683695 | 75 |
| CD40 | Anti-CD40 Ab | NHP: GVHD prophylaxis | Preclinical, combination with CTLA4-Ig and sirolimus, reductions in GVHD | 63 | |
| Anti-CD40 Ab | Rheumatoid arthritis | Phase 2a, safe, not powered for efficacy | NCT01751776 | 105 | |
| CD40L | Anti-CD40L Ab | GVHD prophylaxis | Phase 1/2, recruiting | NCT03605927 | |
| OX40L | Anti-OX40L Ab | NHP: GVHD prophylaxis | Preclinical, combination with sirolimus, no clinical GVHD seen | 113 | |
| Anti-OX40L Ab | Atopic dermatitis | Phase 2, recruiting | NCT03754309 | ||
| DLL4 | Anti-DLL4 Ab | NHP: GVHD prophylaxis | Preclinical, anti-DLL4 Ab monotherapy, prolonged OS | 118 |
| Target . | Reagent . | Disease setting . | Study or clinical trial status/outcome . | Clinical Trial ID . | Reference . |
|---|---|---|---|---|---|
| CD80/86 | CTLA4-Ig | GVHD prophylaxis | Phase 2, low rate of II-IV aGVHD (2 of 10 patients) | NCT01012492 | 65 |
| Phase 2 (randomized), reduced grade III/IV aGVHD and improved GFS, particularly after 7/8 MUD | NCT01743131 | 66 | |||
| CD28 | Anti-CD28 Fab Ab | NHP: GVHD prophylaxis | Preclinical, 100% GVHD-free survival in combination with sirolimus, but infection-related death is high | 68 | |
| Anti-CD28 Fab Ab | Healthy subjects | Phase 1, safe and tolerated | NCT02800811 | 69 | |
| ICOSL | Anti-ICOS ligand Ab | SLE | Phase 1b, safe, not powered for efficacy | NCT01683695 | 75 |
| CD40 | Anti-CD40 Ab | NHP: GVHD prophylaxis | Preclinical, combination with CTLA4-Ig and sirolimus, reductions in GVHD | 63 | |
| Anti-CD40 Ab | Rheumatoid arthritis | Phase 2a, safe, not powered for efficacy | NCT01751776 | 105 | |
| CD40L | Anti-CD40L Ab | GVHD prophylaxis | Phase 1/2, recruiting | NCT03605927 | |
| OX40L | Anti-OX40L Ab | NHP: GVHD prophylaxis | Preclinical, combination with sirolimus, no clinical GVHD seen | 113 | |
| Anti-OX40L Ab | Atopic dermatitis | Phase 2, recruiting | NCT03754309 | ||
| DLL4 | Anti-DLL4 Ab | NHP: GVHD prophylaxis | Preclinical, anti-DLL4 Ab monotherapy, prolonged OS | 118 |
GFS, aGVHD-free survival; MUD, matched unrelated donor; NHP, nonhuman primate; SLE, systemic lupus erythematosus.
IgSF costimulation
CD28 and cytotoxic T-lymphocyte associated protein 4 (CTLA4) are expressed by T cells; CD28 is expressed constitutively on naive CD4+ and CD8+ T cells, and ligation by CD80/86 (B7-1/B7-2) provides the second signal for T-cell growth and survival. In contrast, CTLA4 is induced on activated conventional T cells to provide inhibitory signals52 but is constitutively expressed on Treg.55 Lethal T-cell–mediated aGVHD is almost completely prevented in murine studies when common CD28/CTLA4 ligands, CD80 and CD86, are blocked,56 while aGVHD is only partially reduced when mice received genetically CD28-deficient donor T cells,57,58 likely related to the fact that Treg development and function require constitutional CD28 expression.59-61 Administration of CTLA4-Ig, a fusion protein that binds CD80 and CD86 to block interaction with CD28/CTLA4, does not completely prevent GVHD.62 A nonhuman primate study demonstrated the efficacy of a CTLA4-Ig, anti-CD40 Ab and sirolimus combination for the prevention of aGVHD.63 Indeed, CTLA4-Ig (abatacept) has produced promising clinical results when combined with standard GVHD prophylaxis,64,65 and a randomized phase 2 study found significantly decreased grade II to IV aGVHD after HLA-matched alloSCT.66 Of note, CTLA4-Ig concurrently blocks tolerogenic CTLA4-dependent signaling to Tregs and APCs,67 while CD28-specific inhibition is permissive of this pathway, allowing CD80/86 to continue to bind CTLA4. In nonhuman primates, combining pegylated anti-CD28 Ab (FR104) with sirolimus produced superior GVHD-free survival to a CTLA4-Ig/sirolimus combination, although beneficial effects were limited by infection-related deaths.68 FR104 appears safe in healthy human subjects69 but has not been tested in disease settings. The modulation of this pathway thus shows great promise, although questions in relation to the optimal reagent and timing of administration remain.
Inducible costimulator (ICOS; CD278) binds to ICOS ligand (also known as B7H, B7H2, or B7RP1) and is expressed on activated T cells and constitutively in Tregs and memory CD4+ T cells, where it controls the size of the T-cell pool.70 In murine models, animals receiving ICOS-deficient donor CD4+ T cells or anti-ICOS inhibition develop attenuated aGVHD, with less Th1 cytokine production. In contrast, ICOS-deficient CD8+ T cells (or inhibition during MHC class I–dependent GVHD) promote GVHD by enhancing CD8 T-cell expansion.71-73 This may relate to the fact that CD4+ T cells use PI3K-dependent ICOS signaling, whereas CD8+ T cells use a PI3K-independent pathway.74 Nonetheless, based on these preclinical results, ICOS blockade may attenuate aGVHD when combined with other immune suppressants, such as CTLA4-Ig73 or CD28 inhibition.68 The safety and potential efficacy of anti-ICOS ligand Ab, AMG557, have recently been confirmed in patients with systemic lupus erythematosus.75
The simultaneous interaction of costimulatory/inhibitory molecules often exerts complex effects. B and T lymphocyte attenuator (BTLA) binds to a TNFRSF member, herpes virus entry mediator (HVEM), which binds BTLA and other molecules, lymphotoxin-like inducible protein that competes with glycoprotein D for herpesvirus entry on T cells (LIGHT), and lymphotoxin-α. These ligands and receptors are expressed on T cells and can bind in cis or trans.52 BTLA can deliver inhibitory signals, but76 administration of either non-blocking77 or blocking76,78 anti-BTLA Abs can prevent GVHD in murine models, reducing T-cell cytotoxicity77 while expanding Foxp3+ Tregs,78 independent of HVEM-binding and its known prosurvival effect.77 On the other hand, a BTLA but not LIGHT-blocking anti-HVEM Ab reduced allogeneic T-cell reactions, including IFN-γ production and cytotoxicity,79 suggesting BTLA-HVEM interactions are inflammatory. Inhibitory agonists thus might be effective against aGVHD, but clinical-grade reagents have not yet been tested.
Costimulatory DNAX accessory molecule 1 (DNAM-1; CD226) is expressed primarily by natural killer and T cells.80 The DNAM-1/CD226 subfamily also includes coinhibitory molecules, T-cell immunoglobulin and immunoreceptor tyrosine inhibitory motif domain (TIGIT; VSTM3), and CD96 molecules, with shared receptors (CD155, CD112, CD111).80 DNAM-1–deficient donor CD8+ T cells81 and Tregs82 ameliorate GVHD in murine alloSCT models. At least 1 TIGIT blocking Ab has been shown to accelerate GVHD mortality.83 TIGIT blocking antibodies are currently being tested as checkpoint inhibitors in patients with cancer, but the inhibition of DNAM-1 or activation of TIGIT may be a useful strategy to prevent aGVHD. Indeed, after alloSCT, high levels of soluble DNAM-1 in sera are associated with grade II to IV aGVHD.84 Furthermore, high TIGIT expression in bone marrow after alloSCT was recently shown to correlate with reduced aGVHD but poor progression-free survival due to high levels of relapse.85
Programmed cell death protein 1 (PD-1), which binds to programmed death ligand-1 (PD-L1) and PD-L2, is expressed on activated T cells and is a coinhibitory molecule. When expressed in conjunction with functional defects (eg, cytokine secretion), PD-1 is a marker of T-cell exhaustion. Additional molecules expressed during exhaustion include TIM3 (which binds to Galectin 9) and LAG3 (which binds to MHC II). Thus, while PD-1–PD-L1 and CTLA4 inhibitors are currently the most widely used immune checkpoint inhibitors in clinical oncology practice, trials are underway with reagents that inhibit LAG3 and TIM3. In murine models, PD-1–deficient donor T cells or anti–PD-1 Ab treatment pre- or post-alloSCT increase GVL effects, but also augment GVHD severity86,87 and associated morbidity/mortality.88 PD-1 blockade has been widely used for patients with Hodgkin or non-Hodgkin lymphomas, and pretransplant PD-1 blockade is associated with severe aGVHD.89 These patients should receive posttransplant cyclophosphamide-based immune suppression.90,91 Patients with acute myeloid leukemia or myelodysplastic syndromes frequently undergo alloSCT and have shown susceptibility to PD-1 blockade during induction therapy,92 consistent with high PD-1 and TIGIT expression in CD8+ T cells from acute myeloid leukemia patients.93
V-domain immunoglobulin-containing suppressor of T-cell activation (VISTA) is a novel immune checkpoint ligand/receptor that is expressed on myeloid and T cells. Murine VISTA-Ig fusion protein inhibited cytokine production (IL-2 and IFN-γ) by CD4+ T cells and VISTA-Ig or VISTA-expressing APCs potently suppressed CD4+ and CD8+ T-cell proliferation in vitro.94 Prostate cancers from patients treated with ipilimumab (anti-CTLA4 Ab) increase PD-L1 and VISTA expression on tumor-infiltrated CD4+ and CD8+ T cells and macrophages.95 V-set and Ig domain containing 396,97 and P-selectin glycoprotein ligand-198 have recently been identified as VISTA receptors, and VISTA-Ig may represent a potent therapeutic agent for preventing GVHD. Other suppressive costimulatory pathways, such as B7-H4, have also recently been demonstrated to inhibit GVHD,99 although the cognate receptor on T cells awaits identification.
TNFRSF costimulation
CD40 (TNFRSF5) expressed on APCs binds to its cognate ligand (CD40L; CD154, gp39, or TNFSF5) on activated T cells.54 CD40 signaling potently enhances APC costimulatory expression (CD80/86), and CD40L is critical for B-cell class switching.100 The combination of anti-CD40L Ab and CTLA4-Ig facilitates long-term engraftment in murine cardiac and skin allogeneic transplants101 and is abrogated by calcineurin inhibition. In murine models, CD40L inhibition potently attenuates lethal aGVHD,102,103 especially in combination with CD28 deficiency or LIGHT blockade.103,104 In nonhuman primates, combined (blocking) anti-CD40 Ab, CTLA4-Ig and sirolimus attenuate TRM.63 A phase 2 study has demonstrated the safety of anti-CD40 Ab treatment (BI 655064) and improved end points in patients with rheumatoid arthritis.105 Thus, inhibition of the CD40-CD40L costimulatory pathway seems a highly tractable approach to prevent aGVHD now that a new generation of reagents that do not invoke thrombosis are available (ClinicalTrials: NCT03605927; anti-CD40L Ab; BMS-986004).106-108
The OX40 (CD134, TNFRSF4) is expressed on activated T cells and produces costimulatory signals upon binding to OX40L, expressed on activated APCs.53 Murine models have shown that OX40 induces effector T-cell expansion, without influencing natural Treg generation, and OX40 signaling in Treg perturbs their suppressive function.109,110 In contrast, OX40 inhibits induced Foxp3+ Treg generation from activated CD4+ T cells in a BATF-dependent and independent manner.111,112 This costimulatory pathway is a highly promising target for inhibition, with confirmation of efficacy also demonstrated in nonhuman primate models of alloSCT.113 In contrast to anti-CD40 Ab plus CTLA4-Ig/Sirolimus combinations that induced severe lymphopenia and poor Treg expansion after transplant,63 an OX40L Ab (KY1005) plus sirolimus combination achieved lymphocyte recovery equivalent to that after autologous transplant, and augmented Treg recovery.113 Together, these findings may explain the ability of OX40 inhibition to preserve Treg and T-cell reconstitution, making inhibition of this axis an attractive target after alloSCT.
Notch-dependent costimulation
Notch is an evolutionally conserved element of cell-to-cell interaction. Mammals have 4 Notch receptors (Notch 1 to 4) with 5 ligands of the Jagged (Jagged 1 and 2) and Delta-like (Delta-like ligand 1 [DLL1], DLL3, and DLL4) families.114 T-cell–specific expression of a pan-Notch inhibitor (DNMAML) reduces aGVHD lethality and tissue pathology in murine models, with attenuated cytokine secretion (IFN-γ, TNF-α, IL-2), despite the maintenance of T-cell proliferation and cytotoxicity that is permissive of GVL.115 The effects primarily relate to Notch 1 inhibition in donor T cells, although Notch 1 is also crucial for intestinal regeneration. DLL4 is largely expressed by nonhematopoietic stromal cells116 ; crypt regeneration was preserved and GVHD was inhibited following administration of an anti-DLL4/DLL1 blocking Ab.117 A subsequent nonhuman primate study confirmed this promise for DLL4 blockade as a strategy to prevent GVHD.118
Cytokine-dependent control of T-cell differentiation
IL-6 is a proinflammatory cytokine produced by multiple cell types, including endothelial cells, fibroblasts, keratinocytes, DC, macrophages, and T cells.119 Microbial products enhance IL-6 production in tissue,120,121 and systemic IL-6 levels are increased early after allogeneic transplant in animal models122,123 and patients,24,124 which is most profound after TBI-based conditioning.24 Recipient DC appear to be the major cellular source of IL-6 dysregulation125 but donor DC-derived IL-6 also plays a role in T cell expansion and differentiation during aGVHD.21 IL-6 and transforming growth factor-β (TGF-β) induce Th17 differentiation from naive T cells, and IL-6 inhibits TGF-β–driven Treg differentiation.126 Classical IL-6 signaling in donor T cells is critical for the generation of donor Th17/Tc17 and Th22 differentiation after bone marrow transplantation (BMT).125,127,128 Indeed, the beneficial effects of IL-6 inhibition in preclinical studies are related to increased donor Treg/type 1 regulatory T cells (Tr1) numbers125,129,130 and inhibition of Th17/Th22 differentiation.125 Anti–IL-6 receptor Ab (tocilizumab) shows promise in reducing the incidence of acute GVHD in phase 1/2 studies24,124 but requires confirmation in multicenter phase 3 studies.
IL-12 and IL-23 are proinflammatory cytokines that share p40 subunits and are recognized for their key roles in developing Th1 and Th17 subsets of helper CD4+ T cells, respectively. IL-27 is a heterodimeric member of the IL-12 family that contains the IL-27p28 subunit and is secreted by cells of the monocyte-macrophage lineage to drive both proinflammatory effector differentiation and type-1 regulatory T cells.130-132 In the presence of luminal microbiota, intestinal macrophages secrete IL-12/23p40, that induces IFN-γ by lamina propria lymphocytes to control MHC class II expression by intestinal epithelial cells, and this process is enhanced by conditioning irradiation.22 MHC class II expressed by intestinal epithelial cells can initiate CD4-dependent aGVHD in the GI tract and can be attenuated by IL-12/23p40 inhibition.22 IL-23 consists of p40 and p19 subunits. Mice receiving allografts and anti-p19 Ab administration (or IL-23p19 deficient grafts) have attenuated aGVHD in the colon.133 A randomized placebo-controlled clinical study administered anti–IL-12/23p40 Ab (ustekinumab) 1 day pretransplant and again at day 20 posttransplant, resulted in significant reduction of Th17 and Th1 differentiation ex vivo, although the study was not powered to detect reductions in aGVHD.134 Anti–IL-23p19 Ab (Guselkumab) has demonstrated efficacy in psoriasis.135 Of note, IL-23 promotes IL-22 production in the GI tract early after BMT that protects from epithelial damage,136 and so IL-12 itself may represent the most rational target for inhibition in clinical transplantation.
The impact of T-cell–derived cytokines on GVHD
Alloantigen-exposed donor CD4+ and CD8+ T cells differentiate into pathogenic type-1 cells (IL-2, IFN-γ–secreting Th1, and Tc1, respectively) and type-17 cells (IL-17 secreting Th17 and Tc17, respectively), which expedite rapid host cell elimination, promote engraftment and mediate tissue damage in GVHD target organs.137 Although other T-cell differentiation paradigms may play a role in GVHD (Th2, Tfh), they are generally less relevant to aGVHD, and the reader is referred to reviews of cGVHD for a detailed explanation of these lineages after alloSCT.21,138
IL-2 is primarily secreted by T cells, and particularly CD4+ T cells. The IL-2 receptor, which comprises 3 subunits (the α chain [CD25], β chain [CD122], and the γ subunit [CD132]), is expressed on thymus-derived Treg and antigen-activated T cells.139 IL-2 is critical for thymic Treg development and peripheral homeostasis, including T-cell differentiation. Calcineurin inhibitors, such as tacrolimus and cyclosporin, bind calcineurin and inhibit subsequent nuclear factor of activated T cells (NFAT)–dependent IL-2 transcription, resulting in the blocking of T-cell activation, expansion, and effector function.140 Monoclonal antibodies have been developed to block CD25, and like calcineurin inhibitors, block both conventional T cells but also Treg expansion, creating a potential for both positive and negative effects in transplantation. Conversely, low-dose IL-2 preferentially stimulates Treg and may be tolerogenic, at least when administered late after transplant.141 In contrast, the requirement for IL-2 in effector and regulatory T cell responses has translated to mixed effects on aGVHD when trying to augment or block this cytokine early after transplant.142-144 The effects of therapeutic IL-2 modulation in BMT would appear unpredictable in the absence of mutated IL-2 proteins or Ab with specificity to IL-2 receptor subunits (eg, CD25 agonists to promote Treg expansion).145 In preclinical studies, the transfer of IFN-γ–deficient donor T cells or anti–IFN-γ Ab administration accelerates GVHD lethality due to the promotion of lung injury (idiopathic pneumonia syndrome), but GVHD within the GI tract is markedly attenuated.123,146 Nevertheless, complete blockade of IFN-γ would seem impractical as a clinical strategy.
The IL-17 cytokine family includes 6 members: IL-17A to F; Th17 cell–secreted IL-17A and IL-17F are the best characterized members,147 and the pathogenic role of donor type 17 T cells in aGVHD is well established.127,128,148-150 IL-17 secreted by recipient lymphoid cells, including mucosal-associated invariant T (MAIT) cells, prevent dysbiosis and protect from gut GVHD.150,151 Therefore, although anti-IL17A Ab administration shows the efficacy in multiple autoimmune diseases,152-154 IL-17 blockade itself is unlikely to efficiently prevent GVHD due to the critical role of this cytokine in maintaining mucosal integrity and microbiome diversity.150 The emergence and persistence of donor Th17/Tc17 cells were noted during aGVHD in nonhuman primates following pharmacological GVHD prophylaxis with tacrolimus plus methotrexate.155 Since Tc17 cells exacerbate GVHD but do not mediate GVL, they are good targets to prevent GVHD127 and would require intervention targeting critical transcriptional factors (eg, RORγt) with small molecule inhibitors. During aGVHD, both Th17 and non-Th17 donor lineages are also a major source of granulocyte-macrophage colony-stimulating factor (GM-CSF). GM-CSF expands myeloid populations in peripheral lymph nodes, including donor DC with high alloantigen-presenting capacity that profoundly augments aGVHD in the GI tract.128,156,157 Thus, GM-CSF blockade may be an attractive target to prevent aGVHD, although effects on hematopoiesis would need to be considered.
The immunosuppressive cytokine, IL-10, attenuates tissue damage during inflammation.158 IL-10 is produced predominantly by donor T cells after alloSCT,159 specifically Foxp3+ Treg and Tr1 that are induced in the periphery.130 Although IL-10−/− donor T cells augment lethal GVHD in mice,130,160 systemic IL-10 administration has dose-dependent effects; low doses decrease and high doses enhance aGVHD.160 TGF-β is also an immunosuppressive cytokine that, in the absence of IL-6, promotes Treg differentiation126 and is critical for the induction of tolerance. In murine models, the neutralization of TGF-β early after transplant exacerbates aGVHD, whereas it attenuates fibrosis during chronic skin GVHD.161,162 Thus, inhibition of TGF-β is likely to be difficult to exploit clinically. Given these apparent narrow therapeutic windows, targeting IL-10 delivery to the GI tract, or the adoptive transfer of IL-10/TGF-β–producing regulatory cells (eg, Treg and Tr1) may be the most useful therapeutic approaches after alloSCT.
IL-22 is a member of the IL-20 cytokine subfamily within the larger IL-10 family, which uses receptors with a common subunit (IL-20 receptor β) and links the immune system with epithelial cells to enhance barrier function.158,163 IL-22 secretion by host intestinal innate immune cells (ILCs) protects intestinal stem cells from GVHD damage and promotes epithelial regeneration in a regenerating islet-derived 3γ (REG3γ, mouse homolog of human regenerating islet-derived 3α)-dependent manner, although IL-22–secreting host ILCs are eliminated during GVHD.136,164,165 The systemic administration of F-652, a recombinant human IL-22-dimer–Fc-fusion protein, or mouse recombinant IL-22 reduces gut GVHD in mice when administered after the onset of intestinal GVHD (day 7),164,165 and a clinical trial is underway (Clinical Trials: NCT02406651). In contrast, donor-derived IL-22 can exacerbate GVHD, particularly in the skin, although pathogenic effects in gut have also been suggested.166-168 These effects likely represent IL-22 secretion from pathogenic Th17/Th22 cells125,168 rather than protective ILC3 subsets136 but does represent a theoretical limitation to prolonged IL-22 administration.
Conclusion
The activation, expansion, and differentiation of donor T cells represent the pivotal pathway responsible for acute GVHD. This represents a complex process that starts during conditioning and continues until donor T cells acquire full effector function. The immunological process includes a number of pivotal stimulatory and inhibitory soluble cytokines and surface proteins within the interface of the APCs and T cell that can theoretically be inhibited with clinically available reagents, repurposed for transplantation. Logical targets include pivotal cytokines involved in antigen presentation and T-cell differentiation, such as IL-12, surface costimulatory molecules on APCs, such as CD80/CD86, CD40, OX40L, or their cognate costimulatory T-cell ligands (eg, CD28, CD40L, OX40). Indeed, this field has now generated numerous tractable clinical pathways that are outlined in Table 1 and Table 2. The challenge in the current era is to choose the most appropriate target and concurrent immune suppression, given the recent uptake of augmented immune suppression protocols, including posttransplant cyclophosphamide. It is important to note that meaningful separation of GVHD and GVL has yet to be achieved in the clinic, and early-phase single-arm studies are grossly underpowered and inappropriate to assess relapse. There thus remains a requirement for (ideally blinded) large phase 3 studies to assess promising new approaches to prevent GVHD. Toward the future, it is likely that combinations of therapies that block costimulation and T-cell differentiation may be synergistic; future studies will need to consider a stepwise approach. Finally, we will need to incorporate study end points that measure the potential benefits of using Ab-based approaches that may avoid toxicities inherent in our historical drug-based immune suppression.
Acknowledgments
The authors thank Laura Green of Fred Hutchinson Cancer Research Center for generation of the graphics.
This work was supported by a research grant from the National Institutes of Health (NIH), National Heart, Lung, and Blood Institute grant R01HL148164.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. G.R.H. is an Andy Hill CARE Distinguished Researcher.
Authorship
Contribution: M.K. and G.R.H. wrote the manuscript.
Conflict-of-interest disclosure: M.K. and G.R.H. have submitted a patent application on methods to prevent antigen presentation in the GI tract. G.R.H. has received funding from Roche for a clinical study of Tocilizumab in aGVHD prophylaxis.
Correspondence: Motoko Koyama, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, Seattle, WA 98109; e-mail: mkoyama@fredhutch.org; and Geoffrey R. Hill, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, Seattle, WA 98109; e-mail: grhill@fredhutch.org.