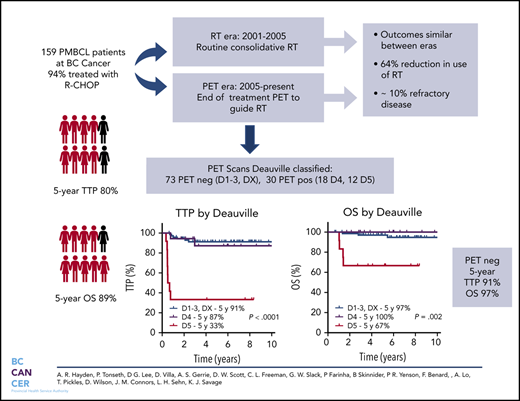
Key Points
PMBCL patients managed with R-CHOP and a PET-guided approach to consolidative RT have favorable outcomes with a 64% reduction in RT use.
Approximately 10% of PMBCL patients have refractory disease and may benefit from integration of novel therapies in the frontline setting.
Abstract
Cure rates for primary mediastinal large B-cell lymphoma (PMBCL) have improved with the integration of rituximab. However, the type of primary therapy and role of radiotherapy (RT) remains ill-defined. Herein, we evaluated the outcome of PMBCL primarily treated with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) and the impact of an end-of-treatment (EOT) 18F-fluorodeoxyglucose positron emission tomography (PET) scan to guide consolidative RT. Patients ≥18 years of age with PMBCL treated with curative intent rituximab-chemotherapy were identified. Prior to 2005, patients were recommended to receive R-CHOP + RT (RT era). Beginning in 2005, EOT PET was used to guide RT and only those with a PET-positive scan received RT (PET era). In total, 159 patients were identified, 94% were treated with R-CHOP and 44% received RT (78% in RT era, 28% in PET era). The 5-year time to progression (TTP) and overall survival (OS) for the entire cohort were 80% and 89%, respectively, similar across treatment eras. Overall, 10% had refractory disease. In total, 113 patients had an EOT PET scan: 63% negative and 37% positive with a 5-year TTP of 90% vs 71% and 5-year OS of 97% vs 88%, respectively. For those with Deauville (D)-scored PET scans (n = 103), the 5-year TTP for PET-negative cases by Deauville criteria (D1-D3, DX) was 91%, with inferior outcomes for D5 vs D4 (5-year TTP 33% vs 87%, P = .0002). Outcomes for PMBCL treated with RCHOP are favorable and use of a PET-adapted approach reduces RT in the majority of patients. A small proportion have refractory disease and may benefit from an alternate treatment.
Introduction
Primary mediastinal large B-cell lymphoma (PMBCL) typically occurs in young females who present with an often bulky anterior mediastinal mass. Morphologically, it is associated with compartmentalizing fibrosis and is often CD30 positive. These distinct clinicopathologic features, along with subsequent studies demonstrating molecular overlap with nodular sclerosis classical Hodgkin lymphoma, have established PMBCL as a distinct entity in the World Health Organization (WHO) classification.1-3
As a result of disease rarity, prospective and, in particular, randomized data are lacking. As a result, practice guidelines vary worldwide.4-6 Rituximab-chemoimmunotherapy is considered standard frontline therapy in PMBCL and is often followed by consolidative radiotherapy (RT) to the anterior mediastinum if rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) or an “R-CHOP equivalent” regimen is used.4-6 Previous studies of R-CHOP with RT demonstrate a 5-year progression-free survival (PFS) of 77% to 81% and a 5-year overall survival (OS) of 84% to 89%7-10 (supplemental Table 1, available on the Blood Web site). In Europe, comparable results are seen with etoposide or methotrexate, doxorubicin, cyclophosphamide, vincristine, prednisone, and bleomycin, which is usually followed by RT.11-13
Recent efforts have focused on reduction of RT exposure because of the potential for long-term toxicities in this typically younger patient population. Dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (DA-EPOCH-R) has been adopted in many North American centers based on promising results from a phase II National Cancer Institute trial of 51 patients with PMBCL, which reported a 5-year event-free survival (EFS) of 93% and 5-year OS of 97%.14 Although it is a more complex regimen associated with increased acute toxicities, it largely mitigates the need for RT.15,16 In subsequent retrospective studies, the 2-year EFS using DA-EPOCH-R has ranged from 81% to 87%10,17,18 (supplemental Table 1).
In British Columbia (BC), a positron emission tomography (PET)-adapted approach has been used to guide consolidative RT in all aggressive large B-cell lymphomas since July of 2005 when centralized 18F-fluorodeoxyglucose (FDG)-PET became available. In this analysis, we evaluated outcomes of PMBCL patients treated in BC during the rituximab era and the impact of a PET-guided approach to consolidative RT.
Patients and methods
The BC Cancer Lymphoid Cancer Database was searched to identify all newly diagnosed PMBCL patients ≥18 years of age who were treated with curative intent during the rituximab era (≥2001) in BC. All medical records were reviewed to confirm that the clinical and pathological features were consistent with the WHO classification.3,19,20 Diagnostic biopsies were reviewed centrally by a BC Cancer hematopathologist, and all PET scans were performed centrally at the Vancouver BC Cancer center. Further details are provided in supplemental Methods. This study was approved by the University of BC/BC Cancer Research Ethics Board and was conducted in accordance with the Declaration of Helsinki.
PMBCL management algorithm in BC
In 2005, a centralized publicly funded PET scan became available at BC Cancer in Vancouver that could be accessed by all physicians in BC. As a limited resource used across all cancer types, it was initially only used in response assessment in aggressive curable lymphomas. With the addition of a second PET scan in 2011 in Vancouver, staging PET scans were introduced, including in PMBCL.
Patients were managed based on BC Cancer era-specific guidelines. From 2001 to July of 2005, patients were recommended to receive 6 cycles of R-CHOP, regardless of stage, followed by consolidative RT. Since the availability of a centralized FDG-PET scan in July of 2005, patients were recommended to receive 6 cycles of R-CHOP, followed by an end of treatment (EOT) PET performed 4 to 6 weeks following completion of chemotherapy. Those with a negative EOT PET (defined in the next paragraph), regardless of initial disease bulk, were observed, whereas those with a positive EOT PET were recommended to receive RT if the disease was radio-encompassable and there was no evidence of disease progression.
Prior to 2014, FDG-PET scans were interpreted based on International Harmonization Project (IHP) guidelines.21 Since 2014, Deauville criteria have been applied: PET negative is associated with a complete remission (CR) Deauville score of 1 to 3 (D1-3) or uptake from an alternate cause (DX), and PET positive (D4-D5)22 is associated with partial remission (PR) or progressive disease (PD; if progressive metabolic disease or new lesions). BC Cancer FDG-PET scans were reviewed by expert nuclear medicine physicians. All FDG-PET scans performed locally prior to 2014 were reclassified for the current analysis, blinded to outcome, in accordance with the Deauville criteria (P.T.). A minority of scans were performed at a private facility through self-pay in the earlier RT era and were not available for re-review for this study.
Statistical analyses
Time to progression (TTP) was defined from the date of diagnosis to disease progression or relapse/death caused by lymphoma/acute treatment toxicity, with death from unrelated causes censored. PFS was measured from the date of diagnosis to the date of disease progression or relapse/death from any cause. OS was determined from the date of diagnosis to the date of last follow-up or death from any cause. Time to central nervous system (CNS) relapse was determined from the date of diagnosis to the date of CNS relapse or progression. Kaplan-Meier curves were used to estimate survival rates, as well as time to CNS relapse (1-Kaplan-Meier estimate), and log-rank testing was used to compare groups. The χ2 test was used to compare prognostic factors between groups. Multivariate analysis was performed using a Cox proportional hazards model and forward selection method including factors with P < .1 on univariate analysis to assess the effect of the prognostic factors on TTP and OS. All analyses were performed using SPSS (version 14.0). Results
Patient characteristics and treatment
A total of 159 patients with PMBCL were identified, who were diagnosed between February of 2001 and January of 2018 and were treated with a rituximab combination with curative intent. There were no exclusions based on this criterion. Median follow-up was 7.9 years in all living patients (range, 0.6-17.8), 14.5 years in the RT era (range, 3.9-17.8), and 6.3 years in the PET era (range, 0.6-13.2). The median age was 36 years (range, 19-84), 57% were female, 70% had a bulky mass (≥ 10 cm), 39% had >1 extranodal site, 41% had a pleural and/or pericardial effusion, 43% had B symptoms, 72% had elevated lactate dehydrogenase (LDH), and 62% had stage I/II disease (Table 1). Of interest, 13 patients had stage III disease (median anterior mediastinal mass size of 12 cm), and 47 patients had stage IV disease, mostly due to extensive intrathoracic disease, but 10 patients had adrenal and/or kidney involvement (median anterior mediastinal mass size of 12.5 cm). R-CHOP was used in the majority of patients (n = 149, 94%); the remaining patients received R-CHOP/R-ICE (rituximab, ifosfamide, carboplatin, etoposide) (n = 8) as part of a clinical trial, and 2 patients received DA-EPOCH-R at the discretion of the treating physician.
Clinical characteristics of all PMBCL patients (N = 159)
| Clinical feature . | Data . |
|---|---|
| Median age, y | 36 (range, 19-84) |
| Females | 91 (57) |
| Bulky (≥10 cm) | 112 (70) |
| B symptoms | 68 (43) |
| PS ≥ 2* | 64 (40) |
| Elevated LDH† | 115 (72) |
| LDH > 2× ULN‡ | 37 (24) |
| Extranodal sites > 1 | 61 (38) |
| Extranodal sites > 2 | 29 (18) |
| Stage I/II disease§ | 99 (62) |
| Extranodal sites | |
| Pleural and/or pericardial effusion | 65 (41) |
| Lung | 44 (28) |
| Bone | 11 (7) |
| Kidney/adrenal | 10 (6) |
| IPI¶ | |
| 0/1 factors | 58 (36) |
| 2/3 factors | 74 (47) |
| 4/5 factors | 22 (14) |
| RT in RT era (n = 50) | 39 (78) |
| RT in PET era (n = 109) | 31 (28) |
| Median follow-up, y | 7.8 (range, 0.6-17.8) |
| Clinical feature . | Data . |
|---|---|
| Median age, y | 36 (range, 19-84) |
| Females | 91 (57) |
| Bulky (≥10 cm) | 112 (70) |
| B symptoms | 68 (43) |
| PS ≥ 2* | 64 (40) |
| Elevated LDH† | 115 (72) |
| LDH > 2× ULN‡ | 37 (24) |
| Extranodal sites > 1 | 61 (38) |
| Extranodal sites > 2 | 29 (18) |
| Stage I/II disease§ | 99 (62) |
| Extranodal sites | |
| Pleural and/or pericardial effusion | 65 (41) |
| Lung | 44 (28) |
| Bone | 11 (7) |
| Kidney/adrenal | 10 (6) |
| IPI¶ | |
| 0/1 factors | 58 (36) |
| 2/3 factors | 74 (47) |
| 4/5 factors | 22 (14) |
| RT in RT era (n = 50) | 39 (78) |
| RT in PET era (n = 109) | 31 (28) |
| Median follow-up, y | 7.8 (range, 0.6-17.8) |
Unless otherwise noted, data are n (%).
IPI, International Prognostic Index; PS, performance status; ULN, upper limit of normal.
PS > 1 missing in 1 patient.
LDH missing in 5 patients (3%).
LDH > 2× ULN missing in 6 patients (4%). For 1 patient, LDH was elevated, but the absolute value is not known.
Stage III (n = 13); stage IV (n = 47).
IPI missing in 5 patients (3%).
Outcome of PMBCL using R-chemotherapy
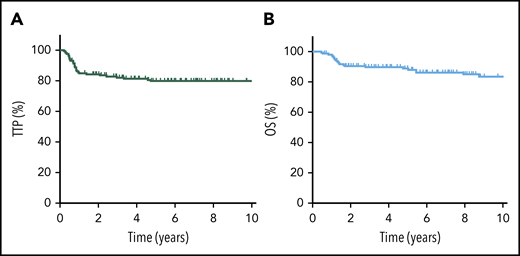
The 5-year TTP, PFS, and OS for the entire cohort were 80% (95% confidence interval [CI], 72-85%), 79% (95% CI, 72-85%), and 89% (95% CI, 83-93%), respectively (Figure 1). Considering only R-CHOP–treated patients (n = 149), outcomes were similar, with a 5-year TTP of 79%, 5-year PFS of 78%, and 5-year OS of 88%. Confining the analysis to the adolescent/young adult population (AYA; age 19-39 years) demonstrated similar findings (5-year TTP of 80%, 5-year OS of 89%).
TTP and OS for all PMBCL patients. TTP (A) and OS (B) for the entire cohort of PMBCL patients (N = 159).
TTP and OS for all PMBCL patients. TTP (A) and OS (B) for the entire cohort of PMBCL patients (N = 159).
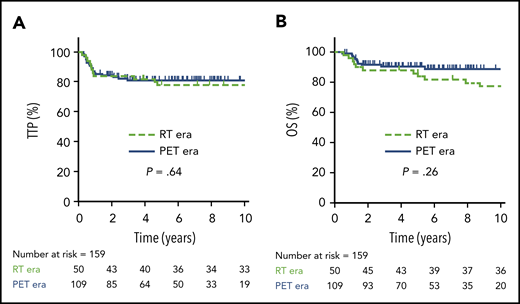
In total, 50 patients were managed during the RT era (2001-2005), 39 (78%) of whom received RT. During the PET era, 109 patients were treated, 31 (28%) of whom received RT, translating into a relative reduction of 64% for the use of RT during the PET era. Comparing outcomes in the RT vs PET era, there was no significant difference in 5-year TTP (78%; 95% CI, 64-87% vs 81%; 95% CI, 72-87%, respectively; P = .64) or 5-year OS (86%; 95% CI, 73-93% vs 91%; 95% CI, 83-95%, respectively; P = .26) (Figure 2). Patients managed in the RT era had a greater frequency of high-risk features, such as the presence of B symptoms (P = .003) and pleural and/or pericardial effusion (P = .05) (supplemental Table 2); however, in the multivariate Cox proportional hazard models, treatment era had no significant effect on TTP or OS (data not shown).
TTP and OS in RT vs PET era. TTP (A) and OS (B) comparing the RT era (n = 50) vs the PET era (n = 109).
TTP and OS in RT vs PET era. TTP (A) and OS (B) comparing the RT era (n = 50) vs the PET era (n = 109).
Considering patients with bulky anterior mediastinal disease (n = 112), there was no difference in TTP between eras (P = .67), despite RT being used in only approximately one third of patients in the PET era. During the RT era, 11 patients did not receive RT because of a negative private EOT PET (n = 4), refractory disease (n = 2), refusal (n = 3), death (n = 1), and reason unknown (n = 1). During the PET era, 102 of 109 patients had an EOT PET. Reasons for not having an EOT PET were patient refusal (n = 3), negative midtreatment PET (n = 2), primary refractory disease (n = 1), and clinical decline in an elderly patient after 1 cycle of R-CHOP with a change to palliative management.
Outcomes in PMBCL by EOT PET status
In total, 113 patients had an EOT PET, including 10 patients who had a private PET outside of BC Cancer, and 2 cases had only a midtreatment negative PET that was not repeated. Of these, 71 (63%) patients had a negative EOT PET, of whom only 1 patient (1%) received RT at the discretion of the physician. In contrast, 42 (37%) patients had a positive EOT PET, of whom 33 (79%) received RT; reasons for not receiving RT included PD (n = 5), physician discretion (EOT PET D4, n = 3, including 1 post–DA-EPOCH-R), and patient refusal (n = 1). Thus, 30% of patients received RT almost exclusively for a PET positive scan.
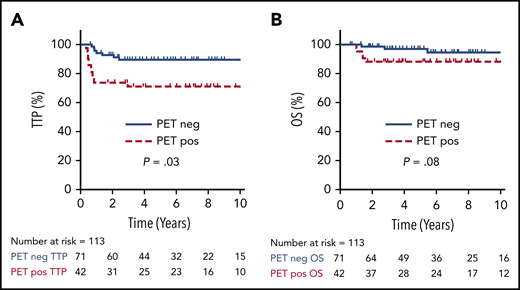
For all 113 patients treated with a PET-guided approach, the 5-year TTP and OS were 82% (95% CI, 73-88%) and 94% (95% CI, 87-97%), respectively. Cases with an EOT PET negative scan had a superior TTP compared with those with an EOT PET positive scan (5-year TTP of 90%; 95% CI, 74-95% vs 71%; 95% CI, 55-82%; P = .01), but this comparison did not reach statistical significance for 5-year OS (97%; 95% CI, 88-99% vs 88%; 95% CI, 74-95%; P = .08) (Figure 3).
TTP and OS by EOT PET. TTP (A) and OS (B) comparing positive vs negative EOT PET in all patients who underwent an EOT PET scan (n = 113).
TTP and OS by EOT PET. TTP (A) and OS (B) comparing positive vs negative EOT PET in all patients who underwent an EOT PET scan (n = 113).
Deauville reclassification in centrally performed PET scans
During the treatment period, reporting criteria for lymphoma patients undergoing FDG-PET has evolved, and Deauville criteria are now considered the standard grading tool.22 In total, 103 of 113 FDG-PET scans were performed centrally at BC Cancer. Earlier central scans reported in accordance with the IHP (n = 59) were reclassified using Deauville criteria by an expert nuclear medicine physician (P.T.) (supplemental Figure 1). Only 1 patient in the PET era did not have an EOT PET scan because of PD and is not included in this analysis. Through the reclassification process, 10 PET scans (10%) were reclassified: 8 were reassigned from positive to negative (reclassified as D3, n = 7; reclassified as D2, n = 1), and 2 were reassigned from negative to positive (both reclassified as D4) (supplemental Figure 1). Taken together, 71% were PET negative (n = 73: D1, n = 21; D2, n = 24; D3, n = 18; DX, n = 10; 8 whom received RT because of an earlier classification as PET positive along with 1 other PET-negative case who received RT by physician discretion [total = 9; 12%]), and 29% were PET positive (n = 30: D4, n = 18; D5, n = 12), of whom 20 (67%) received RT (supplemental Figure 1).
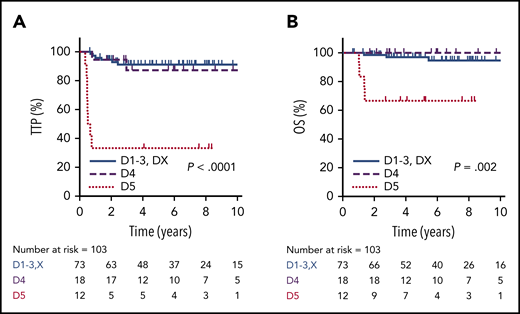
Using the Deauville criteria, the 5-year TTP in PET-negative patients was superior compared to PET-positive cases (91%; 95% CI, 81-96% vs 68%; 95% CI, 50-83%, respectively; P = .001) and the OS was 97% (95% CI, 89-100%) vs 87% (95% CI, 69-95%, respectively; P = .17). Considering only the PET-negative cases, there was no significant difference in 5-year TTP among the Deauville scores (D1, 85.7%; D2, 85.7%; D3, 93.3%; DX, 100%; P = .86). For the PET-positive cases, outcomes for D4 patients (n = 18), of whom 13 (72%) received RT, were comparable to PET-negative patients, with a 5-year TTP of 87% (95% CI, 57-98%) and 5-year OS of 100%. In contrast, patients with an EOT D5 PET scan (n = 12) had a significantly inferior outcome compared to D4 cases, with a 5-year TTP of 33% (95% CI, 10-59%; P < .001) and 5-year OS of 67% (95% CI, 35-86%; P = .002) (Figure 4). For all EOT PET-positive cases who received RT, the 5-year TTP was superior to those who did not receive RT (80% vs 40%, P = .008), and was superior for D4 cases (7/12) vs D5 cases (13/18) who received RT (92% vs 57%; P = .045).
TTP and OS by EOT PET according to Deauville criteria. TTP (A) and OS (B) in PET-negative patients (D1-3 + DX, n = 73) compared with EOT PET D4 (n = 18) and D5 (n = 12) patients.
TTP and OS by EOT PET according to Deauville criteria. TTP (A) and OS (B) in PET-negative patients (D1-3 + DX, n = 73) compared with EOT PET D4 (n = 18) and D5 (n = 12) patients.
Of interest, 4 R-CHOP–treated patients with an EOT score of D4 did not receive consolidative RT: 2 patients were previously classified as PET negative by IHP criteria and were observed as per guidelines, and the other 2 patients were observed at the discretion of the physician, and short-interval repeat PET scan was D2 in both cases. The final patient with an EOT D4 score had received DA-EPOCH-R but ultimately relapsed on serial PET imaging and biopsy.
Evaluation of the impact of bulky disease using a PET-guided approach for RT
The impact of a bulky anterior mediastinal mass (≥10 cm) was assessed in the 103 Deauville scored cases. Of these, 64% (n = 66) had a bulky mass at diagnosis and were more likely to have an EOT PET-positive scan (38%) compared with those with non-bulky disease (14%; P = .009). Consequently, there was a trend toward a greater use of consolidative RT in those with bulky disease (33% vs 16%; P = .06). Of note, 9 of 73 patients (6 bulky, 3 nonbulky) received RT in the PET-negative group: 8 as a result of reclassification and 1 because of physician choice (supplemental Figure 1).
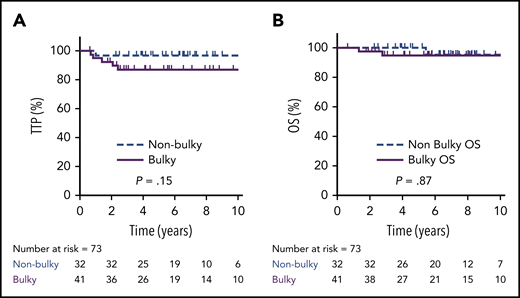
Overall, the 5-year TTP was 80% (95% CI, 68 88%) vs 92% (95% CI, 77-97%) in bulky vs nonbulky cases, respectively (P = .11), and 5-year OS was 91% (95% CI, 80-96%) vs 100%, respectively (P = .43). Considering only EOT PET-negative cases (n = 73; D1-3, DX), the 5-year TTP was 87% in bulky cases (n = 41; 95% CI, 71-94%) vs 97% (95% CI, 80-100%) in nonbulky cases (n = 32; P = .15), and the 5-year OS was 95% (95% CI, 80-100%) vs 100%, respectively (P = .87) (Figure 5). For all 6 PET-negative Deauville scored cases who relapsed (5 bulky), 2 occurred exclusively in the mediastinum, and 3 involved sites outside of the mediastinum, including 1 sole CNS relapse.
TTP and OS by bulky disease in PET-negative patients. TTP (A) and OS (B) in patients with a bulky (≥10 cm) mediastinal mass (n = 41) compared with those without a bulky mass (n = 32) in EOT PET-negative cases (D1-3 + DX).
TTP and OS by bulky disease in PET-negative patients. TTP (A) and OS (B) in patients with a bulky (≥10 cm) mediastinal mass (n = 41) compared with those without a bulky mass (n = 32) in EOT PET-negative cases (D1-3 + DX).
Prognostic factors in PMBCL
Prognostic factors for TTP were evaluated in the entire cohort. On univariate analysis, pleural and/or pericardial effusion (P < .007), extranodal sites > 1 (P = .02), extranodal sites > 2 (P = .02), stage III/IV disease (P = .003), lung or kidney/adrenal involvement, and International Prognostic Index (IPI) were all associated with an inferior TTP (Table 2). In the multivariate analysis, stage III/IV disease (P = .02) and the presence of a pleural and/or pericardial effusion (P = .05) were associated with an inferior TTP (Table 3). For OS, no factor demonstrated a significant relationship (data not shown).
Univariate analyses of prognostic factors’ effect on TTP in all PMBCL patients (N = 159)
| Clinical feature . | P . |
|---|---|
| Age > 40 y | .89 |
| Age > 60 y | .29 |
| Bulky mass (≥10 cm) | .12 |
| B symptoms | .05 |
| PS ≥ 2 | .68 |
| Elevated LDH | .06 |
| Elevated LDH > 2× ULN | .30 |
| Extranodal sites > 1 | .02 |
| Extranodal sites > 2 | .02 |
| Stage III/IV disease | .003 |
| Pleural and/or pericardial effusion | .007 |
| Lung | .16 |
| Bone | .46 |
| Kidney/adrenal | .05 |
| IPI 0/1 vs 2/3 vs 4/5 | .02 |
| RT vs PET era | .60 |
| Clinical feature . | P . |
|---|---|
| Age > 40 y | .89 |
| Age > 60 y | .29 |
| Bulky mass (≥10 cm) | .12 |
| B symptoms | .05 |
| PS ≥ 2 | .68 |
| Elevated LDH | .06 |
| Elevated LDH > 2× ULN | .30 |
| Extranodal sites > 1 | .02 |
| Extranodal sites > 2 | .02 |
| Stage III/IV disease | .003 |
| Pleural and/or pericardial effusion | .007 |
| Lung | .16 |
| Bone | .46 |
| Kidney/adrenal | .05 |
| IPI 0/1 vs 2/3 vs 4/5 | .02 |
| RT vs PET era | .60 |
ULN, upper limit of normal.
Multivariate analyses of prognostic factors’ effect on TTP in all PMBCL patients (N = 159)
| Clinical feature . | HR (95% CI) . | P . |
|---|---|---|
| Pleural and/or pericardial effusion | 2.09 (1.00-4.37) | .05 |
| B symptoms | 1.94 (0.96-3.95) | .06 |
| Stage III/IV disease | 2.30 (1.11-4.75) | .02 |
| Clinical feature . | HR (95% CI) . | P . |
|---|---|---|
| Pleural and/or pericardial effusion | 2.09 (1.00-4.37) | .05 |
| B symptoms | 1.94 (0.96-3.95) | .06 |
| Stage III/IV disease | 2.30 (1.11-4.75) | .02 |
HR, hazard ratio; IPI, International Prognostic Index; ULN, upper limit of normal.
Considering only PET-negative cases by Deauville criteria, there was a marginally significant trend toward an inferior TTP with the presence of B symptoms (P = .09), pleural/pericardial effusion (P = .06), and extranodal sites > 1 (P = .06) (data not shown).
Outcome in relapsed/refractory PMBCL and causes of death
In total, 31 patients had recurrent lymphoma: 15 with relapsed disease and 16 with primary refractory disease. Among the relapses, 12 occurred within 2 years of completion of chemotherapy, whereas 1 occurred at 3 years, and 2 occurred after 4 years. Of the late relapses, 1 involved the CNS, 1 was classic Hodgkin lymphoma, and 1 was high-grade B-cell lymphoma not otherwise specified. There were 4 CNS relapses (2.5%), 1 of whom had concurrent systemic disease, for a 5-year time to CNS relapse of 3%. Three cases involved the brain parenchyma, and 1 was solely in the leptomeninges. None of these patients had received CNS prophylaxis.
Among all relapsed/refractory patients, 25 of 31 were intended for high-dose chemotherapy and autologous stem cell transplant (ASCT), and the majority received gemcitabine, dexamethasone, and cisplatin, with or without rituximab (n = 20). The majority (20/25; 80%) proceeded to ASCT (relapsed, n = 8; refractory, n = 12), with consolidative RT post-ASCT (n = 9) if not given in the frontline setting. Only 2 patients did not receive consolidative RT post-ASCT for unclear reasons; both are relapse free. For the remaining 6 of 31 patients not considered candidates for ASCT, 3 were older than age 70 years, 1 had only CNS-directed therapy, 1 refused chemotherapy, and 1 died suddenly from superior vena cava syndrome posttreatment, despite evidence of CR on EOT PET (considered a lymphoma recurrence).
For all 31 relapsed/refractory patients, the 5-year OS from the time of first relapse/progression was 40% (95% CI, 25-60%), and was similar in those with relapsed disease (43%; 95% CI, 23-72%) compared to refractory disease (37.5%; 95% CI, 16-59%; P = .87). For the 25 patients intended for ASCT, 5-year OS from first progression was 48% and for those who underwent ASCT (n = 20), the posttransplant 5-year OS was 57% (95% CI, 36-77%): 71% vs 50% for relapsed vs refractory patients, respectively (P = .28). Of the 4 patients with CNS recurrence, 2 were managed palliatively and died. The other 2 patients received high-dose methotrexate (8 g/m2), which was followed by ASCT in 1 patient; both remain alive and free of disease after 7.4 and 2.1 years of follow-up since relapse.
There was a total of 24 deaths, the majority of which were due to lymphoma (18/24; including the patient who died of superior vena cava syndrome). Other causes of death included pulmonary embolism (n = 1), acute myelogenous leukemia (n = 1), arrhythmia (n = 1), esophageal carcinoma within the RT field (n = 1), cerebrovascular accident (n = 1), and unknown (n = 1).
Discussion
We report the largest single-center analysis to date evaluating outcomes in PMBCL patients using R-CHOP with integration of a PET-guided approach to the use of consolidative RT. In keeping with most other studies of PMBCL patients treated with R-CHOP, outcomes were favorable overall, with a 5-year TTP of 80% and 5-year OS of 89% (supplemental Table 1). Using a PET-adapted approach to guide administration of consolidative RT resulted in a 64% reduction in use without compromising cure. Patients with a negative EOT PET scan have excellent outcomes, with a 5-year TTP of 90% and 5-year OS of 97%, despite omission of RT in the majority. Patients with a positive EOT PET scan with D4 score had similarly excellent outcomes, although it remains uncertain whether this represents, in some cases, a false-positive posttreatment inflammatory state14 or conversion from a PR to a CR as a result of RT. In contrast, patients with a D5 positive EOT PET scan had inferior outcomes, with the majority experiencing disease progression or relapse, resulting in a 5-year TTP of only 33%.
The optimal frontline regimen in PMBCL remains unknown. Recently, DA-EPOCH-R use has increased because of a phase 2 prospective trial showing excellent outcomes, with PFS and OS > 90%.14 Although it is associated with a greater frequency of acute toxicities, including deep vein thrombosis in up to 30% to 40% of patients from mandatory central venous access,16,18 it largely obviates the need for consolidative RT.15 A recent multicenter retrospective analysis that compared the outcome of PMBCL using R-CHOP or DA-EPOCH-R did not show any significant difference in 5-year PFS (76% vs 85%; P = .28) (supplemental Table 1), but the use of RT was more frequent with R-CHOP (59% vs 13%).10 Neutropenic fever, hospitalization for acute toxicities, and infection rate were more frequent with the use of DA-EPOCH-R.10 Similar efficacy results were seen in another multicenter United States retrospective study of 156 pediatric and adult PMBCL patients treated with DA-EPOCH-R in which the 3-year EFS was 85%.18 The phase 3 CALGB trial comparing these 2 regimens showed no difference in outcomes, including in the PMBCL subgroup; however, patient numbers were small (N = 35).23
The role of RT in PMBCL patients after immunochemotherapy remains controversial. Excellent outcomes have been shown in several studies using dose-intensive regimens without RT,14,18,24 but few studies have assessed forgoing RT in R-CHOP–treated patients, particularly in those with bulky disease. Recognizing limited patient numbers, we did not note any statistical difference in TTP or OS in bulky vs nonbulky PET-negative cases. Further, of the PET-negative cases who relapsed, a minority occurred exclusively in the mediastinum or what would have been the irradiated field, suggesting that other risk factors contributed. Although our data support that cases with an EOT PET-negative scan may be safely observed, the ongoing phase 3 IELSG-37 study (www.clinicaltrials.gov, #NCT01599559) will definitively establish whether consolidative RT is beneficial in this setting.
The role of RT in cases with a positive EOT PET scan also remains undefined, particularly with enhanced granularity using Deauville scoring into 2 PET-positive categories, D4 and D5. Studies support omission of RT in most cases when DA-EPOCH-R is used in patients with either D4 or D5 on EOT PET, although rates of progression are higher in those with D5 disease.14,15 In our study, patients with a positive EOT PET scan who received RT had a more favorable outcome than those who did not, but the latter also includes cases whose disease was not radio-encompassable or had PD and combined Deauville scores of 4 and 5. Considering only D4 cases, the outcome was very favorable, similar to PET-negative cases. It is likely that that many of these cases are false positives, similar to reports using DA-EPOCH-R.14,15 This is supported by the absence of relapse in the 4 cases who did not receive RT, 2 of whom were downgraded to a D2 score on repeat imaging. Given the logistical challenges of obtaining a mediastinal biopsy in PET-positive cases with risk of delay of RT, along with the potential for tumor-sampling errors, our policy did not include pathologic confirmation of PMBCL prior to RT. This deserves additional study to further reduce the proportion of patients who ultimately need RT, which is particularly relevant in the AYA population.
Although the majority of patients treated with R-CHOP had favorable outcomes, ∼20% experienced disease progression or relapse, half with refractory disease. Refractory cases were largely captured by an EOT PET D5 score, which we observed in 12% of cases managed with a PET scan, of whom 67% ultimately had primary refractory disease. Giulino-Roth et al also reported that 11% of PMBCL patients had an EOT D5 score following DA-EPOCH-R, and this group had an inferior outcome, with a 3-year EFS of ∼30% and a 3-year OS of 74%.18 Thus, R-CHOP and, in some cases, DA-EPOCH-R may be insufficient in a small subset of PMBCL patients with highly aggressive disease, further supporting the evaluation of novel frontline therapies. Checkpoint inhibitors, such as pembrolizumab, have shown efficacy in the relapsed/refractory setting, leading to US Food and Drug Administration approval in PMBCL patients who have failed 2 lines of therapy.25,26 Response rates are high using nivolumab in combination with brentuximab vedotin (overall response rate, 70%; CR, 43%), supporting a synergistic mechanism of action, particularly in light of the poor efficacy of brentuximab vedotin alone.27,28 Ongoing trials are evaluating nivolumab with R-CHOP (study #NCT03704714) or DA-EPOCH-R (study #NCT03749018) in patients with aggressive B-cell non-Hodgkin lymphoma, including PMBCL.
A tailored approach to treatment choice is appealing to identify patients at risk for failure after R-CHOP and who may be more appropriate candidates for a dose-intensified approach. However, clinical prognostic factors are unreliable, and more objective biomarkers are needed.29 Emerging studies have shown the utility of PET radiomics in predicting outcome. The prospective IELSG-26 trial evaluated functional FDG-PET parameters at diagnosis in 103 PMBCL patients treated with R-CHOP, MACOP-B (methotrexate, doxorubicin, cyclophosphamide, vincristine, prednisone, and bleomycin), or VACOP-B (etoposide, doxorubicin, cyclophosphamide, vincristine, prednisone, and bleomycin), with RT in most cases. High total lesion glycolysis (TLG) on staging PET was associated with an inferior PFS; however, the positive predictive value was only 36% and, thus, did not adequately discriminate high-risk patients at diagnosis.30 A subsequent study found that PMBCL patients with a low metabolic heterogeneity and TLG had excellent outcomes (5-year PFS, 100%; negative predictive value, 100%), whereas the 9 patients with both high metabolic heterogeneity and TLG at diagnosis had poor outcomes (5-year PFS, 11%; positive predictive value, 89%).31 Although validation is required, this model may identify high-risk patients at diagnosis who might benefit from an alternate treatment approach.
Our study has some limitations, including insufficient power to adequately assess prognostic factors, as well as to definitively rule out differences for some survival analyses. Despite this, there are also a number of strengths. Selection bias is minimized by capturing >90% of patients diagnosed with PMBCL in BC and, with uniform application of practice guidelines across the province, we can systematically evaluate the impact of a treatment recommendation. Finally, pathology and PET scans were centrally reviewed, thereby reducing the risk of inappropriate inclusion.
In summary, use of R-CHOP chemotherapy in PMBCL with selective PET-guided consolidative RT maintains excellent outcomes, despite a significant reduction in the use of RT. This approach obviated the need for RT in approximately two thirds of patients with bulky disease. Further evaluation of serial imaging and repeat biopsy is warranted in D4 PET-positive cases because of the high risk of false positives. Finally, ∼10% of patients exhibit refractory disease, including the majority with an EOT D5 scan. Identifying these high-risk patients at diagnosis and evaluating the integration of novel therapies, including PD-1 inhibitors, is of high priority in PMBCL.
Please send data sharing requests to Kerry J. Savage (ksavage@bccancer.bc.ca).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
The authors acknowledge Rebekah Bahr’s contribution to the reclassification of the PET scans.
Authorship
Contribution: K.J.S. and A.R.H. conceived and designed the study, collected and analyzed data, and wrote the manuscript; D.G.L. performed statistical analyses and wrote the manuscript; P.T. performed the centralized PET rereview and analyzed/wrote the manuscript; D.W. and F.B. contributed to the PET review and guidelines; G.W.S., B.S., and P.F. performed centralized pathology review and analyzed/wrote the manuscript; and D.V., A.S.G., D.W.S., C.L.F., P.R.Y., F.B., A.L, T.P., L.H.S., and J.M.C. analyzed/wrote the manuscript.
Conflict-of-interest disclosure: A.R.H. has received honoraria from Seattle Genetics, Janssen, and AbbVie. K.J.S. has received honoraria from Seattle Genetics, Bristol Myers Squibb, Merck, AbbVie, Astra Zeneca, and Gilead Sciences; has acted as a consultant for Servier; and has received institutional funds from Roche. J.M.C. has received honoraria from Seattle Genetics and Takeda Pharmaceuticals. D.W.S. has acted as a consultant for AbbVie, Janssen, and Celgene; has received research funding from Janssen, NanoString, and Roche/Genentech; and is a named inventor on patents, including 1 licensed to NanoString. A.S.G. has acted as a consultant for and received honoraria from Janssen, AbbVie, Novartis, and Celgene. D.V. has served on advisory boards and received honoraria from Roche, Celgene, AbbVie, Seattle Genetics, Gilead Sciences/Kite, Lundbeck, AstraZeneca, Janssen, NanoString Technologies, and Novartis. C.L.F. has received honoraria from Seattle Genetics, Janssen, Amgen, Celgene, Sanofi, and AbbVie and has received research funding from Roche and Teva Pharmaceutical Industries. L.H.S. has acted as a consultant for and received honoraria from Roche/Genentech, AbbVie, Amgen, Apobiologix, Astra Zeneca, Acerta, Celgene, Gilead Sciences, Janssen, Kite, Karyopharm, Lundbeck, Merck, MorphoSys, Seattle Genetics, Teva Pharmaceutical Industries, Takeda, TG Therapeutics, and Verastem Oncology and has received research funding from Roche and Genentech. The remaining authors declare no competing financial interests.
Correspondence: Kerry J. Savage, BC Cancer, 600 West 10th Ave, Vancouver, BC V5Z 4E6, Canada; e-mail: ksavage@bccancer.bc.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal