Key Points
Most of the 245 patients with essential thrombocythemia who were treated with once-daily low-dose aspirin exhibit incomplete platelet inhibition.
In this randomized study, platelet inhibition was improved by twice-daily dosing, with no further inhibition using a shorter interval.
Abstract
Essential thrombocythemia (ET) is characterized by abnormal megakaryopoiesis and enhanced thrombotic risk. Once-daily low-dose aspirin is the recommended antithrombotic regimen, but accelerated platelet generation may reduce the duration of platelet cyclooxygenase-1 (COX-1) inhibition. We performed a multicenter double-blind trial to investigate the efficacy of 3 aspirin regimens in optimizing platelet COX-1 inhibition while preserving COX-2–dependent vascular thromboresistance. Patients on chronic once-daily low-dose aspirin (n = 245) were randomized (1:1:1) to receive 100 mg of aspirin 1, 2, or 3 times daily for 2 weeks. Serum thromboxane B2 (sTXB2), a validated biomarker of platelet COX-1 activity, and urinary prostacyclin metabolite (PGIM) excretion were measured at randomization and after 2 weeks, as primary surrogate end points of efficacy and safety, respectively. Urinary TX metabolite (TXM) excretion, gastrointestinal tolerance, and ET-related symptoms were also investigated. Evaluable patients assigned to the twice-daily and thrice-daily regimens showed substantially reduced interindividual variability and lower median (interquartile range) values for sTXB2 (ng/mL) compared with the once-daily arm: 4 (2.1-6.7; n = 79), 2.5 (1.4-5.65, n = 79), and 19.3 (9.7-40; n = 85), respectively. Urinary PGIM was comparable in the 3 arms. Urinary TXM was reduced by 35% in both experimental arms. Patients in the thrice-daily arm reported a higher abdominal discomfort score. In conclusion, the currently recommended aspirin regimen of 75 to 100 once daily for cardiovascular prophylaxis appears to be largely inadequate in reducing platelet activation in the vast majority of patients with ET. The antiplatelet response to low-dose aspirin can be markedly improved by shortening the dosing interval to 12 hours, with no improvement with further reductions (EudraCT 2016-002885-30).
Introduction
Essential thrombocythemia (ET) is a chronic Philadelphia-negative myeloproliferative neoplasm (MPN) that is characterized by clonal thrombocytosis and an enhanced risk for arterial and venous thrombosis,1-5 with ≈20% of patients presenting with mainly arterial thrombosis at diagnosis.3 The incidence of thrombosis during the course of the disease averages 2% per year.1,6 Thrombosis recurrence reaches up to 8% per year.2 Increased thromboxane A2 (TXA2) biosynthesis has been reported in patients with ET,1,7-9 suggesting a potential link between persistently enhanced platelet activation and vascular complications.2
Current recommendations for low-dose aspirin in patients wtih ET are largely based on aspirin trials in non-MPN subjects, as well as on a phase 3 trial in polycythemia vera.10 However, the efficacy of aspirin for cardiovascular prevention in ET has been questioned,6 and it appears to be limited to subgroups of patients based on retrospective and observational studies with intrinsic limitations and low-quality evidence.11-13
Using the same aspirin dose (75-100 mg) and dosing regimen (once daily) for patients with ET as for patients without ET implies that the 2 populations have similar aspirin pharmacodynamics. Aspirin permanently acetylates platelet and megakaryocyte cyclooxygenase-1 (COX-1), the enzyme catalyzing the first committed step in TXA2 biosynthesis.14 Under physiological megakaryopoiesis, the irreversible nature of COX-1 inactivation in bone marrow progenitors leads to a new platelet progeny with largely nonfunctioning enzyme throughout the 24-hour dosing interval.14,15 However, the accelerated formation and release of platelets with unacetylated COX-1 and/or COX-2 in ET8 have been suggested to impair inhibition and hasten the recovery of TXA2-dependent platelet function during the 24-hour aspirin-dosing interval.1,15 Consistent with this hypothesis, 2 independent studies of relatively small sample size have reported incomplete inhibition of platelet TXA2 biosynthesis by a standard aspirin regimen in ≥80% of patients with ET.16,17 The antiplatelet effect of low-dose aspirin could be improved more effectively by more frequent dosing (ie, every 12 hours [twice daily]) than by doubling the once-daily dose.16,17 Therefore, multiple daily dosing has been suggested to represent a more effective antiplatelet strategy in ET15-17 ; however, this hypothesis has never been tested in a large study. Moreover, the potential inhibitory effect of more frequent aspirin dosing on vascular prostacyclin (prostaglandin I2 [PGI2]) biosynthesis should also be considered,18 given the enhanced thrombotic risk for patients with ET.1 Under physiological shear stress conditions, the COX-2 isozyme of vascular endothelial cells largely drives PGI2 biosynthesis, which has clinically relevant vasorelaxant and platelet-inhibiting effects, as suggested by the cardiovascular toxicity of COX-2 inhibitors.19 Low-dose aspirin has been shown to have limited inhibitory effects on in vivo PGI2 biosynthesis, possibly because of differential rates of recovery of endothelial COX-2 and platelet COX-1 during the 24-hour dosing interval.20-22 However, it is unknown whether multiple aspirin dosing may reduce endothelial PGI2 production in patients with ET.
To address the unmet need of an optimized antiplatelet regimen in ET patients, we conceived the Aspirin Regimens in Essential Thrombocythemia (ARES) phase 2 trial.23 Here, we report the results of the dose-finding component of the ARES trial that addressed, in a randomized double-blind fashion, the following main objectives: (1) to define the most effective dosing regimen of aspirin to suppress platelet COX-1 activity in patients with ET, as reflected by a surrogate biomarker of aspirin efficacy (ie, ex vivo TXB2 production during whole blood clotting)24,25 and (2) to assess the vascular safety of an improved aspirin regimen, as reflected by a biomarker of endothelial COX-2 activity (ie, urinary prostacyclin PGI2 metabolite [PGIM] excretion).26
Patients and methods
Study design
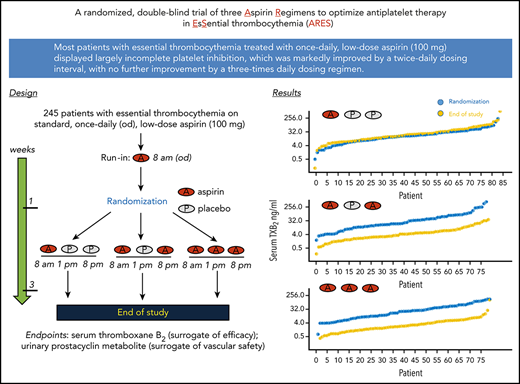
The rationale, design, main objectives, and inclusion/exclusion criteria for the ARES trial (EudraCT 2016-002885-30) have been detailed elsewhere.23 This article relates to the dose-finding component of ARES, consisting of a multicenter randomized parallel-arm double-blind controlled study. Eligible patients with ET23 on chronic aspirin (100 mg, once daily) for primary or secondary cardiovascular prevention were enrolled. At visit 1, all patients were instructed to take their usual aspirin tablet at breakfast (7-9 am) for 7 to 10 consecutive days. Upon run-in completion, patients were randomized 1:1:1 to enteric-coated aspirin (CardioAspirin; Bayer Italy) 100 mg, once daily (breakfast), twice daily (every 12 ± 2 hours; ie, breakfast and dinner), or thrice daily (every 6 ± 2 hours; ie, breakfast, lunch, and dinner) for 2 weeks (Figure 1) and matching placebo, so that all patients took a thrice-daily regimen, including aspirin with or without placebo tablets, according to the randomized arm. Therefore, aspirin or matching placebo tablets were taken at breakfast (7-9 am), lunch (1-2 pm), and after dinner (8-9 pm). The prescribed cytoreductive regimen, if any, was kept unchanged for the 2-week study period. At randomization (visit 2) and after 2 weeks of randomized treatment (visit 3), patients underwent blood and urine sampling in a fasting state before the next aspirin dosing (Figure 1). Then, patients resumed their open-label aspirin regimen (100 mg, once daily). The Ethics Committee of the Fondazione Policlinico Universitario A. Gemelli Istituto di Ricerca e Cura a Carattere Scientifico (IRCCS) approved the study (Protocol #28371/16, ID 1285, final approval 2 August 2016). All participating institutions approved the protocol. All patients signed an informed consent. The study was conducted between December of 2017 and July of 2018.
Study design. Design, visits, and biological sample collection of the first phase of the ARES trial.
Study design. Design, visits, and biological sample collection of the first phase of the ARES trial.
The coprimary end points were the pharmacodynamics efficacy of the aspirin regimens (as reflected by residual serum TXB2 [sTXB2]) and vascular safety (as assessed by urinary PGIM excretion).
The secondary end point was the urinary excretion of a stable enzymatic metabolite of TXA2/TXB2 (ie, the 11-dehydro-TXB2 [urinary TX metabolite (TXM)]), reflecting the actual rate of in vivo TXA2 biosynthesis.27 Gastrointestinal (GI) tolerance and microvascular symptoms were also recorded during the week preceding visit 3 with ad hoc questionnaires: the Severity of Dyspepsia Assessment questionnaire, previously validated for patients taking nonsteroidal anti-inflammatory drugs (NSAIDs),28,29 a validated questionnaire assessing MPN-related symptoms,30 and a patient’s self-scored pain numeric rating scale (0 [no pain] to 10 [worst imaginable pain]) for erythromelalgia of hands and feet.
Methods
Each participating institution performed routine hematochemical analyses in their own laboratories and determined the mutational profile of the patients. Clinical and laboratory characteristics of the patients were collected through Research Electronic Data Capture software.31
Patients were randomized using a randomization list stratified by sex and participating center, implemented within Research Electronic Data Capture software.
The thrombotic risk was assessed according to the International Prognostic Score of Thrombosis in Essential Thrombocythemia system, a validated prognostic score that includes age, previous thrombosis, cardiovascular risk factors, and the JAK2 V617F mutation.32 Compliance was assessed at visit 3 by pill counting and reviewing the patient’s daily diary, where patients recorded daily timing of tablet intake, any drug other than their usual therapy, and any symptom or comment that they deemed relevant.
For sTXB2 measurements, peripheral venous blood was collected without anticoagulant, incubated within 5 minutes33 for 1 hour at 37°C, and centrifuged for 10 minutes at 1200g; the supernatant serum was stored at −40°C until assayed.23 sTXB2 was measured using a previously described liquid chromatography-tandem mass spectrometry–validated immunoassay.24,33,34
The major urinary PGIM, 2,3-dinor-6-keto-PGF1α,35 was measured by liquid chromatography-tandem mass spectrometry, as previously described.22 The major urinary TXM, 11-dehydro-TXB2, was measured in 1-mL urine samples using a gas chromatography-mass spectrometry–validated immunoassay.36,37 Urinary prostanoid values are expressed as pg/mg of urinary creatinine, measured by a commercial kit (Creatinine Colorimetric Detection Kit; Enzo Life Sciences, Farmingdale, NY).
Statistical analyses
Based on previous findings,8,17 we assumed that the mean ± standard deviation of sTXB2 in patients with ET on aspirin, 100 mg once daily, would be ∼22 ± 33 ng/mL. We planned to test 2 hypotheses: (1) the 100-mg twice-daily aspirin regimen is more effective than 100 mg once daily, yielding a ≥50% reduction in sTXB2 and (2) the 100-mg thrice-daily aspirin regimen is more effective than 100 mg twice daily, yielding a ≥50% reduction in sTXB2. Testing these hypotheses with an α-error of 0.05 and a β-error of 0.2 (80% power) required 70 patients per treatment arm. Anticipating a 30% dropout, we estimated that 100 patients in each arm would ensure adequate statistical power. For the coprimary end point of urinary PGIM, the study had 80% power to test the hypothesis that any experimental aspirin regimen would reduce urinary PGIM by >30% compared with the standard regimen. This threshold of urinary PGIM reduction was selected based on the following considerations: urinary PGIM is minimally affected (20-40% variation) by 75 to 100 mg of aspirin daily in healthy subjects, this threshold corresponds to the intrasubject coefficient of variation upon repeated measurements of PGIM excretion over time, and traditional NSAIDs, including high-dose aspirin, reduce urinary PGIM excretion by 60% to 80%.26
Differences between qualitative and quantitative variables were tested with the χ2 and Wilcoxon signed-rank tests, respectively. A linear-regression model was used to evaluate possible differences in sTXB2 response in relation to platelet count and cytoreductive therapy. R 3.6.1 was used for data analysis and plotting (The R Foundation for Statistical Computing, Vienna, Austria).38
Results
A total of 251 eligible aspirin-treated (the vast majority for primary prevention; Table 1), consenting patients with ET was enrolled and started the run-in phase. Six patients withdrew their consent during this phase for personal reasons; thus, 245 patients underwent randomization at visit 2 (Figure 2). The demographic, clinical, and laboratory characteristics of these patients are detailed in Table 1. There were no statistically significant differences among the 3 treatment groups. One patient assigned to aspirin, 100 mg once daily, exited the study before visit 3 because of abdominal pain, and 1 patient did not have a serum sample available at visit 3 (Figure 2). Thus, 243 patients were evaluable at the end of the study and were included in the analyses.
Characteristics of 245 randomized patients with ET overall and according to the assigned treatment
| . | All (n = 245) . | 100 mg, once daily(n = 86) . | 100 mg, twice daily(n = 79) . | 100 mg, thrice daily(n = 80) . |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Male | 112 (45.7) | 40 (46.5) | 36 (45.6) | 36 (45) |
| Female | 133 (54.3) | 46 (53.5) | 43 (54.4) | 44 (55) |
| Age at diagnosis, y | 53 (42-63) | 52 (41.2-62.8) | 59 (43.5-65.5) | 48.5 (39.8-58) |
| Age at enrollment, y | 60 (51-67) | 59 (50.2-66) | 62 (53-69) | 58 (49.8-66) |
| BMI, kg/m2 | 24.9 (22.7-27.3) | 24.9 (22.7-26.9) | 24.5 (22.5-26) | 25.2 (23-28.7) |
| Leukocytes, ×109/L | 7 (5.6-8.5) | 7.3 (5.6-8.3) | 6.9 (5.4-8.8) | 7.1 (5.8-8.4) |
| Platelet count, ×109/L | 521 (422-641) | 512 (418-629) | 521 (404-622) | 532 (424-660) |
| Hematocrit, % | 41.7 (39.1-44.3) | 41.4 (38.3-44.4) | 42.2 (39.5-44.3) | 41.4 (39.6-43.8) |
| JAK2 genotype, n (%) | ||||
| Wild-type | 99 (40.4) | 38 (44.2) | 31 (39.2) | 30 (37.5) |
| Mutated | 145 (59.2) | 48 (55.8) | 48 (60.8) | 49 (61.25) |
| Not available | 1 (0.4) | 0 (0) | 0 (0) | 1 (1.25) |
| CALR mutation, n (%) | ||||
| Type 1 | 19 (7.8) | 7 (8.1) | 6 (7.7) | 6 (7.5) |
| Type 2 | 16 (6.5) | 5 (5.8) | 6 (7.7) | 5 (6.25) |
| Other | 95 (38.8) | 31 (36.1) | 29 (37.2) | 35 (43.75) |
| Not available | 115 (46.9) | 43 (50) | 38 (47.4) | 34 (42.5) |
| IPSET thrombosis score, n | ||||
| 0 | 34 | 14 | 11 | 9 |
| 1 | 41 | 16 | 9 | 16 |
| 2 | 77 | 23 | 26 | 28 |
| 3 | 45 | 20 | 13 | 12 |
| 4 | 42 | 11 | 19 | 12 |
| 5 | 5 | 2 | 1 | 2 |
| 6 | 1 | 0 | 0 | 1 |
| Microvascular symptoms, n (%) | 25 (10.2) | 10 (11.6) | 9 (11.4) | 6 (7.5) |
| Previous thrombosis, n (%) | ||||
| MPN related* | 10 (4.1) | 3 (3.5) | 2 (2.5) | 5 (6.2) |
| Any thrombosis | 28 (11.4) | 10 (11.6) | 8 (10.1) | 10 (12.5) |
| Cytoreductive therapy, n (%) | ||||
| No | 98 (40) | 41 (47.7) | 28 (35.4) | 29 (36.25) |
| Yes | 147 (60) | 45 (52.3) | 51 (64.6) | 51 (63.75) |
| TXB2 before randomization, ng/mL | 19 (9.3-43.2) | 17.1 (8.3-32.8) | 20 (11.6-56.4) | 23.5 (9.8-47.8) |
| . | All (n = 245) . | 100 mg, once daily(n = 86) . | 100 mg, twice daily(n = 79) . | 100 mg, thrice daily(n = 80) . |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Male | 112 (45.7) | 40 (46.5) | 36 (45.6) | 36 (45) |
| Female | 133 (54.3) | 46 (53.5) | 43 (54.4) | 44 (55) |
| Age at diagnosis, y | 53 (42-63) | 52 (41.2-62.8) | 59 (43.5-65.5) | 48.5 (39.8-58) |
| Age at enrollment, y | 60 (51-67) | 59 (50.2-66) | 62 (53-69) | 58 (49.8-66) |
| BMI, kg/m2 | 24.9 (22.7-27.3) | 24.9 (22.7-26.9) | 24.5 (22.5-26) | 25.2 (23-28.7) |
| Leukocytes, ×109/L | 7 (5.6-8.5) | 7.3 (5.6-8.3) | 6.9 (5.4-8.8) | 7.1 (5.8-8.4) |
| Platelet count, ×109/L | 521 (422-641) | 512 (418-629) | 521 (404-622) | 532 (424-660) |
| Hematocrit, % | 41.7 (39.1-44.3) | 41.4 (38.3-44.4) | 42.2 (39.5-44.3) | 41.4 (39.6-43.8) |
| JAK2 genotype, n (%) | ||||
| Wild-type | 99 (40.4) | 38 (44.2) | 31 (39.2) | 30 (37.5) |
| Mutated | 145 (59.2) | 48 (55.8) | 48 (60.8) | 49 (61.25) |
| Not available | 1 (0.4) | 0 (0) | 0 (0) | 1 (1.25) |
| CALR mutation, n (%) | ||||
| Type 1 | 19 (7.8) | 7 (8.1) | 6 (7.7) | 6 (7.5) |
| Type 2 | 16 (6.5) | 5 (5.8) | 6 (7.7) | 5 (6.25) |
| Other | 95 (38.8) | 31 (36.1) | 29 (37.2) | 35 (43.75) |
| Not available | 115 (46.9) | 43 (50) | 38 (47.4) | 34 (42.5) |
| IPSET thrombosis score, n | ||||
| 0 | 34 | 14 | 11 | 9 |
| 1 | 41 | 16 | 9 | 16 |
| 2 | 77 | 23 | 26 | 28 |
| 3 | 45 | 20 | 13 | 12 |
| 4 | 42 | 11 | 19 | 12 |
| 5 | 5 | 2 | 1 | 2 |
| 6 | 1 | 0 | 0 | 1 |
| Microvascular symptoms, n (%) | 25 (10.2) | 10 (11.6) | 9 (11.4) | 6 (7.5) |
| Previous thrombosis, n (%) | ||||
| MPN related* | 10 (4.1) | 3 (3.5) | 2 (2.5) | 5 (6.2) |
| Any thrombosis | 28 (11.4) | 10 (11.6) | 8 (10.1) | 10 (12.5) |
| Cytoreductive therapy, n (%) | ||||
| No | 98 (40) | 41 (47.7) | 28 (35.4) | 29 (36.25) |
| Yes | 147 (60) | 45 (52.3) | 51 (64.6) | 51 (63.75) |
| TXB2 before randomization, ng/mL | 19 (9.3-43.2) | 17.1 (8.3-32.8) | 20 (11.6-56.4) | 23.5 (9.8-47.8) |
Quantitative values are reported as median (interquartile range), unless otherwise indicated. There were no significant differences between the randomized groups, based on the Kruskal-Wallis test or χ2 test for continuous or discrete variables, respectively.
BMI, body mass index; IPSET, International Prognostic Score of Thrombosis in Essential Thrombocythemia; TX, thromboxane.
Any major thrombosis occurring within 2 years before diagnosis and any time afterward.
Compliance at visit 3, as assessed by pill count and the patient’s diary, is reported in Table 2: 218 of 243 patients (90%) took all 9 pills in the 3 days preceding visit 3 and were considered fully compliant. None of the patients reported taking any NSAID in the 3 days preceding visit 3.
Compliance with aspirin treatment according to pill counting in 243 evaluable patients with ET
| . | Fully compliant (n =218)* . | Partially compliant (n = 21)† . | Noncompliant (n = 4)‡ . | Global P . |
|---|---|---|---|---|
| Sex | .23 | |||
| Male | 104 (47.7) | 6 (28.6) | 2 (50) | |
| Female | 114 (52.3) | 15 (71.4) | 2 (50) | |
| Age at enrollment, median (IQR), y | 60 (51.3-67) | 54 (45-66) | 54 (43.3-64) | .21 |
| TXB2 at visit 2, median (IQR), ng/mL | 18.6 (8.9-42.9) | 22.8 (13.6-37) | 46.5 (24-107.8) | .40 |
| Treatment, 100 mg | .60 | |||
| Once daily | 73 (33.5) | 10 (47.6) | 2 (50) | |
| Twice daily | 71 (32.6) | 7 (33.3) | 1 (25) | |
| Thrice daily | 74 (33.9) | 4 (19.1) | 1 (25) |
| . | Fully compliant (n =218)* . | Partially compliant (n = 21)† . | Noncompliant (n = 4)‡ . | Global P . |
|---|---|---|---|---|
| Sex | .23 | |||
| Male | 104 (47.7) | 6 (28.6) | 2 (50) | |
| Female | 114 (52.3) | 15 (71.4) | 2 (50) | |
| Age at enrollment, median (IQR), y | 60 (51.3-67) | 54 (45-66) | 54 (43.3-64) | .21 |
| TXB2 at visit 2, median (IQR), ng/mL | 18.6 (8.9-42.9) | 22.8 (13.6-37) | 46.5 (24-107.8) | .40 |
| Treatment, 100 mg | .60 | |||
| Once daily | 73 (33.5) | 10 (47.6) | 2 (50) | |
| Twice daily | 71 (32.6) | 7 (33.3) | 1 (25) | |
| Thrice daily | 74 (33.9) | 4 (19.1) | 1 (25) |
Data are n (%), unless otherwise indicated. The P values were calculated using the Kruskal-Wallis test or χ2 test for continuous or discrete variables, respectively.
All 9 pills in the 3 days before visit 3.
Six to 8 pills in the 3 days before visit 3.
No pill in the 3 days before visit 3.
Coprimary end points
The sTXB2 level at visit 2 averaged 19 (3.4-140.4) ng/mL (median and interquartile range [IQR]; n = 245) and was similar across the 3 treatment arms (Table 3). As shown in Figure 3, sTXB2 at visit 2 showed substantial interindividual variability, spanning 2 to 3 orders of magnitude, with the vast majority of ET patients showing evidence of incomplete platelet COX-1 inactivation.
TXB2 and urinary PGIM and TXM values before (visit 2) and after (visit 3) the randomized aspirin regimen in 243 evaluable patients with ET
| . | 100 mg, once daily (n = 85) . | 100 mg twice daily(n = 79) . | 100 mg thrice daily (n = 79) . | Global P* . | P† . |
|---|---|---|---|---|---|
| sTXB2 at V2 (ng/mL) | 17 (8.2-33) | 20 (11.6-6.4) | 23.3 (9.6-46.4) | .098 | .41 |
| sTXB2 at V3 (ng/mL) | 19.3 (9.7-40) | 4 (2.1-6.7) | 2.5 (1.4-5.7) | <.001 | .04 |
| sTXB2 V3/V2 ratio | 1 (0.77-1.5) | 0.1 (0.08-0.3) | 0.1 (0.08-0.2) | <.001 | .24 |
| PGIM at V2 (pg/mg creatinine) | 84 (50-123) | 76 (47-132) | 83 (53-123) | .96 | .74 |
| PGIM at V3 (pg/mg creatinine) | 89 (54-127) | 87 (46-121) | 80 (47-131) | .70 | .90 |
| PGIM V3/V2 ratio | 1.1 (0.7-1.5) | 0.9 (0.7-1.4) | 0.9 (0.6-1.6) | .48 | .88 |
| TXM at V2 (pg/mg creatinine) | 485 (336-693) | 641 (437-864) | 515 (379-738) | .02 | .09 |
| TXM at V3 (pg/mg creatinine) | 457 (313-674) | 367 (237-541) | 344 (229-487) | .001 | .37 |
| TXM V3/V2 ratio | 0.9 (0.7-1.3) | 0.7 (0.5-0.8) | 0.7 (0.5-0.8) | <.001 | .71 |
| . | 100 mg, once daily (n = 85) . | 100 mg twice daily(n = 79) . | 100 mg thrice daily (n = 79) . | Global P* . | P† . |
|---|---|---|---|---|---|
| sTXB2 at V2 (ng/mL) | 17 (8.2-33) | 20 (11.6-6.4) | 23.3 (9.6-46.4) | .098 | .41 |
| sTXB2 at V3 (ng/mL) | 19.3 (9.7-40) | 4 (2.1-6.7) | 2.5 (1.4-5.7) | <.001 | .04 |
| sTXB2 V3/V2 ratio | 1 (0.77-1.5) | 0.1 (0.08-0.3) | 0.1 (0.08-0.2) | <.001 | .24 |
| PGIM at V2 (pg/mg creatinine) | 84 (50-123) | 76 (47-132) | 83 (53-123) | .96 | .74 |
| PGIM at V3 (pg/mg creatinine) | 89 (54-127) | 87 (46-121) | 80 (47-131) | .70 | .90 |
| PGIM V3/V2 ratio | 1.1 (0.7-1.5) | 0.9 (0.7-1.4) | 0.9 (0.6-1.6) | .48 | .88 |
| TXM at V2 (pg/mg creatinine) | 485 (336-693) | 641 (437-864) | 515 (379-738) | .02 | .09 |
| TXM at V3 (pg/mg creatinine) | 457 (313-674) | 367 (237-541) | 344 (229-487) | .001 | .37 |
| TXM V3/V2 ratio | 0.9 (0.7-1.3) | 0.7 (0.5-0.8) | 0.7 (0.5-0.8) | <.001 | .71 |
Data are median (IQR).
V, visit.
Spearman test.
Twice-daily vs thrice-daily aspirin, Wilcoxon test.
Individual sTXB2 values according to the randomized treatment. Individual values for sTXB2 at randomization (visit 2) and at the end of the 2-week treatment (visit 3) (left panels) and the corresponding distribution of the data (right panels) for the once-daily regimen (A), the twice-daily regimen (B), and the thrice-daily regimen (C).
Individual sTXB2 values according to the randomized treatment. Individual values for sTXB2 at randomization (visit 2) and at the end of the 2-week treatment (visit 3) (left panels) and the corresponding distribution of the data (right panels) for the once-daily regimen (A), the twice-daily regimen (B), and the thrice-daily regimen (C).
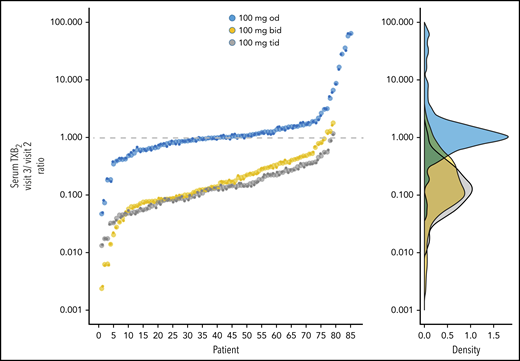
After 2 weeks of randomized aspirin treatment, sTXB2 values for patients assigned to the 100-mg twice-daily or thrice-daily regimen were reduced by 80% to 90% compared with their baseline values and were significantly lower than sTXB2 values for patients assigned to 100 mg once daily (Figure 3; Table 3). In the last group, sTXB2 values showed remarkably similar interindividual variability before and after the 2-week treatment (Figure 3A), indicating the stability of the poor aspirin-responsiveness phenotype in ET. Patients assigned to the twice-daily (Figure 3B) and thrice-daily (Figure 3C) regimens showed substantially and significantly reduced interindividual variability, in addition to lower median values for sTXB2 (Table 3). Data were also analyzed as the individual ratio of sTXB2 values at visit 3 vs visit 2, considering that all patients at visit 2 were on 100 mg of aspirin once daily (Figure 3). This analysis was performed to minimize the effect of variables, such as the platelet count, turnover rate, and body weight, that are known to influence aspirin responsiveness.17,39 In fact, we found that platelet count had a slight, but statistically significant, effect on the response to twice-daily and thrice-daily dosing (β coefficient, −0.02, for every 100 × 109/L platelet increase; P = .049). We found no effect of cytoreduction (β coefficient, −0.06; P = .23). Patients randomized to the once-daily regimen had a mean sTXB2 visit 3/visit 2 ratio of 1.03 (Table 3), indicating no appreciable short-term change in platelet COX-1 inhibition. The visit 3/visit 2 ratios for the twice-daily and thrice-daily regimens averaged 0.14 and 0.13, respectively (Figure 4; Table 3), consistent with comparable profound suppression of residual platelet TXA2 production in the 2 experimental aspirin regimens. The improved pharmacodynamics response was independent of previous thrombosis (data not shown).
Individual ratios of sTXB2 values. Individual ratios of sTXB2 values measured at visit 3 vs visit 2 for each treatment arm (left panel) and the corresponding data distribution (right panel).
Individual ratios of sTXB2 values. Individual ratios of sTXB2 values measured at visit 3 vs visit 2 for each treatment arm (left panel) and the corresponding data distribution (right panel).
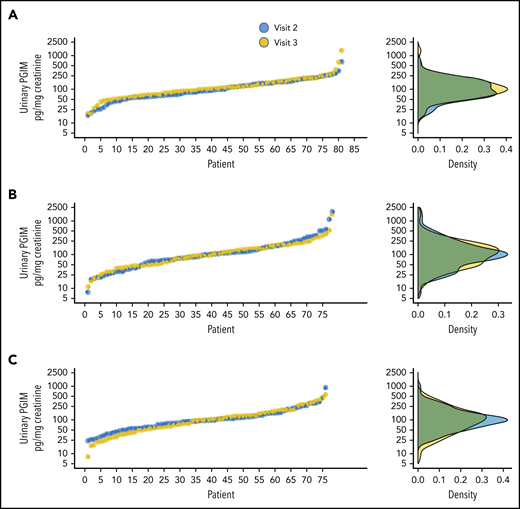
Urinary PGIM excretion, a noninvasive index of endothelial COX-2 activity,18 was similar across the treatment groups at visit 2 (Table 3) and was not affected by the once-daily, as well as by the twice-daily experimental regimens, as compared with the respective baseline excretion rate, to any statistically significant extent (Table 3; Figure 5).
Individual urinary PGIM values according to the randomized treatment. Individual values for urinary PGIM excretion at visit 2 (randomization) and visit 3 (end of treatment) (left panels) and the corresponding distribution of the data (right panels) for the once-daily regimen (A), the twice-daily regimen (B), and the thrice-daily regimen (C).
Individual urinary PGIM values according to the randomized treatment. Individual values for urinary PGIM excretion at visit 2 (randomization) and visit 3 (end of treatment) (left panels) and the corresponding distribution of the data (right panels) for the once-daily regimen (A), the twice-daily regimen (B), and the thrice-daily regimen (C).
Secondary end points
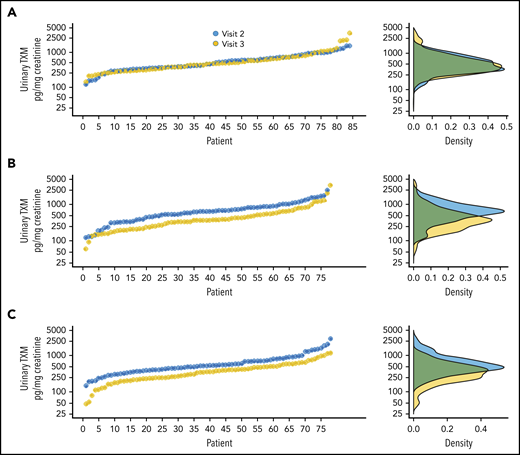
Baseline urinary TXM excretion, a noninvasive index of platelet activation,18 averaged 428 (IQR, 158.8-1063.7) pg/mg of creatinine (n = 245), with no significant differences among the 3 treatment groups (Table 3). As shown in Figure 6, urinary TXM at visit 2 displayed substantial interindividual variability, spanning 1 to 2 orders of magnitude, as would be expected for patients with variably and incompletely reduced TXA2 biosynthesis.34 After 2 weeks of randomized aspirin treatment, urinary TXM excretion rates for patients assigned to the 100-mg twice-daily or thrice-daily regimen were reduced by 30 to 40% vs their baseline values (Figure 6B-C), with reduced interindividual variability, and they were significantly lower than TXM excretion of patients assigned to 100 mg once daily (Table 3). In the once-daily group, TXM values were remarkably superimposable between visits 2 and 3 (Figure 6A), confirming the stability of the rate of platelet activation in vivo. Moreover, there was a positive significant association between individual sTXB2 ratios at visit 3/visit 2 and the corresponding urinary TXM ratios (Figure 7; correlation coefficient, r2 = +0.12; P < .0001). Therefore, optimization of aspirin pharmacodynamics results in reduced in vivo platelet activation in patients with ET.
Individual urinary TXM values according to the randomized treatment. Individual values for urinary TXM excretion at visit 2 (randomization) and visit 3 (end of treatment) (left panels) and the corresponding distribution of the data (right panels) for the once-daily regimen (A), the twice-daily regimen (B), and the thrice-daily regimen (C).
Individual urinary TXM values according to the randomized treatment. Individual values for urinary TXM excretion at visit 2 (randomization) and visit 3 (end of treatment) (left panels) and the corresponding distribution of the data (right panels) for the once-daily regimen (A), the twice-daily regimen (B), and the thrice-daily regimen (C).
Correlation between visit 3/visit 2 individual ratios for sTXB2 and urinary TXM values. The plot shows the linear correlation (blue line) between the ratios of sTXB2 and urinary TXM values at visit 3/visit 2 in all patients, according to the randomized treatment (correlation coefficient, r2 = +0.12; P < .0001). bid, twice daily; od, once a day; tid, thrice daily. The shaded area represents the 95% confidential interval of the linear regression line.
Correlation between visit 3/visit 2 individual ratios for sTXB2 and urinary TXM values. The plot shows the linear correlation (blue line) between the ratios of sTXB2 and urinary TXM values at visit 3/visit 2 in all patients, according to the randomized treatment (correlation coefficient, r2 = +0.12; P < .0001). bid, twice daily; od, once a day; tid, thrice daily. The shaded area represents the 95% confidential interval of the linear regression line.
A total of 239 patients (98%) completed the Severity of Dyspepsia Assessment questionnaire,28 the MPN Symptom Assessment Form questionnaire,30 and the pain numeric rating scale for erythromelalgia at visits 2 and 3. Patients who took aspirin, 100 mg thrice daily, had significantly higher scores for GI disturbances compared with the other groups (supplemental Table 1, available on the Blood Web site), even though none of the patients experienced GI adverse events requiring medical intervention. No major differences were observed in the microvascular disturbance scores, with the exception of 1 query related to sleeping difficulties, which were reduced in the twice-daily arm (supplemental Tables 2 and 3).
There were no major bleeding (defined according to the International Society of Haemostasis and Thrombosis)40 or adverse cardiovascular events during the 2-week randomized treatment or during the 2 weeks of observation after visit 3.
Discussion
The way in which low-dose aspirin prevents atherothrombosis is through permanent inactivation of platelet COX-1, resulting in virtually complete (ie, >97%) suppression of TXA2 production throughout the 24-hour dosing interval.20 There is consistency in the saturability of the acetylation of platelet COX-1,41 suppression of TXA2 formation,42 and reduction in atherothrombotic events at daily doses of aspirin in the range of 75 to 100 mg.20 Although the clinical efficacy of low-dose aspirin has been evaluated in subjects at variable risk for vascular occlusion, spanning the spectrum from asymptomatic healthy subjects43 to patients with acute ischemic syndromes,44 its use in MPNs has been largely based on extrapolation from non-MPN trials and from a single trial in polycythemia vera.10 In the absence of any aspirin trial in ET patients, justification for its use based on extrapolation from other clinical settings would require demonstrating comparable pharmacodynamics responses (ie, platelet TXA2 suppression) in ET and non-ET subjects. We reported preliminary evidence for aspirin-resistant platelet TXA2 production in patients with ET8 and showed that aspirin responsiveness could be rescued, at least in part, by shortening the dosing interval.17 We suggested that this reversible phenotype of biochemical “resistance” could be explained by accelerated renewal of the drug target (ie, platelet COX-1) because of pathological megakaryopoiesis.17
We designed the ARES study with 2 main objectives: (1) to demonstrate improved antiplatelet efficacy and preserved endothelial safety of an optimized aspirin-dosing regimen for primary and secondary prevention in a large population of patients with ET and (2) to assess long-term compliance with, and tolerability of, the selected regimen.23 This article deals with the results of the first component of the study.
We found high absolute values and marked interindividual variability in sTXB2, a validated biomarker of low-dose aspirin efficacy,24,25 with the vast majority of patients with ET displaying biochemical evidence of inadequate platelet inhibition when treated with a standard low-dose aspirin regimen. In fact, only ≈5% of non-ET subjects in previous studies had sTXB2 levels > 10 ng/mL at 24 hours after dosing, corresponding to <97% COX-1 inhibition,34,45 compared with 72% of patients with ET in the present study taking the same once-daily aspirin regimen. It should be emphasized that most traditional NSAIDs (with the possible exception of high-dose naproxen),46 inhibit platelet TXA2 production by <95%, which would correspond to a residual sTXB2 > 15 to 30 ng/mL (depending on platelet count), a level comparable to the average basal value (19 ng/mL) measured in our aspirin-treated ET patients. Incomplete platelet COX-1 inhibition by NSAIDs is not sufficient to exert a cardioprotective effect or to protect against COX-2–dependent cardiotoxicity.47
We could demonstrate with high statistical confidence that twice-daily aspirin administration reduced interindividual variability in sTXB2 and lowered the residual sTXB2 level by ≈90%. However, no further improvement in antiplatelet pharmacodynamics was achieved by a thrice-daily regimen, suggesting that a ceiling was reached in matching accelerated renewal of the drug target with a shortened dosing interval. Both experimental regimens similarly reduced in vivo TXA2-dependent platelet activation, as reflected by urinary TXM excretion, consistent with saturability of platelet COX-1 inactivation with twice-daily aspirin administration in ET. The apparent endothelial safety of such a regimen in sparing PGI2 biosynthesis confirms the preliminary findings in a small sample of ET patients,22 apparently at odds with earlier findings in healthy subjects.26 It remains to be established whether the markedly different study design and size and/or the enteric-coated vs plain aspirin formulation used in these studies accounts for the apparent discrepancy.
Based on the present results, we have chosen aspirin, 100 mg twice daily, as the experimental regimen to be compared with the standard once-daily 100-mg regimen for maintenance of superior antiplatelet efficacy, compliance, and tolerability in the long-term phase of the ARES study, in which the same patients with ET are being rerandomized to 1 of the 2 aspirin regimens.
We conclude that (1) the currently recommended aspirin regimen, 75 to 100 once daily, for primary or secondary cardiovascular prophylaxis is largely inadequate in reducing platelet activation in the vast majority of ET patients; (2) the antiplatelet response to low-dose aspirin can be markedly improved by shortening the dosing interval to 12 hours, with no significant improvement seen with additional decreases; and (3) the long-term superiority, compliance, and tolerability of an optimized aspirin regimen remain to be investigated in the ongoing phase of the ARES trial.23
Deidentified individual participant data that underlie the reported results will be made available 3 months after publication for a period of 3 years after the publication date. The study dataset is available at www.osf.io, upon request. Requests for access should be sent to Alberto Tosetto (alberto.tosetto@aulss8.veneto.it); they will be subjected to review and approval by the Steering Committee of the study.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Aspirin and placebo used in the trial were generously provided by Bayer AG, courtesy of Elmar Detering and Paolo Montanari.
The ARES trial was funded by the Italian Medicines Agency (study code FARM12Y8H).
Authorship
Contribution: V.D.S., B.R., A. Tosetto, F.R., and C. Patrono designed the study, analyzed the data, and wrote the manuscript; D.S. and A. Timillero coordinated the study; G.P., V.C., and B.P. measured serum and urine biomarkers; and all authors recruited patients and collected data and samples.
Conflict-of-interest disclosure: V.D.S. has received consulting and lecture fees from Amgen, Bayer, Celgene, and Novartis and has received institutional research grants from Novartis. E.M.E. has received advisory board and project fees from Novartis. F.P. has received consulting and lecture fees from Novartis. C. Patrono has received consulting and lecture fees from Acticor Biotech, Amgen, Bayer, GlaxoSmithKline, and Zambon and has received institutional research grants from Bayer; he serves as Chairperson of the Scientific Advisory Board of the International Aspirin Foundation. B.R. has received consulting and lecture fees from Bayer AG and Novartis. F.R. has received consulting and lecture fees and institutional research grants from Novartis and Amgen. A.M.V. has received consulting and lecture fees from Italfarmaco, Novartis, Shire, and Celgene. The remaining authors declare no competing financial interests.
A complete list of the members of the Aspirin Regimens in Essential Thrombocythemia (ARES) Investigators appears in “Appendix.”
Correspondence: Valerio De Stefano, Department of Radiological and Hematological Sciences, Section of Hematology, Catholic University, Fondazione Policlinico Universitario A. Gemelli IRCCS, Largo Gemelli, 8, 00168 Rome, Italy; e-mail: valerio.destefano@unicatt.it.
Appendix: study group members
The members of the Aspirin Regimens in Essential Thrombocythemia (ARES) Investigators are: Valerio De Stefano, Elena Rossi, Silvia Betti, Tommaso Za, Angela Ciminello, Francesca Bartolomei, Denise Soldati, Giulia De Santis (Fondazione Policlinico Universitario A. Gemelli IRCCS and Catholic University School of Medicine, Rome, Italy); Carlo Patrono, Bianca Rocca, Giovanna Petrucci (Catholic University School of Medicine, Rome, Italy); Marco Ruggeri, Alberto Tosetto, Giuseppe Carli, Ilaria Nichele, Stefania Bellesso (Ospedale San Bortolo, Vicenza, Italy); Francesco Rodeghiero, Andrea Timillero (Hematology Project Foundation, Vicenza, Italy); Viviana Cavalca, Benedetta Porro (Centro Cardiologico Monzino IRCCS, Milan, Italy); Alessandra Iurlo, Daniele Cattaneo, Cristina Bucelli, Silvia Artuso (Foundation IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy); Alfredo Dragani, Mauro Di Ianni, Paola Ranalli, Giovanna Summa, Celeste Santone (Santo Spirito Hospital, Pescara, Italy); Francesca Palandri, Nicola Vianelli, Giuseppe Auteri, Stefania Giaquinta (Sant’Orsola-Malpighi Hospital, Bologna, Italy); Eloise Beggiato, Giuseppe Lanzarone, Miriana Arminio (University of Torino, Torino, Italy); Elena Maria Elli, Monica Carpenedo, Stefania Priolo (Ospedale San Gerardo ASST, Monza, Italy); Maria Luigia Randi, Irene Bertozzi, Giulia Bogoni (University of Padova, Padova, Italy); Alessandro Maria Vannucchi, Paola Guglielmelli, Francesco Mannelli, Giacomo Coltro, Chiara Paoli, Enrica Ravenda (CRIMM-Center of Research and Innovation of Myeloproliferative Neoplasms, Azienda Ospedaliera Universitaria Careggi and University of Firenze, Firenze, Italy); Giorgina Specchia, Francesco Albano, Alessandra Ricco, Paola Carluccio, Marianna Gentile (University of Bari, Bari, Italy); and Elena Carafelli (Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy).
REFERENCES
Author notes
B.R. and A. Tosetto contributed equally to this work.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal