Abstract
Lymphomas afflict all age groups of people, with certain types demonstrating a female predilection in adolescents and young adults. A proportion of lymphomas that are diagnosed in this population demographic occur in the setting of pregnancy. Most of these behave aggressively at presentation and require immediate or urgent therapy. Treatment must consider both maternal and fetal health, and management approaches are therefore influenced by gestational age at diagnosis and treatment and timing of delivery. Although there is a paucity of literature on how to treat these patients, limited retrospective reports demonstrate generally good outcomes and highlight the necessity of an experienced multidisciplinary team approach to management.
Introduction
A diagnosis of cancer, as a complication of pregnancy, is an uncommon occurrence with an incidence of approximately 1 in 1000.1-3 Lymphoma is the fourth most common cancer diagnosis in pregnancy, with Hodgkin lymphoma (HL) occurring with the highest frequency, followed by non-Hodgkin lymphoma (NHL), a high proportion of which is primary mediastinal B-cell lymphoma (PMBCL).4,5 HL and NHL represent approximately 6% and 5% of all pregnancy-related cancers, respectively. A diagnosis of lymphoma during pregnancy presents many challenges, and the delivery of optimal curative therapy has to consider unique risks with respect to the mother and developing fetus. In that respect, it is critical that a multidisciplinary team including specialists from maternal-fetal medicine, anesthesiology, neonatology, and hematology/oncology, with experience providing care for this unique population, are involved in major decision making. A diagnosis of lymphoma in pregnancy raises unique and nuanced clinical and ethical dilemmas that require careful discussion and consideration of the best treatment approach to ensure optimal outcomes for the woman and her unborn child. Although the aggressiveness and extent of the lymphoma are important determinants of the need for urgent initiation of treatment, the therapeutic approach is significantly impacted by the trimester in which the lymphoma diagnosis occurs. Given the rarity of a lymphoma diagnosis in pregnancy, there is a paucity of literature on how best to treat these complex cases, and prospective studies are lacking. We present 2 cases of lymphoma in women during pregnancy and discuss our approach to their management that considers maternal and fetal needs.
Case 1
A 37-year-old woman, in her second ongoing pregnancy, presents to the emergency room at 30 weeks and 6 days (30+6 weeks) gestation with a history of chest pain and shortness of breath that woke her from sleep. She also has cramps in her right calf. She has been experiencing increasing shortness of breath and palpitations when mobilizing over a few weeks. She is a nonsmoker who drinks no alcohol. There are no concerns with her baby, and her first pregnancy and birth were uncomplicated. On examination, she is tachycardic at 120 beats per minute, normotensive with a blood pressure of 114/60 mm Hg, oxygen saturations of 97% on room air, and afebrile. Distended jugular veins are noted bilaterally, and she has no chest wall tenderness. Chest auscultation revealed crackles in the right lower zone. Heart sounds are dual with no murmurs. She has no lymphadenopathy, and abdominal examination reveals an enlarged uterus as expected for her dates with no other abnormalities. Complete blood count is normal. A comprehensive metabolic panel is normal other than an elevated lactate dehydrogenase (448 U/L; reference range, 120-250 U/L) and C-reactive protein (70 mg/L; reference range, 1-5 mg/L). The initial clinical concern for a pulmonary embolism prompted a computed tomography (CT)-pulmonary angiogram. This shows a large mediastinal mass measuring 11.5 × 8.3 × 8.3 cm causing significant obstruction of the superior vena cava and left main pulmonary artery and narrowing of the left upper lobe bronchi (Figure 1A-B). A small pericardial effusion is noted. Differential diagnosis includes lymphoma with other possibilities of thymic neoplasm, teratoma, or thyroid malignancy. A CT-guided biopsy of the mediastinal mass is performed and confirms focal areas showing proliferation of medium to large lymphoid cells. The large lymphoid cells are positive for CD20, CD79a, and PAX5, as well as BCL6, and have weak BCL2 staining. CD23 and CD30 show weak patchy positivity in the large cells and CD10, CD15, MUM1, cyclin D1, and Epstein-Barr virus in situ hybridisation (ISH) are negative. The Ki67 proliferative fraction is 60%. Fluorescence in situ hybridization studies reveal no rearrangement of MYC, BCL2, or BCL6. Pathologic features are consistent with a diagnosis of PMBCL.
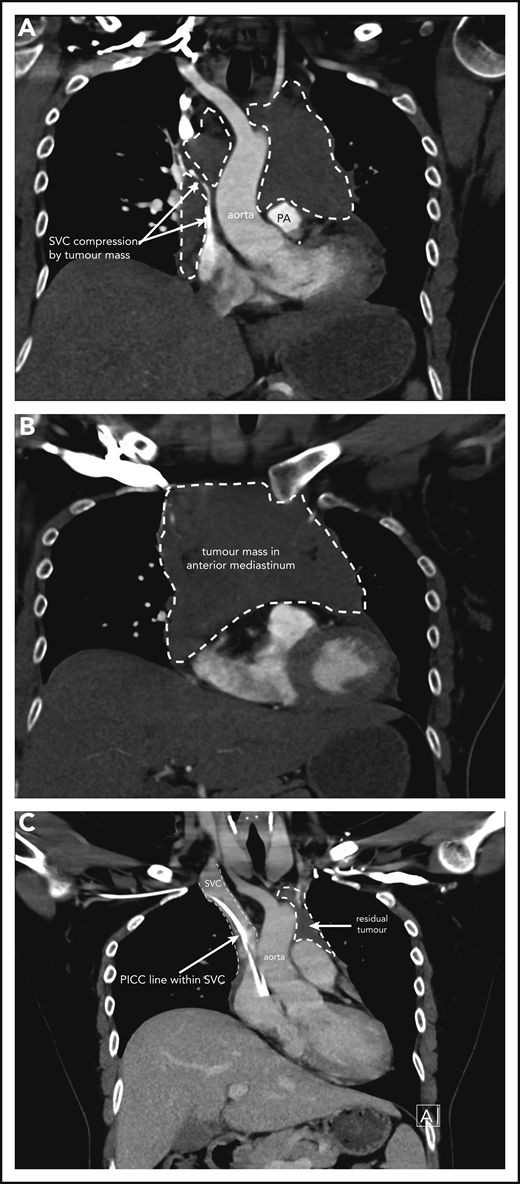
Case 1 of patient with primary mediastinal B-cell lymphoma presenting at 31weeks gestation. (A) Coronal section chest CT scan with contrast at diagnosis, pretreatment. Illustrates significant compression of SVC by tumor mass (within broken lines) on right and demonstrating extent of mass also on left superior to pulmonary artery (PA). (B) Transverse section chest CT scan with contrast at diagnosis, pretreatment. Illustrates extent of anterior mediastinal tumor mass. CT imaging suggested possible moderate-sized pericardial effusion found on echocardiography to be a small effusion. (C) Coronal section chest CT scan with contrast at completion of treatment. Normal caliber SVC with a peripherally inserted central catheter (PICC) line in situ. Small volume of residual tumor (within broken lines). Subsequent PET-CT scan demonstrated no activity confirming a complete metabolic response.
Case 1 of patient with primary mediastinal B-cell lymphoma presenting at 31weeks gestation. (A) Coronal section chest CT scan with contrast at diagnosis, pretreatment. Illustrates significant compression of SVC by tumor mass (within broken lines) on right and demonstrating extent of mass also on left superior to pulmonary artery (PA). (B) Transverse section chest CT scan with contrast at diagnosis, pretreatment. Illustrates extent of anterior mediastinal tumor mass. CT imaging suggested possible moderate-sized pericardial effusion found on echocardiography to be a small effusion. (C) Coronal section chest CT scan with contrast at completion of treatment. Normal caliber SVC with a peripherally inserted central catheter (PICC) line in situ. Small volume of residual tumor (within broken lines). Subsequent PET-CT scan demonstrated no activity confirming a complete metabolic response.
Staging of lymphoma in pregnancy
Staging modalities for lymphoma include CT imaging, 18F-flurodeoxyglucose positron emission tomography (FDG-PET), and less frequently, whole-body magnetic resonance imaging (MRI). Minimizing exposure of the fetus to ionizing radiation is prudent given that there is no defined safe level when considering its carcinogenic risks.6 Radiation doses to the fetus and mother with common staging investigations are outlined in Table 1. The radiation dose can be described as either the dose that is delivered to the tissue, usually reported in milligray or by the effective dose of radiation, reported in millisieverts that describes the biologic effect of the radiation delivered and is dependent on the type of radiation and the tissue that is exposed.7 Whole body MRI is frequently used as an alternative to PET-CT scanning because it does not expose the woman or fetus to potentially harmful ionizing radiation. Mediastinal disease may be assessed by CT imaging, and MRI, without gadolinium, is the preferred imaging modality for the abdomen and pelvis.
Radiation doses to the fetus and mother with common staging investigations
| Investigation . | Effective fetal radiation dose (mSv) . | Effective maternal radiation dose (mSv) . |
|---|---|---|
| Chest X-ray | 0.0005-0.01 | 0.02 |
| CT chest | 0.01-0.66 | 4-18 |
| CT pulmonary angiogram | 0.01-0.66 | 13-40 |
| CT abdomen and pelvis | 13-25 | 3-45 |
| Nuclear medicine | ||
| 18FDG-PET | 1.4-5.2 | 3-9 |
| 18FDG-PET/CT | 10-22 | 13-32 |
| Whole body MRI | None | None |
| Abdominal ultrasound | None | None |
| Investigation . | Effective fetal radiation dose (mSv) . | Effective maternal radiation dose (mSv) . |
|---|---|---|
| Chest X-ray | 0.0005-0.01 | 0.02 |
| CT chest | 0.01-0.66 | 4-18 |
| CT pulmonary angiogram | 0.01-0.66 | 13-40 |
| CT abdomen and pelvis | 13-25 | 3-45 |
| Nuclear medicine | ||
| 18FDG-PET | 1.4-5.2 | 3-9 |
| 18FDG-PET/CT | 10-22 | 13-32 |
| Whole body MRI | None | None |
| Abdominal ultrasound | None | None |
The radiotracer used in PET imaging crosses the placenta, and the delivered radiation dose to the fetus is higher in the first and second trimesters.8 There is limited increased benefit with FDG-PET imaging over CT at diagnosis, and its major clinical utility is at end of therapy and interim assessment. This argues against its routine use at the time of diagnosis, and as most women will be postpartum at the time point of end of therapy assessment, it limits the exposure of the fetus to additional radiation.9 We recommend that PET imaging be avoided during pregnancy and replaced by MRI or plain chest radiography for the (interim) assessment of the mediastinum. If bone marrow and/or cerebrospinal fluid assessments are necessary, they should be performed as per the guidelines for nonpregnant patients.
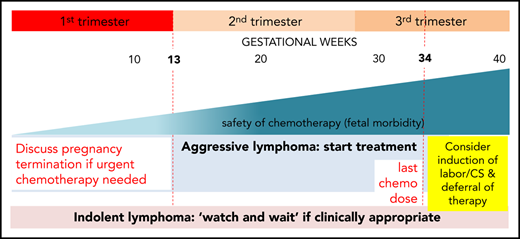
Optimal time to initiate therapy
Although certain cancers that may occur during pregnancy have indolent clinical courses and therefore may not need to be treated urgently, this is typically not the case for lymphoma. Our patient is symptomatic from a large mediastinal mass with symptoms and signs of superior vena cava (SVC) obstruction and therefore requires prompt initiation of treatment. Cesarean section (CS) or induction of labor are both feasible in the third trimester; however, careful consideration must be given to timing of birth to maximize fetal maturity and, as much as possible, avoid preterm birth (before 37 weeks of gestation), which exposes the infant to a higher risk of complications. Short-term complications include respiratory distress syndrome, intraventricular hemorrhage, sepsis, and perinatal mortality, with longer-term complications including cerebral palsy, chronic lung disease, and retinopathy of prematurity. Complications are more common in infants born very preterm and growth restricted, but even infants born late preterm (born between 34 weeks and 36+6 weeks gestation) have higher rates of neonatal complications, feeding problems, and hypoglycemia.10 Potential complications of prematurity argue against immediate induction of labor or CS before administration of chemotherapy in our patient who is 31 weeks of gestation.
Administration of chemotherapy while the woman is pregnant, thus exposing her infant to potential complications with these agents, must be balanced with the very real risks associated with prematurity. It is accepted that the chemotherapy agents will cause neutropenia and immune suppression in the fetus, and it is recommended that delivery is delayed for 3 weeks after administration of chemotherapy to avoid birth when the infant will be immunocompromised. In practice, this generally means that, for most women, chemotherapy would not be given after 34 weeks gestation. It is important to discuss a woman’s preferences for birth and review her prior obstetric history especially a history of preterm birth.
An additional risk is the presence of impending SVC and airway obstruction. If CS is required, although neuraxial anesthesia is usually successful, general anesthesia may be required, and in this woman, the presence of impending SVC and airway obstruction would make administration of a general anesthetic extremely hazardous.
In the case described, we favor planning of birth as soon as it is safe to do so with minimal risks to the fetus and mother. Full diagnosis and staging were complete by 33 weeks of gestation. Giving the first cycle of chemotherapy would have the benefit of reducing the size of the large mediastinal mass and relieving the SVC and airway obstruction. This would make the birth safer for the woman and gain fetal maturity with limited exposure to immunochemotherapy. Birth after 36 weeks of gestation carries a low risk of complications of prematurity, and timing of administration of chemotherapy to plan for birth after this gestation should be the goal.
What therapy should be used?
Because PMBCL is relatively rare and a recently described distinct clinicopathologic entity by the World Health Organization Classification, there are few prospective studies and no randomized prospective trials to inform on what the optimal approach should be. Although once considered to be a subset of diffuse large B-cell lymphoma (DLBCL), the disease is clinically and molecularly very different from other types of DLBCL.11 In fact, its clinical and biologic characteristics resemble classical Hodgkin lymphoma (cHL) much more closely.12,13 Early studies in the disease, albeit retrospective comparisons, suggested that regimens with higher-dose intensity than cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) were associated with improved survival, and hence these have been developed for PMBCL. The important role of dose intensity is not surprising given the close biologic relationship of PMBCL to cHL, where higher dose intensity is beneficial in certain high-risk patients. One single-center prospective study and a large multicenter retrospective study demonstrated high efficacy that obviated the need for radiation therapy with the dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, etoposide, and rituximab (DA-EPOCH-R) regimen.14,15 Recognizing that there has not been a direct comparison in PMBCL specifically of DA-EPOCH-R and rituximab + CHOP (R-CHOP; the standard regimen in DLBCL) but high single-arm efficacy with the former regimen, we would favor instituting DA-EPOCH-R for PMBCL in the third trimester. Etoposide as is the case with most of the other drugs has not been well studied in pregnancy, but limited retrospective experience with it, particularly in the third trimester, suggests that it is safe. Other approaches that are more intensive than R-CHOP such as rituximab + doxorubicin, cyclophosphamide, vindesine, bleomycin, and prednisone (R-ACVBP) have been used successfully in young nonpregnant patients, but to our knowledge, there are little retrospective data on their use in pregnancy. The decision on which regimen to use is more challenging in the earlier trimesters as discussed in the context of case 2. Localized radiation treatment is used extremely rarely in the management of SVC syndrome in the context of lymphoma given its sensitivity to steroids and chemotherapy; we would recommend their use once the diagnosis is made. For most patients presenting with DLBCL in pregnancy, we recommend R-CHOP therapy.16 In a nongestational setting, high-grade B-cell lymphoma with chromosomal rearrangements of MYC and BCL2 and/or BCL6 are rarely encountered in younger patients and may benefit from an alternative approach. This should be no different in the setting of pregnancy.
Case 1 follow-up
The patient initially received high-dose steroids for treatment of her SVC syndrome once the diagnosis was made. She then received her first cycle of DA-EPOCH-R therapy at 33+3 weeks of gestation and tolerated it very well. Additionally, growth colony-stimulating factor was administered with no complications. An MRI of the chest was performed 2 weeks later and showed reduction in the size of the mediastinal mass to 8.6 × 4.5 × 5.4 cm with significant improvement in SVC and airway compression. Labor was induced at 35+5 weeks, and our patient had an uncomplicated vaginal birth to a healthy girl (birth weight, 2585 g) who had no neonatal complications. She received her second cycle of DA-EPOCH-R on day 22 as per the chemotherapy regimen without complication on the fourth postpartum day and subsequently completed a total of 6 cycles of therapy. CT and FDG-PET scans were done at completion of therapy, showing significant shrinkage of the mass on CT (Figure 1C) and complete metabolic response on FDG-PET, and she did not require consolidation mediastinal radiation. She remains in complete remission at last follow-up.
Case 2
A 38-year-old woman at 12+4 weeks of gestation in her third pregnancy presents to the emergency department with a 2-hour history of dull central chest pain that had resolved on her arrival. Her first pregnancy was uncomplicated, with a vaginal birth at 41 weeks of gestation. A few months before this presentation, her second pregnancy ended in miscarriage at 6 weeks of gestation. She has no history of fevers, night sweats, or weight loss. A chest radiograph shows marked abnormality of the cardio-mediastinal contour with an impression of a large anterior mediastinal mass. A subsequent chest CT scan shows a large infiltrating anterior mediastinal mass measuring 17.6 × 9.8 × 8.8 cm, encasing but not compressing vessels of the mediastinum but infiltrating the pretracheal space. There is no pericardial effusion and no supraclavicular, axillary, or hilar lymphadenopathy. Additionally, there is no focal pulmonary abnormality and no pleural fluid. A complete blood count is normal, and there is elevation of C-reactive protein at 53 mg/L (reference range, 1-5 mg/L) and lactate dehydrogenase at 292 U/L (reference range, 120-250 U/L). A core biopsy of the mass is performed and demonstrates cores of tissue that are predominantly fibrotic, with scattered lymphoid aggregates centered on foci of granulomatous inflammation. The granulomata are associated with clustered eosinophils, some of which are degranulated. Moderate numbers of large cells with hyperchromatic nuclei and prominent eosinophilic nucleoli with a moderate amount of amphophilic cytoplasm are present. Reed-Sternberg cells were present, and background lymphocytes reveal a mixture of small- and medium-sized lymphocytes with no atypical features and plasma cells present in very low numbers. The large cells are positive for CD30, CD15, PAX5, and MUM1 and negative for ALK, Epstein-Barr virus ISH, CD23, CD20, CD3, CD68, and CKAE1/AE3. Small background lymphocytes are predominantly T lymphocytes that are positive for CD3. The pan-cytokeratin stain highlights thymic epithelial elements infiltrated by the neoplastic population. The appearances and immune profile are in keeping with HL, nodular sclerosing subtype. She has no disease outside the mediastinum on MRI imaging, and therefore, the isolated mediastinal bulky disease stratifies her to early-stage unfavorable HL.
Management of lymphoma in early pregnancy
Organogenesis is ongoing in the embryo until 10 weeks of gestation (8 weeks after conception), so there is higher potential for teratogenicity from medications or radiation until this period is complete. The fetal phase begins at 10 weeks of gestation until birth, with continued growth and maturation of formed organs and exposure to medications during this phase may result in growth restriction but typically not gross structural abnormalities. Some organs with potential higher vulnerability to chemotherapy include the central nervous system, hematopoietic system, and eyes, but in general, administration of chemotherapy in the second and third trimester has been considered relatively safe.17,18 However, as with other drugs, chemotherapy may affect fetal organ functionality. In 1 large published (retrospective) experience of lymphoma in pregnancy (90 women; Table 2), 11 patients were diagnosed in the first trimester, and of these, 6 pregnancies were terminated to enable immediate chemotherapy.19 In this same series, the median gestational age for women (n = 56) who received antenatal therapy was 22 weeks. Combination chemotherapy was administered to 89% of women, with 37 (66%) receiving therapy in the second trimester. Therapy was deferred in 28 (33%) women until postpartum who were diagnosed at a median of 30 weeks of gestation. There were no increased pregnancy complications such as preterm birth, preterm induction of labor, premature rupture of membranes, or preeclampsia in women who received antenatal vs deferred (postnatal) therapy. Median birth weight was similar, but there was a trend to small-for-gestational age infants in women who received antenatal chemotherapy. This data set did not identify significant increased fetal morbidity or mortality with treatment institution in the second and third trimesters.20,21 Another recent study looking at 134 pregnant women with HL identified that survival did not differ between pregnant and nonpregnant women, but preterm labor and rupture of membranes was higher in HL patients who received antenatal therapy compared with cases where it was deferred until postpartum.22 Institution of chemotherapy in the first trimester is not well studied, and outcomes are likely very dependent on the gestational time point because it is the period of major organogenesis. For patients diagnosed with HL in late first trimester (as our case) with advanced symptomatic disease, urgent therapy initiation (or with short delays to start in the second trimester) is reasonable.
Selected retrospective studies looking at outcome in women with lymphoma diagnosed during pregnancy
| Authors . | Study population . | T1, T2, and T3 . | Outcome/survival . | Fetal morbidity/mortality . |
|---|---|---|---|---|
| Evens et al19 | 90 patients: HL:50; NHL: 40 | T1, 12% | 3-y PFS and OS: 53% and 82% for NHL and 85% and 97% for HL, respectively | Gestation full term in 56% of cases; delivery at median of 37 wk; induction of labor in 33%; 6 patients underwent elective termination |
| T2, 58% | ||||
| T3, 38% | ||||
| Pinnix et al23 | 39 patients: HL 31; NHL 8 | T1, 8% | 5-y PFS and OS: 75% and 82%, respectively | Delivery at a median of 37 wk; 3 patients underwent elective termination; no fetal abnormalities were observed |
| T2, 67% | ||||
| T3, 25% | ||||
| Maggen et al22 | 134 patients with HL | 5-y PFS and OS: 82.6% and 97.3% for early stage HL and 90.9% and 100% for advanced HL, respectively | Preterm contractions (12%) and rupture of membranes (5%), higher in patients receiving antenatal treatment |
| Authors . | Study population . | T1, T2, and T3 . | Outcome/survival . | Fetal morbidity/mortality . |
|---|---|---|---|---|
| Evens et al19 | 90 patients: HL:50; NHL: 40 | T1, 12% | 3-y PFS and OS: 53% and 82% for NHL and 85% and 97% for HL, respectively | Gestation full term in 56% of cases; delivery at median of 37 wk; induction of labor in 33%; 6 patients underwent elective termination |
| T2, 58% | ||||
| T3, 38% | ||||
| Pinnix et al23 | 39 patients: HL 31; NHL 8 | T1, 8% | 5-y PFS and OS: 75% and 82%, respectively | Delivery at a median of 37 wk; 3 patients underwent elective termination; no fetal abnormalities were observed |
| T2, 67% | ||||
| T3, 25% | ||||
| Maggen et al22 | 134 patients with HL | 5-y PFS and OS: 82.6% and 97.3% for early stage HL and 90.9% and 100% for advanced HL, respectively | Preterm contractions (12%) and rupture of membranes (5%), higher in patients receiving antenatal treatment |
HL, Hodgkin lymphoma; NHL non-Hodgkin lymphoma; OS, overall survival; PFS, progression-free survival; T1, first trimester; T2, second trimester; T3, third trimester.
Choice of treatment approach for HL
Adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD) administered every 2 weeks is the most commonly used regimen in the treatment of HL during pregnancy.23 Several reports demonstrate that it can be safely administered in the second and third trimesters. For patients with advanced stage disease, alternatives such as brentuximab with AVD (omission of bleomycin) or escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone may be considered for HL outside of pregnancy.24 However, these are significantly more toxic than ABVD, there is a paucity of experience using these platforms in pregnancy, and we would therefore recommend avoiding their use in pregnancy at this point in time. For novel agents such as brentuximab, there are no data regarding their use in pregnancy, and their potential teratogenic and toxic effects are unknown.25,26 In terms of duration of therapy, for early-stage favorable patients, shorter duration of ABVD may be feasible, and for advanced-stage patients who are disease negative after 2 cycles, the bleomycin in ABVD may be dropped if interim PET results are negative.27,28 There is little experience using radiation as a therapeutic modality for lymphoma during pregnancy. We discuss its use below, but in this setting, we favor avoiding its antenatal use other than in exceptional circumstances.
Case 2 follow-up
Once the diagnosis was confirmed, and after careful counseling and an opportunity for the woman and her family to discuss the implications with therapy, our patient elected to proceed with ABVD chemotherapy. Critical to her decision was that she understood that in choosing to continue her pregnancy, she would receive the standard recommended chemotherapy regimen that would offer the best chance of cure, which at the same time would be highly unlikely to have a negative impact on the health of her unborn child. She had her first dose of ABVD at 15+4 weeks of gestation and tolerated it well with no significant complications. She continued to receive ABVD therapy every 2 weeks throughout the pregnancy, and an interim chest radiograph demonstrated significant reduction in the size of the mediastinal mass. Because of toxicity, shivers, and aches with chemotherapy, she stopped after 5 cycles of ABVD. She had spontaneous rupture of membranes at 37+1 weeks of gestation and went on to have an uncomplicated vaginal birth of a healthy boy (birth weight, 4195 g), and there were no neonatal complications. An FDG-PET/CT scan done after completion of therapy showed a complete metabolic remission, and the patient remains well with no evidence of disease at the most recent follow-up.
Other lymphomas in pregnancy
From the limited series that have looked at this, T-cell lymphomas make up a significant proportion of NHLs that occur during pregnancy. The principles of management of T-cell lymphomas diagnosed during pregnancy are the same as discussed above for our 2 cases; however, when these diseases occur in this age group, presentations are typically highly aggressive needing emergent chemotherapy, and frequently multiagent intensive regimens are necessary making the risk of complications to the mother and fetus high. This is also the case for Burkitt’s lymphoma diagnosed during pregnancy. Other rarer lymphomas that are encountered in this setting are follicular lymphoma and other indolent lymphomas such as mucosal-associated lymphoid tissue associated lymphoma: these diseases tend to have a much less aggressive presentation at diagnosis and mostly can be managed with a watch-and-wait approach. We would not recommend extensive radiologic work-up of these diseases when they are diagnosed in pregnancy, and this should be deferred (as should therapy if possible) to the postpartum period.
Radiation use during pregnancy
The role of radiation therapy in this age group of patients with a diagnosis of aggressive lymphoma is typically to improve localized disease control in the case of early-stage disease and occasionally for palliative goals. Although historical data have demonstrated that administration of supra-diaphragmatic radiation with shielding of the abdomen and pelvis can be a safe and feasible modality, considering the high efficacy of systemic therapy alone and albeit limited data on its safety in this setting, we recommend avoiding radiation if possible and, if needed, deferring its use to the postpartum period.29
Novel agent use for lymphoma treatment in pregnancy
Rituximab, the anti-CD20 monoclonal antibody, is an integral component of curative therapy for aggressive B-cell lymphomas. Its use has been described extensively in the literature for treating pregnant women with lymphoma and autoimmune diseases. Its association with hypogammaglobulinemia suggests that it could potentially augment the rate of neonatal infections, but this has not been clearly demonstrated.30 We recommend always using it in settings where it may augment the curability of chemotherapy. Brentuximab vedotin, an antibody-drug conjugate, is now US Food and Drug Administration approved for the front-line treatment of HL, but at this time, there is a lack of safety data with respect to its effect on the fetus, and we would therefore not favor its use in pregnancy. We have not discussed the management of relapsed and refractory lymphoma in pregnancy extensively because it is exceedingly rare. Given that and the many different treatment options compared with the newly diagnosed setting, each case should be tackled individually with consideration to the aforementioned approaches to treating lymphoma in pregnancy.
Supportive treatment
Granulocyte-colony stimulating factor (G-CSF) is frequently used in patients receiving certain chemotherapy regimens. Animal models suggest that G-CSF crosses the placenta and causes neutrophilia, but it has not been associated with adverse outcomes when used in pregnancy.31 An international registry of women who received G-CSF during pregnancy for severe chronic neutropenia reported no increase in rates of adverse outcomes.32 Its use in the second and third trimesters is considered safe, but it should be avoided or used very cautiously in the first trimester given the paucity of available data. The development of venous thromboembolism is more common in pregnancy, and a diagnosis of cancer is additionally a risk factor for this. We do not definitively recommend venous thromboembolism prophylaxis, and the decision to institute this and the type of prophylaxis given should be according to guidelines such as those recently published by the American Society of Hematology.33 During chemotherapy with typical regimens for HL and common types of NHL seen in this age group, a few supportive medications are commonly given. These include serotonin 5-HT3 receptor antagonists such as ondansetron, proton pump inhibitors such as omeprazole or pantoprazole, and antihistamines in patients who are receiving growth factors. Careful consideration should be given to instituting other classes of drugs, and we recommend consultation with a pharmacist familiar with obstetric prescribing. No class of drug should be prescribed unless the benefit outweighs the potential risks.
Fertility, future pregnancies, and survivorship
Recommended options for fertility preservation in women receiving chemotherapy include embryo and egg freezing.34 The efficacy of ovarian protection techniques such as gonadotropin-releasing hormone analogs and hormonal suppression is uncertain, and they are not routinely recommended. Advances in cryopreservation and transplantation of ovarian tissue continue, but there is a lack of mature data to guide their application. These options were not available in either of the women presented because pregnancy precluded the use of these modalities for fertility preservation.
No data have demonstrated an increased risk of relapse in this population. Postpartum surveillance imaging in follow-up should be no different from other settings and in accordance with National Comprehensive Cancer Network or other similar guidelines. Survivorship follow-up should also be similar.
Conclusions
Lymphoma occurring in the setting of pregnancy represents a unique disease setting where optimal management requires close collaboration between a multidisciplinary team including maternal-fetal medicine specialists, anesthesiologists, and a hematologist/oncologist with expertise in the management of lymphoid diseases. Albeit limited retrospective experience, the maternal and fetal outcomes for most women diagnosed with lymphoma during pregnancy are excellent, and standard curative therapies are well tolerated for the most part. Some of the most difficult challenges are the management of lymphoma in the first trimester and deciding about the optimal time of delivery in the third trimester. As novel agents become more widely used in the treatment of lymphoma, it is vital that experience and safety data with their use during pregnancy are captured to inform on future management of this patient population.
Authorship
Contribution: K.D. and C.M. wrote the review and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Claire McLintock, Women's HealthNB, Auckland City Hospital, Grafton Rd, Auckland 1143, New Zealand; e-mail: doctorclaire@redhealth.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal