Abstract
Thrombotic thrombocytopenic purpura (TTP) is an acute, life-threatening thrombotic microangiopathy (TMA) caused by acquired or congenital severe deficiency of ADAMTS13. Pregnancy is a recognized risk factor for precipitating acute (first or recurrent) episodes of TTP. Differential diagnosis with other TMAs is particularly difficult when the first TTP event occurs during pregnancy; a high index of suspicion and prompt recognition of TTP are essential for achieving a good maternal and fetal outcome. An accurate distinction between congenital and acquired cases of pregnancy-related TTP is mandatory for safe subsequent pregnancy planning. In this article, we summarize the current knowledge on pregnancy-associated TTP and describe how we manage TTP during pregnancy in our clinical practice.
Introduction
Thrombotic thrombocytopenic purpura (TTP) is a rare, potentially life-threatening acute thrombotic microangiopathy (TMA). It is caused by a severe deficiency of the von Willebrand factor (VWF)-cleaving protease ADAMTS13, resulting in the presence of highly adhesive unusually large VWF (ULVWF) multimers, which causes inappropriate platelet clumping in the microvasculature under high shear stress conditions. The subsequent ischemic damage can affect almost any organ but generally affects the brain, digestive tract, heart, and/or kidneys.
In the vast majority of cases, severe ADAMTS13 deficiency (ie, activity levels <10%) is autoantibody mediated (acquired TTP [aTTP]), while congenital defects (congenital TTP [cTTP]) account for <5% of cases.
Pregnancy (including the postpartum period) is a recognized risk factor for acute (first or recurrent) episodes of TTP.
As an autoimmune disorder, aTTP typically affects young women, mainly in their third and fourth decades. On the other hand, a large proportion of adult-onset cTTP patients experience their debut during their first pregnancy.1 Overall, it is estimated that almost half of all acute TTP episodes occur in women of childbearing age and that pregnancy-associated TTP accounts for 12% to 25% of adult-onset TTP cases.1-5 Moreover, after being diagnosed with a pregnancy-onset TTP, the likelihood of having a congenital form is much higher (≤24% in the French TMA National Registry and ≤66% in the UK TTP Registry) than expected in adulthood-onset TTP in general (<5%).6,7
The association between TTP and pregnancy no longer seems to be a coincidence but is related to the hemostatic and immunologic changes that characterize pregnancy and the postpartum period. Physiologic gestation is accompanied by extensive changes in all aspects of hemostasis, leading to a state of hypercoagulability8,9 that is most pronounced in the late third trimester to decrease bleeding complications during delivery, followed by a gradual normalization during the first 4 to 6 weeks postpartum. With regard to the main actors of TTP pathogenesis, VWF concentrations progressively rise throughout pregnancy, reaching the maximum increase in the last trimester. Conversely, ADAMTS13 levels progressively decrease from the second trimester (>12 weeks’ gestation) through the end of the early postnatal period (1-3 days after delivery), reaching values as low as 25% to 30%, and then returning to normal levels by the late puerperium period (7-21 days after delivery).10 The progressive decrease in ADAMTS13 levels during gestation has been associated with consumption of the protease by the higher VWF levels.10 In addition, estrogen control over the protease may also play a role, as suggested by the detection of lower ADAMTS13 levels in primigravidae, whose estradiol levels are significantly higher than in subsequent pregnancies.11 These physiological gestational changes in the VWF-ADAMTS13 balance clearly explain why every single pregnancy represents a challenge for severely ADAMTS13-deficient women, especially for congenital cases, whose risk of pregnancy-associated TTP is almost 100%.12,13
Changes in the maternal immune system may also play a role. Immune tolerance toward the semiallogeneic fetus is necessary for the physiologic progression of gestation14 and the postpartum period is associated with the recovery of gestational immune tolerance, which can lead to autoimmune disorders, either de novo (such as acquired factor VIII inhibitors) or relapsing (such as lupus erythematosus systemic recrudescence). Autoimmune aTTP could behave in a similar manner. Moreover, TTP can be associated to other autoimmune disorders (secondary TTP), which can manifest before, during, or after pregnancy, claiming for a complete evaluation of the autoantibody panel and subsequent appropriate management.
Although gravidic changes in the maternal hemostatic and immune systems clearly support the pathogenic link between pregnancy and TTP, they do not fully explain why a subset of women will experience acute aTTP only in association with pregnancy but never outside of pregnancy, claiming for further yet-unknown explanations (ie, additional role of hormonal regulation or genetic factors). Gravidic TTP represents a unique entity with distinguishing features in the field of TTP, not only for the specific pathogenetic mechanisms but also for its peculiar difficulties in differential diagnosis and management. Pregnancy-associated TTP is a medical emergency for both the affected woman and the fetus with specific disease-related and treatment-related risks. Therefore, the unicity of gravidic TTP needs a multidisciplinary approach involving expert hematologists and obstetricians, together with a laboratory team with expertise in hemostasis. Moreover, women with a history of TTP who are planning pregnancy should be offered a counseling, as the risk of gravidic TTP recurrence needs to be considered.
In this paper, we describe how we currently manage women with TTP during pregnancy, using clinical cases to highlight the features and dilemmas encountered when facing this challenging situation in clinical practice.
Discussion
Presentation of pregnancy-associated TTP
Case 1 is a 28-year-old woman admitted to the hospital at 13 weeks gestation during her first pregnancy for fatigue, headache, low-grade fever, nausea, and palpitations. Her medical history was uneventful before pregnancy. Physical examination was unremarkable except for tachycardia (110 beats per minute). Laboratory tests showed severe anemia and thrombocytopenia (hemoglobin [Hb], 4.4 g/dL; mean corpuscular volume [MCV], 98.5 fL; platelets, 8 × 103/μL). Lactate dehydrogenase (LDH) levels were 3× upper limit of normal (ULN) levels, while renal and liver functions, coagulation parameters, and C reactive protein (CRP) levels were normal. Coombs test result was negative, and schistocytes were detectable at blood smear. Fetal and placental scan results were normal.
Case 2 is a 38-year-old woman admitted to the hospital at 20 weeks gestation during her first pregnancy for fatigue and laboratory abnormalities (Hb, 8.1 g/dL; MCV, 94 fL; platelets, 20 000/μL). Her personal and family medical histories were uneventful before pregnancy. Physical examination was unremarkable except for isolated petechiae on her limbs; further laboratory examinations showed increased LDH levels (4.5× ULN), while renal and liver functions, coagulation parameters, and CRP levels were normal. Coombs test result was negative, and schistocytes were detected at blood smear. Fetal ultrasounds showed signs of intrauterine growth retardation (IUGR).
At disease onset, clinical features of acute TTP vary according to the degree of tissue ischemic injury (widespread but mainly involving central nervous system, digestive tract, heart, and kidneys), together with symptoms and signs related to anemia (fatigue and pallor) and thrombocytopenia (bleeding diathesis, mainly cutaneous). Patients usually present with petechiae, cerebral manifestations (mainly headache and confusion or behavioral changes but also seizure, focal deficits, and coma), or splanchnic disturbances (mainly nausea, vomiting, abdominal pain, or nonbloody diarrhea) and less frequently with cardiac symptoms (ie, palpitations, pain chest, or shortness of breath) or renal involvement (sometimes referred as dark urine, but usually mild and only detected by urine dipstick showing hematuria/proteinuria or increased serum creatinine). Peripheral edema and high blood pressure are not typical features of acute TTP.
When evaluating a pregnant woman with any of the problems above, a complete blood count (CBC) is mandatory; the presence of low platelets and low Hb levels requires further examinations to confirm microangiopathic hemolysis (increased total and indirect bilirubin, low haptoglobin, high LDH, high reticulocytes, negative Coombs test result, and schistocytes on peripheral blood smear), define the presence and severity of organ damage (liver, renal, and cardiac tests), and rule out other conditions (ie, CRP to exclude sepsis, coagulation screening tests for disseminated intravascular coagulation [DIC], and autoimmune screening for acute systemic vasculitis); an urgent ultrasound is also needed to assess fetoplacental status (ie, abruptio placentae or retention of dead fetus, which can trigger DIC) (Table 1).
Investigations required in the initial evaluation of gravidic TMAs
| Diagnostic parameters . | Role . |
|---|---|
| CBC (PLT, Hb, MCV), RET; LDH; bilirubin (total, indirect), haptoglobin; Coombs test; blood smear | To diagnose a TMA; MAHA with thrombocytopenia |
| Fetal ultrasound/uterine artery Doppler scan | To assess fetoplacental status |
| ALT, AST; creatinine, urinalysis, 24-h urine proteins; cardiac troponin, ECG; coagulation screening (PT, aPTT, D-dimer, fibrinogen); CRP, WBCs; autoimmune screening (aPL, ANA, ANCA); stool culture/STEC testing (if indicated) | To assess organ damage and make differential diagnosis |
| ADAMTS13 (activity and anti-ADAMTS13 antibody) | To confirm TTP clinical diagnosis |
| Diagnostic parameters . | Role . |
|---|---|
| CBC (PLT, Hb, MCV), RET; LDH; bilirubin (total, indirect), haptoglobin; Coombs test; blood smear | To diagnose a TMA; MAHA with thrombocytopenia |
| Fetal ultrasound/uterine artery Doppler scan | To assess fetoplacental status |
| ALT, AST; creatinine, urinalysis, 24-h urine proteins; cardiac troponin, ECG; coagulation screening (PT, aPTT, D-dimer, fibrinogen); CRP, WBCs; autoimmune screening (aPL, ANA, ANCA); stool culture/STEC testing (if indicated) | To assess organ damage and make differential diagnosis |
| ADAMTS13 (activity and anti-ADAMTS13 antibody) | To confirm TTP clinical diagnosis |
ANA, anti-nuclear antibody; ANCA, anti-neutrophil cytoplasmic autoantibody; aPL, anti-phospholipid antibodies (lupus anticoagulant, anticardiolipin, anti-β2-glycoprotein 1 antibodies); aPTT, activated partial thromboplastin time; ECG, electrocardiogram; PLT, platelet; RET, reticulocytes; PT, prothrombin time; STEC, Shiga toxin Escherichia coli; WBC, white blood cell.
In a case of acute TTP, particularly at the first episode, laboratory analyses usually show very severe thrombocytopenia (<30 000/μL, but often <10 000/μL) with Hb <8 g/dL and LDH >2× ULN and absent-to-mild increase of creatinine and transaminases. For aTTP, the onset of disease is typically hyperacute, while in congenital cases, isolated thrombocytopenia usually precedes overt hemolysis, mainly in the second and third trimester, and is potentially misdiagnosed as common gestational or immune thrombocytopenia. At that stage, cTTP women may be asymptomatic; therefore, only an accurate obstetric history (previous fetal loss or severe IUGR, pregnancy-related neurologic disturbances such as severe headaches, migraine, or depression),15 together with biochemical monitoring, may help to make the correct diagnosis. In practice, we advise for CBC serial monitoring in any pregnant woman with thrombocytopenia <100 000/μL, adding a blood film and LDH in case of platelets <75 000/μL, given the high prevalence of cTTP (as high as 5%) reported in a large obstetric population with such a low platelet count.16
In clinical practice, it is much more likely to face preeclampsia (PE) or hemolysis, elevated liver enzymes, low platelet count (HELLP) syndrome rather than pregnancy-associated TTP, given their reported incidences of 3% to 5%, 0.1% to 0.8%, and probably <0.00004%, respectively.15 However, the high fetomaternal mortality rate if unrecognized and untreated requires a high index of suspicion for TTP.
In the acute phase, it is crucial to distinguish TTP from other pregnancy- and postpartum-related TMAs, but a quick differential diagnosis is often challenging due to overlap in gestational age of onset and clinical and laboratory features; a close cooperation between the hematologist and the obstetrician is vitally important for both diagnosis and management, especially in those cases with concomitant disorders (ie, TTP plus PE). The list of gravidic TMAs is long, but those that concretely enter into differential diagnosis with TTP are essentially PE with severe features (PE-SF), HELLP syndrome, and complement-mediated hemolytic uremic syndrome (cHUS)17,18 ; acute systemic vasculitis (both ANCA associated and catastrophic anti-phospholipid syndrome) and DIC may also mimic gravidic TTP (Table 2).
Tips and tricks for differential diagnosis in pregnancy-associated TMAs
| . | Gestational age of onset . | Symptoms and signs . | Laboratory . | Effect of delivery . |
|---|---|---|---|---|
| Distinctive features (do help in differentiating) | ||||
| PE-SF | Do not consider if <20 wk or >72 h pp | New-onset hypertension | AST/ALT increase >>LDH increase | Resolution (<48-72 h pp) |
| Peripheral edema | ||||
| HELLP | Do not consider if <20 wk or >72 h pp | Predominant splanchnic involvement, jaundice | AST/ALT increase >>LDH increase | Resolution (<48-72 h pp) |
| Visual disturbances | ||||
| TTP | Strongly consider in late third trimester or >72 pp | Severe neurological impairment | PLT <30 000/μL | Possible worsening >48-72 h pp |
| Almost the only TMA in the first trimester | LDH increase, >>AST/ALT increase | |||
| Creatinine usually <1.1 mg/dL | ||||
| cHUS | Strongly consider in late third trimester or >72 pp | Hypertension | Creatinine usually >2 mg/dL | Possible worsening >48-72 h pp |
| Rare in the first half of pregnancy | LDH ≥8× ULN (>>AST/ALT increase) | |||
| Acute systemic vasculitis | All trimesters and postpartum | Hypertension | MAHA rare | Possible worsening >48-72 h pp |
| Acute nephritis | Creatinine usually >2 mg/dL | |||
| Small- and large-vessel thrombosis | aPL/ANA/ANCA positivity | |||
| aPTT may be increased | ||||
| Acute DIC | All trimesters and postpartum | Critical settings | MAHA rare | Possible worsening >48-72 h pp |
| Severe bleeding | PT, aPTT, D-dimer increase | |||
| Low fibrinogen | ||||
| Common features (do not help in differentiating) | ||||
| All the above TMAs | ≥20 wk to <48 h pp | Abdominal disturbances such as nausea, vomiting, abdominal pain | PLT <100 000/μL | Possible worsening <48-72 h pp |
| MAHA, Coombs negative | ||||
| Neurological disturbances such as headache, mild confusion | Creatinine >ULN <2 mg/dL | |||
| LDH > ULN <600 U/L | ||||
| AST/ALT >ULN |
| . | Gestational age of onset . | Symptoms and signs . | Laboratory . | Effect of delivery . |
|---|---|---|---|---|
| Distinctive features (do help in differentiating) | ||||
| PE-SF | Do not consider if <20 wk or >72 h pp | New-onset hypertension | AST/ALT increase >>LDH increase | Resolution (<48-72 h pp) |
| Peripheral edema | ||||
| HELLP | Do not consider if <20 wk or >72 h pp | Predominant splanchnic involvement, jaundice | AST/ALT increase >>LDH increase | Resolution (<48-72 h pp) |
| Visual disturbances | ||||
| TTP | Strongly consider in late third trimester or >72 pp | Severe neurological impairment | PLT <30 000/μL | Possible worsening >48-72 h pp |
| Almost the only TMA in the first trimester | LDH increase, >>AST/ALT increase | |||
| Creatinine usually <1.1 mg/dL | ||||
| cHUS | Strongly consider in late third trimester or >72 pp | Hypertension | Creatinine usually >2 mg/dL | Possible worsening >48-72 h pp |
| Rare in the first half of pregnancy | LDH ≥8× ULN (>>AST/ALT increase) | |||
| Acute systemic vasculitis | All trimesters and postpartum | Hypertension | MAHA rare | Possible worsening >48-72 h pp |
| Acute nephritis | Creatinine usually >2 mg/dL | |||
| Small- and large-vessel thrombosis | aPL/ANA/ANCA positivity | |||
| aPTT may be increased | ||||
| Acute DIC | All trimesters and postpartum | Critical settings | MAHA rare | Possible worsening >48-72 h pp |
| Severe bleeding | PT, aPTT, D-dimer increase | |||
| Low fibrinogen | ||||
| Common features (do not help in differentiating) | ||||
| All the above TMAs | ≥20 wk to <48 h pp | Abdominal disturbances such as nausea, vomiting, abdominal pain | PLT <100 000/μL | Possible worsening <48-72 h pp |
| MAHA, Coombs negative | ||||
| Neurological disturbances such as headache, mild confusion | Creatinine >ULN <2 mg/dL | |||
| LDH > ULN <600 U/L | ||||
| AST/ALT >ULN |
According to the American College of Obstetricians and Gynecologists, PE-SF is currently diagnosed when new-onset hypertension (≥160 mm Hg systolic or ≥110 mm Hg diastolic) occurs ≥20 wk of pregnancy, in conjunction with ≥1 severe feature (creatinine >1.1 mg/dL or 2× baseline; cerebral or visual disturbances; pulmonary edema; liver enzymes ≥2× ULN; severe or persistent abdominal pain in epigastric or right upper quadrant; platelets <100 000/μL), regardless of urine protein assessment. There is no consensus among the American College of Obstetricians and Gynecologists regarding the definition for HELLP.
pp, postpartum.
Pregnancy-associated TTP is most common in the third trimester and postpartum period but may occur throughout gestation.1 In practice, if the timing of presentation is the first trimester, TTP is the most likely diagnosis; between mid-gestation (>20 weeks) and the immediate postpartum period (<48 hours), all the aforementioned gravidic TMAs are equally likely, while >48 to 72 hours after delivery, both TTP and cHUS need to be ruled out, together with acute vasculitis. The effect of delivery on the clinical and biochemical course of gravidic TMAs is also relevant, since the absence of stabilization or improvement within 48 hours following delivery usually rules out PE-SF and HELLP syndrome. However, both TTP and cHUS do not improve or could even worsen after delivery in the absence of a concomitant targeted therapy.
The most frequent neurologic and splanchnic manifestations (headache, confusion, abdominal pain, nausea, or vomiting) do not help to distinguish TTP from other TMAs.
Thrombocytopenia is common to all TMAs with significant low platelet counts in TTP, which becomes the most likely diagnosis if platelets are <30 000/μL. Microangiopathic hemolysis is marked in TTP and cHUS, while it is usually milder in PE, DIC, and acute systemic vasculitis. LDH, a marker of both hemolysis and tissue ischemic damage, is markedly increased in TTP (and even more in cHUS, with mean levels >8× ULN in a large series), with a high LDH/aspartate aminotransferase (AST) ratio (>10:1) being proposed to distinguish TTP from PE-SF/HELLP syndrome, where liver damage prevails over hemolysis.19 Kidney injury (creatinine >2 mg/dL, proteinuria) and high blood pressure (≥160 mm Hg systolic or ≥110 mm Hg diastolic) may sometimes be detected in TTP but are far more common in cHUS, PE-SF, and in acute systemic vasculitis. With regard to acute systemic vasculitis, the differential diagnosis is based on the different patterns of organ damage (single and/or multiorgan dysfunction) related to the different size of affected vessels, with specific histopathological and imaging findings. Moreover, subsets of patients develop a vasculitis in association with other autoimmune rheumatic diseases (including systemic lupus erythematosus). The close cooperation with an experienced immunologist is crucial to properly frame these disorders.
Assessing ADAMTS13 activity levels (before any plasma therapy) is mandatory to confirm (if <10%) or rule out TTP, but a low-to-moderate decrease (10% to 40%) has also been reported in other gravidic TMAs. However, we have to remember that the diagnosis of TTP is still based on clinical and laboratory features and, as soon as the clinical diagnosis has been postulated, the appropriate treatment of acute TTP should be started and should not be delayed until ADAMTS13 results are obtained. ADAMTS13 activity and anti-ADAMTS13 antibodies will also help in differentiating congenital vs acquired TTP. Therefore, it is important to take some plasma samples before the start of plasma treatment. Several scores have been recently developed (out of the gravidic setting) to predict the risk of severe deficiency of ADAMTS13, with the primary aim to distinguish TTP from cHUS, in order to administer the targeted available treatment; in these scores (plasmic and French scores), platelet count and creatinine levels are crucial, since platelets >30 000/μL and creatinine >2 mg/dL almost eliminates a TTP diagnosis.20 In fact, platelets are usually not severely decreased in cHUS, while the hallmark of disease is kidney damage, with most cases showing severe kidney injury requiring dialysis and a high risk of progression to end-stage kidney disease if unrecognized and untreated appropriately (ie, eculizumab and supportive care for renal failure).
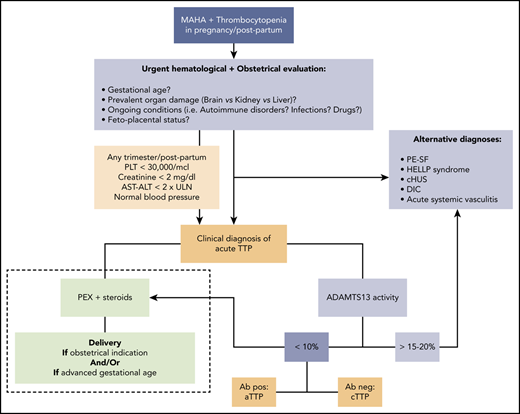
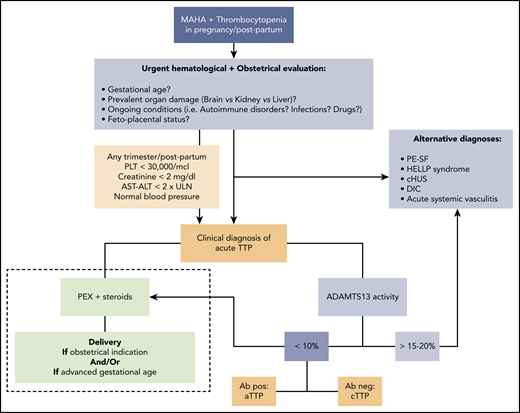
In practice, when we are facing a previously healthy pregnant woman with biochemical features of acute TMA with severe thrombocytopenia (especially <30 000/μL) and absent-to-mild renal and hepatic involvement (creatinine <2 mg/dL, AST/alanine aminotransferase (ALT) <2× ULN), especially if neurological impairment is present in the absence of hypertension, we assume acute TTP until proven otherwise, and we start the appropriate treatment as soon as possible if the fetal parameters are not life threatening (Figure 1).
Approach to acute TTP: differential diagnosis and treatment (dashed line). Ab, anti-ADAMTS13 antibody; cHUS, complement-mediated hemolytic uremic syndrome; MAHA, microangiopathic hemolytic anemia; PEX, plasma exchange; PLT, platelet.
Approach to acute TTP: differential diagnosis and treatment (dashed line). Ab, anti-ADAMTS13 antibody; cHUS, complement-mediated hemolytic uremic syndrome; MAHA, microangiopathic hemolytic anemia; PEX, plasma exchange; PLT, platelet.
Treatment of pregnancy-associated TTP
Back to our cases
In cases 1 and 2, clinical and biochemical features were highly suggestive of acute TTP. After sampling for ADAMTS13, daily PEX and steroids were started immediately.
Case 1 achieved clinical and biochemical remission, with a total of 18 PEX sessions, and steroids were continued after PEX withdrawal. Thereafter, the pregnancy proceeded normally for both the mother and the fetus. Baseline pretreatment ADAMTS13 activity levels were <10% in the presence of anti-ADAMTS13 autoantibodies; the diagnosis of aTTP was confirmed. ADAMTS13 was regularly checked after achieving remission (fortnightly for the first 2 months and then monthly); activity levels remained >40% during steroids tapering but, once stopped, gradually decreased to 23%. In the absence of overt TTP relapse, only steroids were restarted; subsequent fortnightly ADAMTS13 controls demonstrated no further drop until vaginal delivery (at 38 weeks gestation) and also in the postpartum period.
Case 2 also demonstrated baseline pretreatment undetectable ADAMTS13 activity levels, but in the absence of anti-ADAMTS13 autoantibodies; the diagnosis of TTP was confirmed and, given the suspicion of cTTP, genetic analysis was requested. Despite achieving a persistent and complete normalization of LDH and ADAMTS13 activity levels (>50%) on plasma therapy, the patient had an additional obstetrical complication that delayed her platelet normalization (maximum platelet count of 110 000/μL); she developed high blood pressure and transaminase increase (AST and ALT ≤7× and 11× ULN, respectively), with only mild proteinuria (0.455 g/24 hours). A diagnosis of concomitant PE-SF/HELLP was made and caesarian section performed at 23 + 2 weeks gestation, since continuation of pregnancy would have exposed the mother to high risks with no benefit to fetal health, which was already compromised by severe IUGR. Blood pressure, liver test results, and platelets were completely normalized by 72 hours postpartum. Plasma therapy was continued for 4 weeks after delivery due to recurrence of isolated thrombocytopenia after a first attempt at therapy withdrawal at 2 weeks postpartum. Genetic analysis revealed double heterozygous ADAMTS13 mutations, one on exon 19 (c.2281G>A, p.Gly761Ser) and a novel one on exon 18 (c.2115G>A, p.Trp705*), confirming a case of cTTP.
A correct and prompt diagnosis of gravidic TTP is crucial for maternal and fetal outcome, since untreated women develop impaired placental circulation due to microvascular thrombosis, including placental ischemia and infarction leading to IUGR and/or miscarriage or stillbirth.
Regarding treatment choices, a quick differential diagnosis is essential for deciding whether urgent delivery is needed. Delivery is a therapeutic tool for PE-SF/HELLP syndrome, whereas it is not for TTP (and cHUS), where delivery should be considered in emergency only if required by fetal conditions (fetal distress or severe IUGR) or in case of maternal worsening despite proper therapy. However, in all TTP cases occurring near term, it would be wise to perform delivery as soon as maternal conditions allow it.
Apart from the decision on delivery, the treatment of pregnancy-associated TTP does not differ from non–pregnancy-associated TTP, taking into account the potential drug toxicity on the fetus (Figure 1, dashed line). Daily therapeutic PEX has to be started as soon as possible (at least 1 but preferably 1.5 plasma volume exchanges daily), with plasma infusion to be considered only if PEX is not available. In those cases where the diagnosis of cTTP is ascertained, plasma infusion is sufficient to correct ADAMTS13 severe deficiency; the typical initial schedule requires 10 to 15 mL/kg daily until clinical remission and platelet and LDH normalization and then regular maintenance infusions throughout pregnancy and puerperium (usually twice a month; see next paragraph for more details). For autoimmune TTP, immunosuppressive therapy is also required; corticosteroids are used as first-line therapy (we usually prescribe oral prednisolone, 1 mg/kg daily), with association of azathioprine as steroid-sparing drug, if appropriate. Although anti-CD20 therapy has been reported as a salvage therapy in life-threatening gravidic TTP,21 rituximab should be avoided based on the lack of safety evidence in pregnancy and should only be considered postpartum.
If remission is achieved (clinical stability, platelet normalization, and absence of hemolysis), then pregnancy is carried on as long as maternal and fetal conditions allow it. Along with maternal clinical and biochemical monitoring (including serial ADAMTS13 evaluations), fetal conditions need to be regularly assessed through fetal ultrasound examination and uterine artery Doppler scan.15 The type and frequency of maintenance treatment will be customized but will be needed in almost all women throughout the postpartum period; in the more benign cases, steroids will be sufficient to suppress autoantibody production, while in the more severe cases, PEX will be continued throughout pregnancy. In our clinical practice, we monitor ADAMTS13 activity level at least monthly after remission with the aim of maintaining it >10% or preferably >20% to 25%. The latter alarm threshold for ADAMTS13 activity has been chosen for 2 reasons: (1) the physiological decline of ADAMTS13 reaches a minimum activity of 25% to 30% during regular pregnancies in healthy women,10 and (2) reduced levels of ADAMTS13 activity (<25%) in the first trimester may be associated with an increased risk of gravidic complications (both gravidic TTP and miscarriage).22
Once the platelet count is >50 000/μL, low-dose aspirin and/or thromboprophylaxis with low-molecular-weight heparin can be considered to reduce the risk of additional placental vascular damage, based on fetal and placental scan and overall venous thrombotic risk. In our clinical practice, we do not routinely prescribe aspirin to all TTP women during pregnancy, unless there is a specific gynecologic indication, since the pathogenesis of clots in acute TTP does not depend on cyclooxygenase-mediated platelet–platelet aggregation but on inappropriate ULVWF-mediated platelet clumping.
Last, but not least, we have to consider also the risk of concurrent TMAs during the same pregnancy (as in case 2) and the risk of different gravidic TMAs in subsequent pregnancies. In particular, the risk of PE seems to be higher in women with a history of aTTP (31% vs 3% in the general population, according to the results of the Oklahoma TTP-HUS registry).23,24 The association may be at least partially explained by common risk factors (ie, black race and obesity, both associated with PE and TTP), but also by the possible overlap of pathogenetic mechanisms (for example, through complement activation). Obviously, the coexistence of different TMAs requires a complex multidisciplinary evaluation and appropriate treatment choices.
Management of subsequent pregnancy
Case 3 is a young woman affected by acquired TTP, diagnosed after a single non–pregnancy-related event. Two years after acute TTP, she experienced an early miscarriage (at 7 weeks gestation), in the absence of overt gravidic TTP relapse, while her ADAMTS13 activity levels were 20%. In anticipation of a subsequent pregnancy, she was referred to our center. We discussed pros and cons and proposed antiCD20 therapy, which achieved complete normalization of her ADAMTS13 activity levels (≤95%). Her subsequent pregnancy was strictly monitored and uneventful (except for gestational diabetes), with vaginal delivery at term. During pregnancy, she received no treatment other than low-dose aspirin due to her previous obstetrical history. Her ADAMTS13 activity levels were checked monthly and showed a slow but progressive decrease (from a high of 61% shortly before conception to a low of 23% at 38 weeks gestation in the absence of anti-ADAMTS13 antibodies), requiring no specific therapy. One week after delivery, ADAMTS13 levels were as low as 10% in the absence of clinical or biochemical signs of active disease, so we decided to take a wait-and-see approach, and TTP relapse did not occur.
Clinical practice on how to manage subsequent pregnancy in women with TTP has significantly changed in the last few decades. For many years, data on the risk of gravidic TTP recurrence were conflicting, mainly because the conclusions were drawn from mixed populations, including not only aTTP and cTTP but also non-TTP gravidic TMAs.12,25-31 Because of this uncertainty and the high mortality and morbidity rates of acute TTP, women with a previous diagnosis of TTP (both pregnancy related and non–pregnancy related) were discouraged from having a subsequent pregnancy.
Nowadays, it is clear that the risk of recurrence is not related to the history of TTP per se but is strictly dependent on the persistence of severe ADAMTS13 deficiency. In cTTP, the risk of gravidic relapse is almost 100%, since severe ADAMTS13 deficiency persists in the absence of adequate replacement therapy; on the contrary, ADAMTS13 levels in women with aTTP may or may not recover sufficiently to minimize the risk of recurrence in future pregnancy, carrying a risk of gravidic relapse. Until now, there was no available tool to predict ADAMTS13 persistent normalization rather than its periodic evaluation. In aTTP patients, a regular ADAMTS13 monitoring before, during, and after pregnancy is essential for assessing the risk of relapse and choosing appropriate therapeutic strategies. It would be cautious to keep ADAMTS13 activity higher than 20% to 25%.
For the reasons above, in our clinical practice, we generally propose proper counseling with our female patients of childbearing age to discuss the option of gestation, preferably before they plan to conceive, and then we follow them together with our Obstetrics Unit for regular multidisciplinary controls. Women are informed that (1) the risk of TTP recurrence during pregnancy will never be zero but will be minimized through careful evaluation and a close ADAMTS13 monitoring during pregnancy, with maintenance of ADAMTS13 activity levels >20% to 25%22 ; (2) in case of gravidic TTP recurrence, even despite proper treatment, the risk of fetal morbidity is not zero, especially for IUGR, miscarriage, and second-trimester stillbirth6,22 ; and (3) there is an increased risk of other pregnancy complications (ie, PE), requiring close obstetrical follow-up.
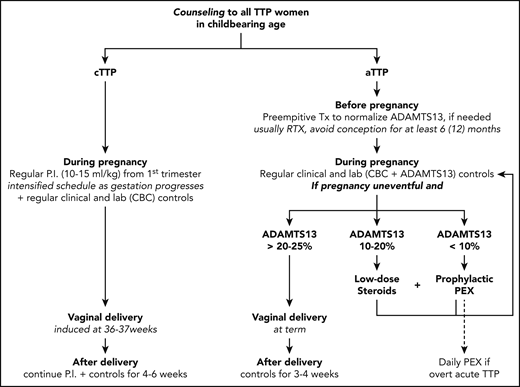
Management of women with TTP in subsequent pregnancies is reported in Figure 2.
Management of women with TTP in subsequent pregnancies. P.I., plasma infusion; lab, laboratory; Tx, therapy; RTX, rituximab.
Management of women with TTP in subsequent pregnancies. P.I., plasma infusion; lab, laboratory; Tx, therapy; RTX, rituximab.
In aTTP, anti-CD20 therapy is offered to those cases with persistent severe ADAMTS13 deficiency, with the aim of normalizing the protease level before conceiving. After rituximab administration, we advise waiting at least 6 (to 12) months before attempting pregnancy.32 During pregnancy, CBC should be monitored monthly and ADAMTS13 activity levels at least once per trimester (monthly in our practice), with intensification of controls in case of biochemical abnormalities (platelet reduction/LDH increase), especially if ADAMTS13 levels decrease.
If the pregnancy is uneventful and the ADAMTS13 activity level remains >20% to 25%, then vaginal delivery at term is advised, with monitoring of CBC at least every other day for 3 to 5 days after delivery and of ADAMTS13 levels within 3 weeks postpartum.
If ADAMTS13 activity levels decrease to <20% to 25% during pregnancy but in the absence of overt acute TTP (normal platelet count and LDH levels, no hemolysis), we generally start low-dose corticosteroids (oral prednisolone, 0.5 mg/kg daily) to reduce autoantibody production; if this approach is insufficient to correct ADAMTS13, or if levels fall to <10%, we start prophylactic PEX, to be continued weekly or fortnightly based on its efficacy in preventing TTP relapse and correcting ADAMTS13 activity. The PEX schedule becomes daily in case of overt acute TTP. In a gravidic case with severe TTP and no response to PEX and immunosuppressive therapy, maternal and fetal health should be monitored very closely by maternal clinical symptoms and laboratory evaluation (platelet reduction/LDH increase), together with fetal ultrasound. In case of a dramatic worsening, delivery might be considered near term or premature termination of pregnancy at earlier stages of gestation.
In cTTP, regular plasma infusions (standard dose of 10-15 mL/kg) should be started at the very outset of pregnancy and continued until 4 to 6 weeks postpartum. The infusion schedule must be intensified as gestation progresses; usually, the initial frequency of infusion is fortnightly, while in the second/early third trimester it is weekly or even every other day. Moreover, regardless of gestational age, the infusion schedule is intensified in case of clinical and/or laboratory worsening (platelet reduction/LDH increase). In some very severe cases, near the end of pregnancy, even daily plasma infusion might not be enough to avoid recurrence of disease, and PEX may help remove ULVWF multimers. For this reason, vaginal delivery is generally induced at 36 to 37 weeks gestation, and plasma infusions are continued for at least 3 weeks after delivery.
As mentioned above, we do not usually prescribe low-dose aspirin and/or thromboprophylaxis with low-molecular-weight heparin, except to women with a previous history of gravidic complications related to placental flow impairment and/or higher thrombotic risk.
Conclusion
Acute TTP is a medical emergency and requires a multidisciplinary team approach, especially when occurring during pregnancy or in the postpartum period. Clinical judgment and ADAMTS13 measurement are crucial for a correct diagnosis and an appropriate therapeutic approach. Current available treatments (plasma therapy and ikmmunosuppressors) dramatically improved maternal and fetal prognosis of acute pregnancy-associated TTP and, together with ADAMTS13 monitoring, allow planning a safe subsequent pregnancy in women affected by this rare disease.
Acknowledgments
The authors thank the entire staff of Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Fondazione Luigi Villa, for their invaluable everyday work, which allows ADAMTS13 testing to be performed in a timely manner. Special thanks go to Luigi Flaminio Ghilardini for his help in preparing the figures. The authors are also grateful to all researchers and patients who participate in the Milan TTP Registry, contributing to knowledge of this rare disorder.
This work was (partially) supported by the Italian Ministry of Health–Bando Ricerca Corrente.
Authorship
Contribution: F.P. and B.F. wrote this paper.
Conflict-of-interest disclosure: F.P. has received honoraria for participating as a speaker at satellite symposia and educational meetings organized by Bioverativ, Grifols, Roche, Sanofi, Sobi, Spark, and Takeda and reports participation on the advisory boards of Sanofi and Sobi. B.F. has received honoraria for scientific consultancy at educational meetings organized by Sanofi Genzyme and received travel support from Sanofi Genzyme and Bayer.
Correspondence: Flora Peyvandi, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center; Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Via Pace 9, 20122, Milan, Italy; e-mail: flora.peyvandi@unimi.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal