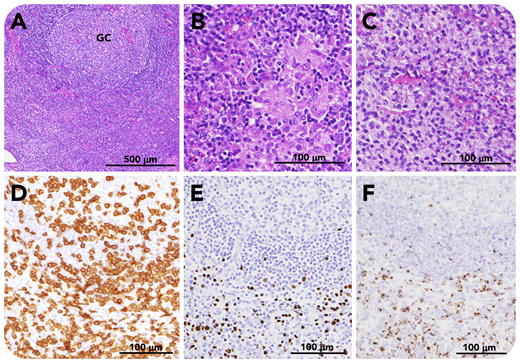
A 47-year-old man presented with persistent fever and fatigue. Laboratory findings included thrombocytopenia, hepatic dysfunction, polyclonal hypergammaglobulinemia, and evidence of systemic inflammation. Computed tomography scanning demonstrated anasarca, hepatosplenomegaly, and generalized lymphadenopathy. Viral screening revealed positive Epstein-Barr virus (EBV) capsid antigen (VCA) immunoglobulin G (IgG; 1:20 480); nuclear antigen antibody (1:40) and early antigen-diffuse/restricted type IgG (1:1280); and negative VCA-IgM. EBV-DNA of peripheral blood mononuclear cells was also positive (11 000 copies per 106 cells). Axial lymph node biopsy shows preserved nodal structure, paracortical hyperplasia, and an enlarged lymphoid follicle (panel A, hematoxylin and eosin stain; GC, germinal center) with epithelioid granuloma (panel B, hematoxylin and eosin stain) and monocytoid B-cell infiltration (panel C, hematoxylin and eosin stain). CD3+ T cells are present in the interfollicular zone (panel D, CD3 stain), where EBV-encoded small RNA (EBER)-positive cells are distributed (panel E, EBER in situ hybridization (ISH)). T cells are positive for granzyme B (panel F, granzyme B stain) (scale bars: panel A, 500 μm; panels B-F, 100 μm). No cells expressed EBV latent membrane protein or EBNA-2. The patient was diagnosed with chronic active EBV infection (CAEBV). Cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisone (CHOEP) chemotherapy was given followed by allogeneic hematopoietic stem cell transplantation.
CAEBV is a rare, but life-threatening, lymphoproliferative disorder with persistent infectious mononucleosis-like symptoms. Although the pathological findings lack distinctive histological features, EBER-ISH to identify EBV-infected cells and EBV-DNA viral load are critical in making the diagnosis of CAEBV.
A 47-year-old man presented with persistent fever and fatigue. Laboratory findings included thrombocytopenia, hepatic dysfunction, polyclonal hypergammaglobulinemia, and evidence of systemic inflammation. Computed tomography scanning demonstrated anasarca, hepatosplenomegaly, and generalized lymphadenopathy. Viral screening revealed positive Epstein-Barr virus (EBV) capsid antigen (VCA) immunoglobulin G (IgG; 1:20 480); nuclear antigen antibody (1:40) and early antigen-diffuse/restricted type IgG (1:1280); and negative VCA-IgM. EBV-DNA of peripheral blood mononuclear cells was also positive (11 000 copies per 106 cells). Axial lymph node biopsy shows preserved nodal structure, paracortical hyperplasia, and an enlarged lymphoid follicle (panel A, hematoxylin and eosin stain; GC, germinal center) with epithelioid granuloma (panel B, hematoxylin and eosin stain) and monocytoid B-cell infiltration (panel C, hematoxylin and eosin stain). CD3+ T cells are present in the interfollicular zone (panel D, CD3 stain), where EBV-encoded small RNA (EBER)-positive cells are distributed (panel E, EBER in situ hybridization (ISH)). T cells are positive for granzyme B (panel F, granzyme B stain) (scale bars: panel A, 500 μm; panels B-F, 100 μm). No cells expressed EBV latent membrane protein or EBNA-2. The patient was diagnosed with chronic active EBV infection (CAEBV). Cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisone (CHOEP) chemotherapy was given followed by allogeneic hematopoietic stem cell transplantation.
CAEBV is a rare, but life-threatening, lymphoproliferative disorder with persistent infectious mononucleosis-like symptoms. Although the pathological findings lack distinctive histological features, EBER-ISH to identify EBV-infected cells and EBV-DNA viral load are critical in making the diagnosis of CAEBV.
For additional images, visit the ASH Image Bank, a reference and teaching tool that is continually updated with new atlas and case study images. For more information, visit http://imagebank.hematology.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal